Deflagration to detonation transition in weakly confined conditions for a type of potentially novel green primary explosive: Al/Fe2O3/RDX hybrid nanocomposites
2023-05-06QingpingLuoXinpingLongFudeNieGuixingLiuChoWu
Qing-ping Luo ,Xin-ping Long ,Fu-de Nie ,Gui-xing Liu ,Cho Wu
a State Key Laboratory of Environmental-friendly Energy Materials,Southwest University of Science and Technology,Mianyang,621010,China
b Institute of Chemical Materials,Chinese Academy of Engineering Physics,Mianyang,621900,China
Keywords:Green primary explosives Al/Fe2O3/RDX nanocomposites Deflagration to detonation transition Mechanism
ABSTRACT The properties of the combustion and deflagration to detonation transition(DDT)of Al/Fe2O3/RDX hybrid nanocomposites,a type of potentially novel lead-free primary explosives,were tested in weakly confined conditions,and the interaction of Al/Fe2O3 nanothermite and RDX in the DDT process was studied in detail.Results show that the amount of the Al/Fe2O3 nanothermite has a great effect on the DDT properties of Al/Fe2O3/RDX nanocomposites.The addition of Al/Fe2O3 nanothermite to RDX apparently improves the firing properties of RDX.A small amount of Al/Fe2O3 nanothermite greatly increases the initial combustion velocity of Al/Fe2O3/RDX nanocomposites,accelerating their DDT process.When the contents of Al/Fe2O3 nanothermite are less than 20 wt%,the DDT mechanisms of Al/Fe2O3/RDX nanocomposites follow the distinct abrupt mode,and are consistent with that of RDX,though their DDT processes are different.The RDX added into the Al/Fe2O3 nanothermite increases the latter's peak combustion velocity and makes it generate the DDT when the RDX content is at least 10 wt%.RDX plays a key role in the shock compressive combustion,the formation and the properties of the DDT in the flame propagation of nanocomposites.Compared with RDX,the fast DDT of Al/Fe2O3/RDX nanocomposites could be obtained by adjusting the chemical constituents of nanocomposites.
1.Introduction
Primary explosive is a sensitive explosive which is widely used in military ammunitions and civil applications.Relatively slight external stimuli,such as a flame or other heat,friction,static discharge,and so forth,can cause it to deflagrate or detonate and release enough energy to set off other explosives.Traditional primary explosives mostly contain toxic elements such as acid-rich mercury,lead azide,and lead benzoate [1].This inherent shortcoming can cause it to produce pollutants harmful to organisms or the environment[2,3].Therefore,they are restricted in military and civilian applications.Consequently,novel green primary explosive substitutes have attracted much attention from the researchers in the field of primary explosives [4,5].Some studies on green initiating explosive substitutes,such as the tetrazoles and their derivatives [6—11],and furazans [12]have been published.However,the processes for synthesizing these primary explosives are very complicated[6,11,12]and they also generate significant amounts of pollutants [6,9,12].In the past two decades,some novel nanoenergetic materials (e.g.,metastable intermolecular composites)with potential applications in the primary explosives field have also attracted the interest of military experts [13,14],and the energetic nanocomposites with tunable formulation and controllable properties might resolve the issues surrounding current green primary explosives.
As a nano-energetic material,nanothermite with the exceptional ignition and energy release qualities,has gradually attracted the attention of researchers in the fields of propellants,explosives,and pyrotechnics [5,15—18].Nanothermite is eco-friendly and has high thermal stability and a high combustion velocity of~2400 m/s(the combustion velocity refers to the flame propagation distance at certain direction in unit time for loose nanothermite that is confined in cylindrical tubing.),which is in the range of explosive velocity which is the flame propagation velocity of explosive when it explodes [5],so it has the potential for use as a green primary explosive.Nanothermites composed of a metal and a metallic oxide have higher exothermic properties than that composed of a metal and a nonmetallic oxide,but these nanothermites cannot selfdetonate because their thermite reactions do not directly generate gases.Secondary explosives,like RDX and HMX,do generate massive amounts of gases (e.g.,H2,H2O,N2,NO,CO,CO2et al.) and detonate during the chemical reaction,but their combustion velocities are lower than that of nanothermites and therefore cannot quickly cause the deflagration to detonation transition Nanothermites mixed with secondary explosives,however,would possess the characteristics of both nanothermites and high explosives.Based on this idea,some studies on the kind of hybrid nanocomposite have been conducted [19—21],and their combustion characteristics show that they could cause a rapid deflagration-to-detonation transition (DDT) that could be accelerated to the primary explosive level[20]and so used to initiate the detonation of a high explosive [21].In addition,the process for preparing the lead-free hybrid nanocomposite is simple and generates fewer,less-toxic pollutants.Thus,a combination of nanothermite and high explosive might overcome some shortcomings of existing green primary explosives,and so a kind of novel green initiatory substitute for a range of initiatory applications might be achieved by this combination of substances.
Due to its excellent inherent properties,the nitramine explosive RDX is widely used as a high explosive,an additive to powder,and an oxidizer in advanced composite propellants.Al/Fe2O3nanothermite has a high energy release rate and combustion velocity,and could increase the combustion velocity of RDX more effectively than other nanothermites for the catalytic effect of nano-Fe2O3on the decomposition of RDX and thereby causing RDX to generate a faster DDT than that RDX generates by itself.The fast DDT of a primary explosive is crucial to its applications in the initiation devices.Though Qiao et al.[20]investigated the DDT properties of quasi-core/shell structured RDX@Fe2O3—Al composites in strongly confined conditions of steel tube,but we could not effectively observe the DDT process of Al/Fe2O3/RDX hybrid nanocomposite and obtain the mechanism of its DDT process.In addition,the process for preparing RDX@Fe2O3—Al nanocomposite is very complicated.Therefore,the Al/Fe2O3/RDX hybrid nanocomposite was prepared by a simple mixing method in the paper,its DDT properties were studied in weakly confined conditions of transparent quartz glass tube and the effect of the Al/Fe2O3nanothermite on RDX in the DDT process was discussed in detail.
2.Experimental
2.1.Preparation of materials
The super fine RDX (SFRDX) used in our experiment was prepared by spurting RDX solution into deionized water at high pressure in our laboratory and its particle size is in the range of 1—5 μm as described in Ref.[22].The passivated nano-Al powder with the particle size of 40 nm and active aluminum content of 52 wt% was obtained from Wuhan Nanoparticle Co.,Ltd.Nano-Fe2O3was synthesized by the sol-gel method and low temperature CO2supercritical process as described in Ref.[23]and its specific surface area was measured by an American Quantachrome ADSORP gas adsorption analyzer using BET theory.The specific surface area of nano-Fe2O3used in our experiment is 170 m2/g.The Al/Fe2O3/RDX nanocomposites were prepared by mixing RDX with the nanothermite consisted of nano-Fe2O3and nano-Al powder according to the equivalence ratio of 1.1 (Al-rich) in the solvent cyclohexane as described in Ref.[22].An ultrasonification process lasting 3 h was applied to the solution to ensure more homogeneous mixing.A powder consisting of the Al/Fe2O3/RDX nanocomposites was then obtained by drying at 55°C for 48 h in a vacuum drying cabinet in order to eliminate the solvent.The Al/Fe2O3nanothermite prepared by the previous process possesses the specific surface area of 133 m2/g and the pore volume of 0.5766 ml/g,and the specific surface area and pore volume of finished Al/Fe2O3/RDX nanocomposites are proportional to the Al/Fe2O3nanothermite contents.The resulting hybrid nanocomposites were labelled with their RDX contents (for example,10 wt% Al/Fe2O3,90 wt% RDX=R-90).
2.2.DDT testing of the Al/Fe2O3/RDX nanocomposites
The finished Al/Fe2O3/RDX nanocomposites were placed into a 20-(or 40-)cm-long quartz glass tube with the diameter of 2.5 mm at a loading density of 0.4 g/cm3.The initial ignition was achieved by an electric arc stimulus from an initiation probe of a standard common mine detonator (initiation voltage~1500 V).A Photron(Japan) APX-RS high-speed camera captured the visible emission light and recorded the DDT process at 100,000 frames per second(fps).The experimental setup used for the DDT test is shown in Fig.1.
3.Results
3.1.The firing properties of Al/Fe2O3/RDX nanocomposites
Before subsequent testing of the combustion and DDT properties of the Al/Fe2O3/RDX nanocomposites in the quartz glass tube,their firing properties were investigated to determine their firing conditions.First,we tested to see if the electric arc stimulus from the initiation probe alone would ignite them.If not,then ignition charges like B/KNO3or nanothermite were loaded at the front of the explosive bed,and the arc from the initiation probe was applied once more.Table 1 gives the results of these initial tests.
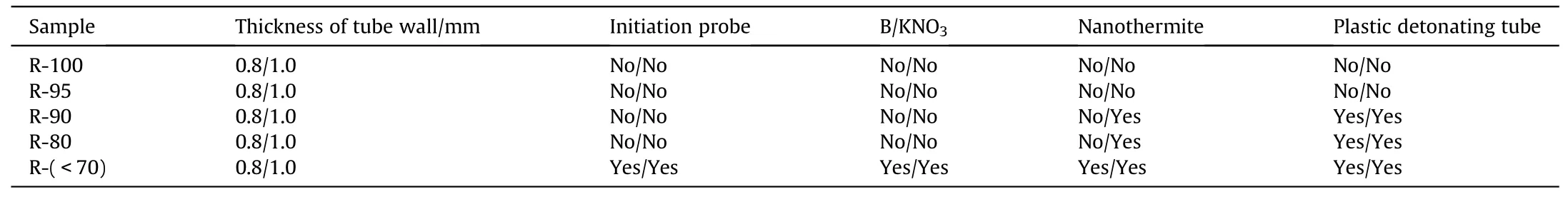
Table 1 Firing properties of Al/Fe2O3/RDX nanocomposites under different conditions.
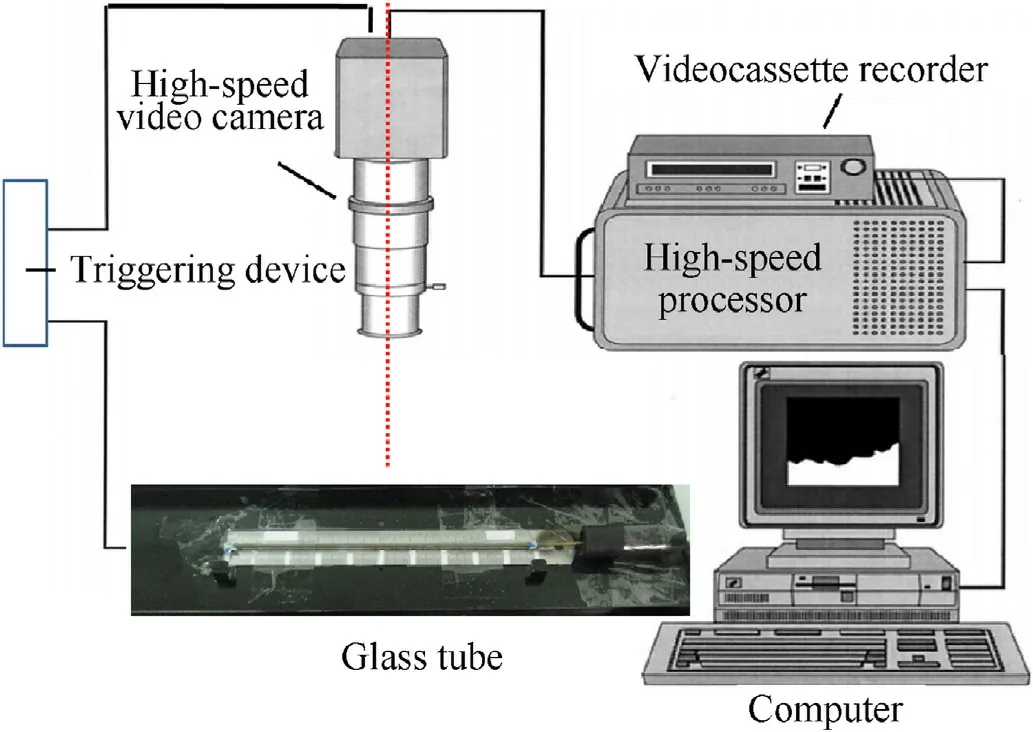
Fig.1.Experimental setup for the DDT performance testing of Al/Fe2O3/RDX hybrid nanocomposites.
As shown in Table 1,the pure RDX in the glass tube is very stable and does not fire when an electric arc stimulus from the initiation probe,a heat stimulus from B/KNO3or nanothermite,or a plastic detonating tube made up of RDX and Al are used during its ignition and the thickness of tube wall is 0.8 mm or 1 mm.The firing property of RDX is greatly improved when Al/Fe2O3nanothermite is added to it.The Al/Fe2O3/RDX nanocomposites fire more and more easily as the amount of Al/Fe2O3nanothermite in them increases.When the amount of Al/Fe2O3nanothermite is less than 20 wt%,the ignition probe is unable to ignite the nanocomposites directly,but it is able to do so when the amount of Al/Fe2O3nanothermite exceeds 30 wt%.This may be due to the fact that the Al/Fe2O3nanothermite consisted of nano-Fe2O3and nano-Al powder has a high specific surface area,and is more sensitive to external electrical or heat stimulus than RDX [22],so the addition of nanothermite to RDX causes an increased reactivity in the resulting Al/Fe2O3/RDX nanocomposites.
Table 1 shows that the presence and amount of Al/Fe2O3nanothermite as well as the thickness of the glass tube wall have a significant effect on the ignition properties of Al/Fe2O3/RDX nanocomposites.When loaded into a tube with a 1 mm-thick wall,the nanocomposites R-80 and R-90 could be ignited by both the nanothermite and the plastic detonating tube,but not if the tube wall is only 0.8 mm thick.These results indicate that the exact nature of the confinement has a major effect on the ignition of nanocomposites-specifically,that the stronger the confinement provided by the tube,the easier the ignition.
Table 1 also shows the combination of the initiation probe and the plastic detonating tube is the best for igniting the nanocomposites,followed by nanothermite and the initiation probe by itself,in that order.But the Al/Fe2O3/RDX nanocomposites with a content of Al/Fe2O3nanothermite of less than 10 wt% would not ignite even when the initiation probe and the plastic detonating tube are both used.To get ignition of the nanocomposites,an ignition powder consisting of two charges of nanothermite(I)/R-80(II) was loaded in front of the nanocomposites being tested in the tube,and then the nanocomposites and pure RDX were ignited by the initiation probe.
3.2.The DDT properties of the Al/Fe2O3/RDX nanocomposites
Using the Al/Fe2O3nanothermite as a matrix,the Al/Fe2O3/RDX nanocomposites were prepared by adding RDX at respectively 1 wt%,5 wt%,10 wt%,20 wt% and 30 wt% to the Al/Fe2O3nanothermite.The finished nanocomposites were then loaded into a 20 cm-long silicon glass tube with a diameter of 2.5 mm and a thickness of 1 mm for the combustion and DDT test.The results appear in Table 2 and Figs.2 and 3.
As shown in Table 2 and Fig.2,the burning velocity of Al/Fe2O3nanothermite is very low with a peak velocity of 700 m/s in a 20 cm-long silicon glass tube.Its burning rate increases greatly when RDX is added,and the time needed for a flame propagation distance of 20 cm decreases greatly,from 580 μs to 260 μs,when the amount of RDX in the nanothermite increases by 5 wt%.The reason may be that RDX,as an energetic gas generator,decomposes after the nanothermite is ignited,giving off considerable amounts of gas and heat.This would enhance the convection combustion mechanism in flame propagation,and increase the burning rate of the Al/Fe2O3nanothermite.As the amount of RDX in the nanothermite increases,the flame front changes more and more sharply(see Fig.2(b)and(c),for instance),and the flame radius grows(see Fig.3(a) and (b)).This indicates that the convection mechanism plays a more and more important role in the burning processes of the Al/Fe2O3/RDX nanocomposites as the amount of RDX increases.Thus,one can conclude that the addition of RDX to Al/Fe2O3nanothermite improves the latter's convective combustion mechanism for gases from RDX decomposition [24],thereby increasing its initial burning rate and peak combustion velocity.
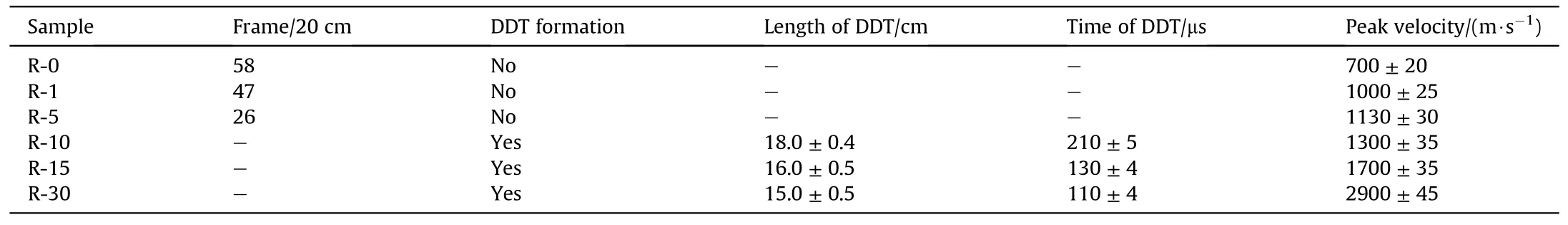
Table 2 Combustion and DDT properties of Al/Fe2O3/RDX nanocomposites with low RDX contents.
When the amount of RDX in the nanothermites exceeds 10 wt%,they generate the deflagration to detonation transition(DDT)with low detonation velocity.As the amount of RDX increases,the peak velocities of flame propagation in the DDT process also increase,and the DDT time and DDT distance decrease.We ascribe these effects to the gas phase and heat produced by the decomposition of RDX.The more RDX in the nanothermal mites,the more gas generated by the decomposition of RDX,which in turn leads to a shorter time for shock formation and from shock compression to detonation.Therefore,the DDT time of R-30 is less than that of R-10 and R-15.The DDT distance is determined by the DDT time and the average burning rate in flame propagation.So,the DDT distance and time of R-10 are the longest,and the DDT distance and time of R-30 are the shortest.
As shown in Fig.3,the DDT process of R-10 differs from that of R-15 and R-30.The initial burning rate of R-10 increases rapidly after ignition,reaching about 1000 m/s after 100 μs The burning rate begins to decrease when it approaches 1300 m/s,and the combustion wave flame begins to expand,implying that the shock compressive combustion stage appears in the DDT process for R-10,with a shock wave following behind the combustion wave(Fig.3(a),Frames 285—287).The observation for R-10 indicates its DDT mechanism follows to the MODE 1(the gradual mode,in which the compression wave produced through penetration of gaseous products after ignition of the explosive accelerates the combustion reaction of the explosive.In turn,the accelerated combustion causes the pressure accumulation to form a stronger compression wave.The pressure accumulation makes the compression wave develop into a strong shock wave.After the shock wave reaches the critical strength,it induces detonation.The combustion wave acceleration of each part is very close and the combustion velocities of explosives increase gradually in the DDT process.) [25]with low detonation velocity.
In the DDT process for R-15,the burning rate begins to decrease when it approaches 1500 m/s,this implies that the compressive combustion stage appears (Fig.3(b),Frames 363—366),which is a significant difference from R-10.The burning rate begins to decrease again when it increases to 1700 m/s,and the shock compressive combustion stage appears (Fig.3(b),Frames 369—372).Then the flame begins to expand,and a low velocity detonation occurs.The change process of combustion wave flame is clearly visible in the DDT processes for R-10 and R-15,and some information about DDT growth characteristics is obtained for future study.The foregoing description of the R-15 DDT process in a glass tube is consistent with the process as described in Ref.[26].
The DDT process of R-30 is the same as for R-15,with both the compressive burning stage and the shock compressive burningstage present.When the shock wave overtakes and coalesces with the combustion wave in the DDT process for R-30,detonation occurs (Fig.3(c)).Thus,the shock wave lies at the surface of the combustion wave,this differs from R-10 and R-15.The increased luminous intensity observed at the combustion wave surface indicates the greatly increased temperature in the zone of coalescence.
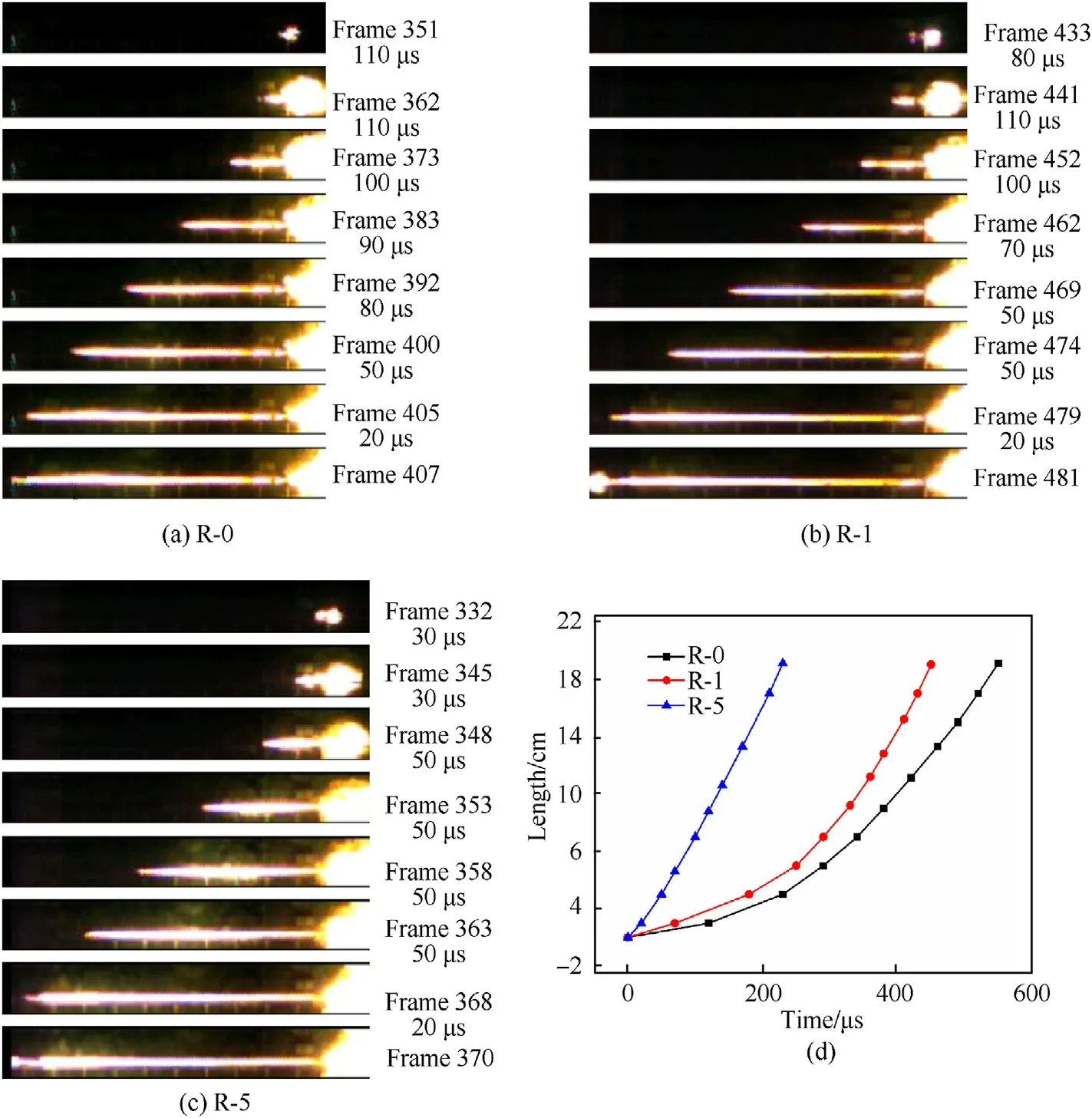
Fig.2.The burning photos for Al/Fe2O3/RDX nanocomposites with low RDX content and their corresponding time-distance curves.
The combustion wave velocity increases gradually in the DDT processes for R-10,R-15,and R-30,respectively,and there is no sudden change in the velocity of flame propagation either.Therefore,their DDT mechanisms all belong to the MODE 1 [25].
For the next phase of our experiment,RDX was used as a matrix again,and the Al/Fe2O3/RDX nanocomposites were again prepared by adding Al/Fe2O3nanothermite at respectively 1 wt%,5 wt%,10 wt%,20 wt%to the RDX.The finished nanocomposites were then loaded into a 40 cm-long silicon glass tube with a tube-wall thickness of 1 mm for the DDT test.As a comparison,the pure RDX was also tested under the same conditions.The results appear in Table 3 and Figs.4 and 5.
As shown in Table 3 and Figs.4 and 5,when a small amount of nanothermite(≤5 wt%)is added to RDX,the peak velocity and the DDT distance and DDT time decrease in the DDT process of the Al/Fe2O3/RDX nanocomposites as increase in the amount of the Al/Fe2O3nanothermite.The reason may be that the added Al/Fe2O3nanothermite has high thermal conductivity [27]and chemical reactivity,and releases a great amount of heat in thermite reaction.These improve the heat transmission by conductivity and also enhance the conduction mechanism for the reaction process of the Al/Fe2O3/RDX nanocomposites.In addition,the nano-Fe2O3in the Al/Fe2O3nanothermite has a catalytic effect on the decomposition of RDX.A small amount of nanothermite added to RDX helps the initial burning rate of RDX,after ignition,to increase rapidly(Figs.4 and 5).It also accelerates the DDT process for the nanocomposite and leads to the formation of DDT at lower detonation velocity and in a shorter time than for pure RDX.The faster DDT of nanocomposite is obtained by adding nanothermite to pure RDX.
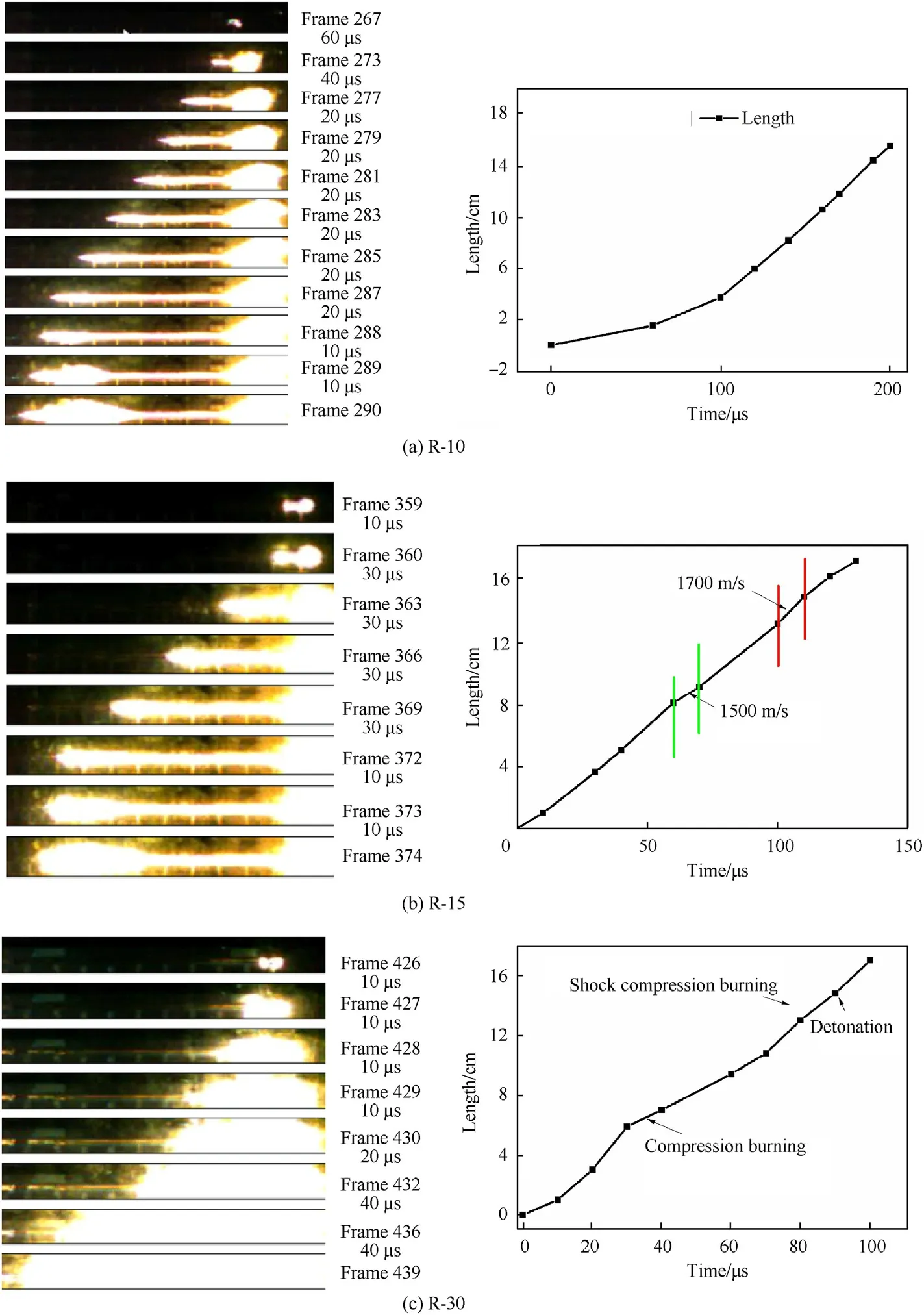
Fig.3.Photos of DDT for Al/Fe2O3/RDX nanocomposites with low RDX content and their corresponding interpretive graphs.
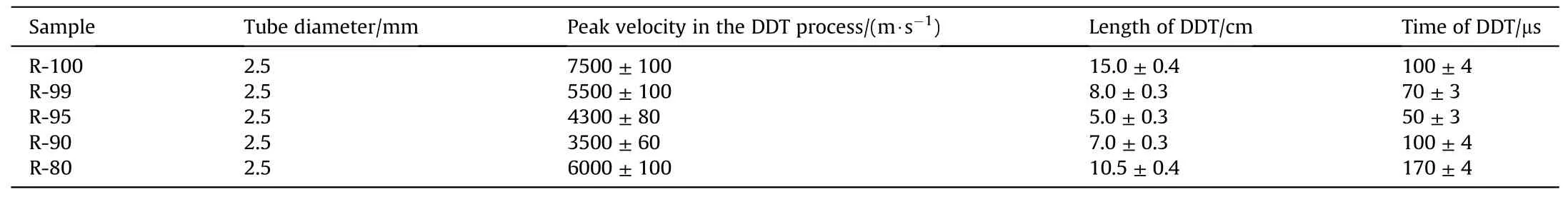
Table 3 The DDT properties of Al/Fe2O3/RDX nanocomposites with high RDX contents.
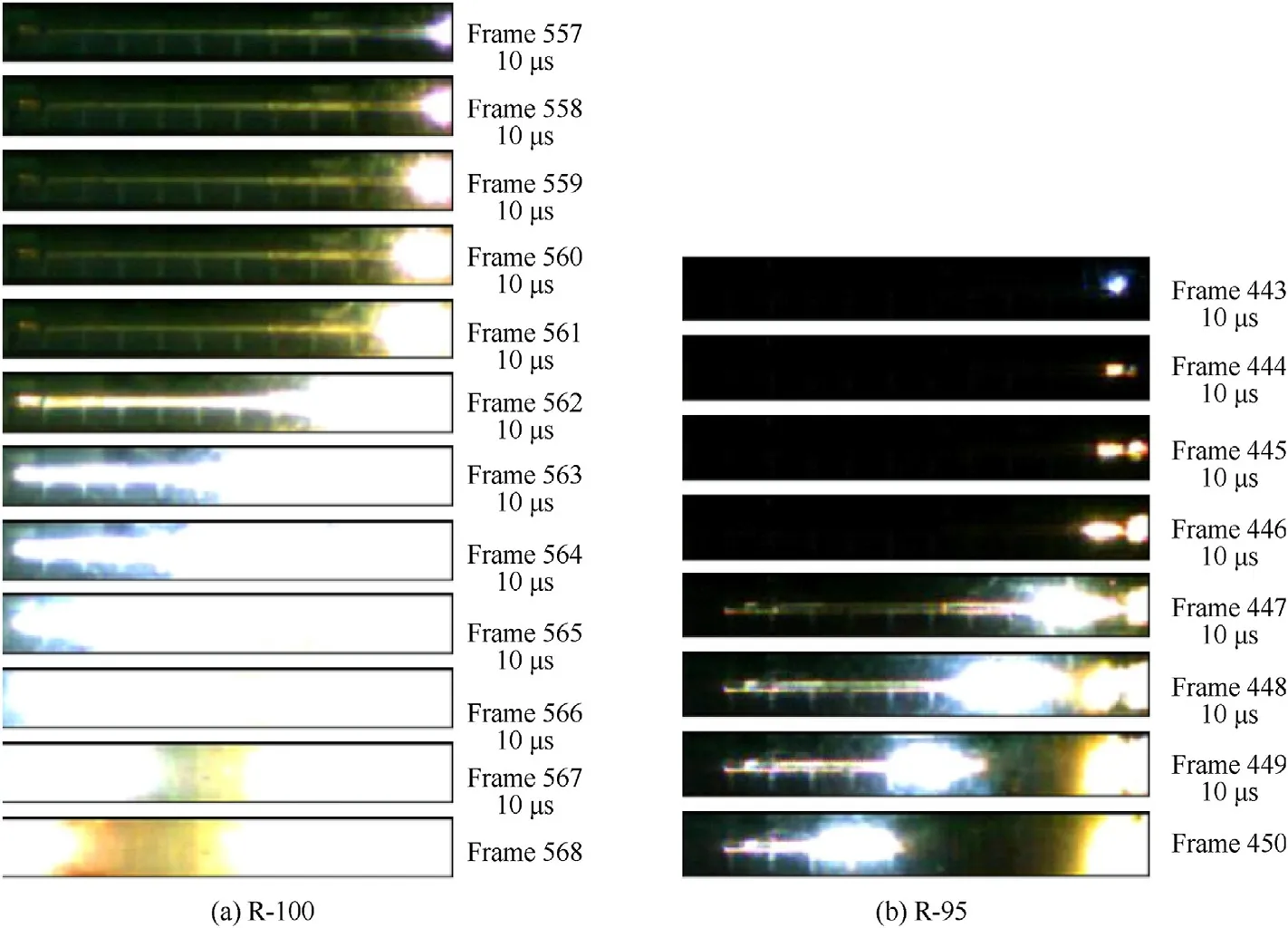
Fig.4.Photos of DDT for pure RDX and Al/Fe2O3/RDX nannocomposites R-95.
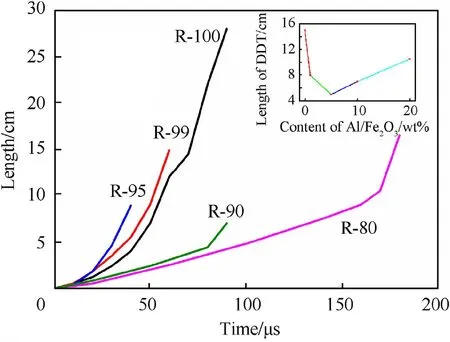
Fig.5.DDT curves for Al/Fe2O3/RDX nanocomposites with low Al/Fe2O3 content.
Up to a certain point—e.g.,20 wt%–as the amount of Al/Fe2O3nanothermite in the Al/Fe2O3/RDX nanocomposites increases,the peak velocity and the DDT distance and time in the DDT process also increase.This may be due to the inability of the nanothermite to generate gas,thereby retarding the convective mechanism in flame propagation.Too much nanothermite leads to a reduction in the total amount of the gas phase produced and a lower combustion velocity than for pure RDX and the nanocomposites with a lower amount of the nanothermite.This delays the formation of the DDT for the nanocomposites and increases their DDT time and distance.Since the adiabatic flame temperature for the thermite reaction of the Al/Fe2O3nanothermite is 3135 K and the products of the thermite reaction are solids,the high flame temperatures coupled with the solid products would provide a more intense source for radiation transport than broadband emitting gas phase products.The sequence of images in the DDT process indicates that the flame is highly luminescent and that radiation transport may play an important role in flame propagation.The increase of peak velocity may result from the coupling of the convective and the radiative mechanisms in flame propagation.
If the amount of the Al/Fe2O3nanothermite in the nanocomposites increases continuously,this further enhances the conduction mechanism and weakens the convective mechanism in flame propagation,leading to the decrease of the peak velocity in the DDT process.DDT with a low detonation velocity eventually appears (see for example the results for R-30).
Figs.4(a)and 6 show the DDT process for pure RDX in a silicon glass tube.As shown in Fig.6,RDX undergoes five stages in flame propagation according to the changes in the burning rate of RDX after ignition,these stages,plus the initial ignition stage and final detonation stage,match the seven stages of the DDT process as outlined by R.R.Bernecker et al.[26]and H.W.Sandusky et al.[28].The observations for pure RDX suggest that the detonation wave forms behind the combustion wave,and the final stage of its DDT mechanism is closer to the MODE 2 (the distinct abrupt mode,in which the pressure increases linearly with time after ignition of the explosive.At the downstream of the ignition area and close to the detonation point(about 3/4 of the induced detonation length),the pressure rises sharply.The compression wave caused by pressure propagates back and forth in the charge bed,and the compression wave propagated forward accumulates the pressure and leads to detonation.In this process,the combustion velocity of explosive possesses distinct abrupt increase when it reaches the adjacent detonation.),which is consistent with the DDT mechanism for RDXwax as observed by M.Samirant[25,29].

Fig.6.DDT curve for ultra-fine RDX in a silicon glass tube.
The time/distance curves for Al/Fe2O3/RDX nanocomposites in Fig.5 shows sudden changes of burning rate during flame propagation,indicating that their DDT mechanisms follow to the MODE 2.The DDT mechanisms for the nanocomposites and pure RDX are consistent with each other when the amount of nanothermite in the nanocomposites is less than 20 wt%.However,some characteristic stages,for example the compressive burning and shock compressive burning stages,are not evident in the DDT process for the nanocomposites.A small amount of Al/Fe2O3nanothermite added to RDX apparently does not affect the DDT mechanism for RDX,but it does have a significant effect on its DDT properties.
4.Discussions
4.1.Analysis of DDT process for Al/Fe2O3/RDX nanocomposites
The previous studies [30]on DDT focus on the modeling of the DDT phenomenon in granular,solid,energetic materials (explosives) confined in a packed bed configuration.Based on foregoing investigation of DDT for Al/Fe2O3/RDX nanocomposites confined in a loosely loaded glass tube configuration,the following physical model of the DDT process in weakly confined conditions is suggested,as shown in Fig.7.
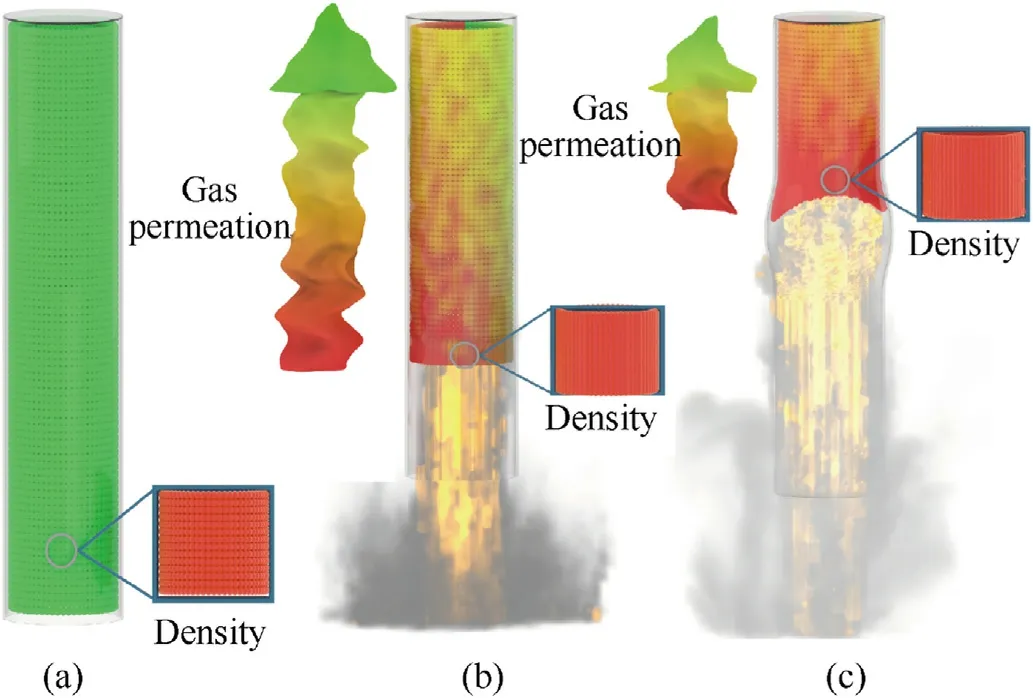
Fig.7.Schematic of DDT process for Al/Fe2O3/RDX nanocomposites in a loose granular bed.
Fig.7(a) shows the initial state of the Al/Fe2O3/RDX nanocomposites.Pressure,temperature,density,velocity and volume fraction in flame propagation all have major effects on the DDT process and mechanisms for the nanocomposites according to the current theory of the multiphase flows in reactive granular media[31].The chemical reaction begins in the granular bed and the flame propagates by conductive heat transfer after the nanocomposites are ignited.The initial flame combustion wave is in a single plane (Fig.7(b)),and the flame propagation resembles the“piston-driving” process in the low-density granular bed as described by J.M.McAfee [32].The boundary between the flame front and the unburned granular bed changes gradually from the plane to the sharp“tower”(Fig.7(c))[30]as the flame continues to propagate.Because the granular bed is loose and porous,the gas phase produced by the combustion of the nanocomposite is able to permeate it easily.Therefore,as the flame propagates,the gas permeates the unburned granular bed,preheating and compressing it,and the compression occurs at the flame front because of the loosely loaded granular bed [33,34].At that point in time,the granular bed at the flame front reaches a maximum density that increases gradually along with the flame propagation [30,32].The accelerating of the combustion wave would enhance the compressive wave encountered by the unburned granular bed[35,36],and the permeation of the high-pressure gas phase at the flame front makes the mechanical load transfer from the gas phase to the solid phase [37],which then results in elastic-plastic deformation of particles and pore collapse in that part of the granular bed.The shock wave forms through the coupling action of the acceleration of the flame wave and the thermal compression produced by gas permeation,and the coalescence of both the flame wave and the shock wave ultimately leads to the formation of DDT in the granular bed,and the convective combustion plays a central role in bridging the gap between the conductive combustion and the transition to detonation[31,38].
The DDT process for the Al/Fe2O3/RDX nanocomposites is observed clearly in the images of the flame propagation in the glass tube.The flame columns of the nanocomposites expand during flame propagation (see,for example,R-10 and R-15 in Fig.3).The high temperature and the accumulating pressure in the expansion area make the glass tube generate the elastic-plastic deformation through the lateral expansion (Fig.7(c)).The higher the RDX content in the nanocomposite is,the greater the flame column radius,and the stronger the compressive effect on the granular bed.When the flame column expands to a certain extent,the detonation occurs.The higher the flame expansion velocity,the shorter the time of DDT.From the macro-perspective,the DDT process for nanocomposites in weakly confined conditions can be attributed to the flame and pressure changes and the coupling process of flame propagation(gas permeation)and pressure(mechanical load).It is difficult to approach to the 100% theoretical maximum density(TMD) of nanocomposite in the DDT process because of the tube deformation in weakly confined conditions.The gas plays a key role in the flame wave velocity and the compression of the granular bed,and the convective transfer of heat in the hot gas may be one of the main driving mechanisms in the DDT process [38].
4.2.Analysis of the mechanism of the DDT process
The DDT mechanisms for explosives are classified as either the gradual mode (MODE 1) or the distinct abrupt mode (MODE 2)according to how combustion velocity changes during their DDT process [26].The chemical constituents of Al/Fe2O3/RDX nanocomposites have a large effect on their DDT mechanisms.
In the DDT processes of Al/Fe2O3/RDX nanocomposites,there are three different heat transfer mechanisms: conduction,convection and radiation.The effects of the gas phase on the solid phase show the convective thermal transfer and compression through the gas permeation,representing the mechanism of combustion by the convective heat transfer in the DDT process.While the behavior of the solid phase represents the mechanism of combustion by conductive heat transfer.The radiative mechanism also may have a significant effect on flame propagation in the DDT process for nanocomposites because of the high temperature of the thermite reaction,the solid products of the reaction,and the loose particulate bed,and therefore it should be included when modeling the DDT process.
When the Al/Fe2O3nanothermite is added to RDX,the improvement in the thermal conductivity and firing properties of RDX may stem from the fact that the nanothermite possesses far higher thermal conductivity and chemical reactivity than does RDX,and this will enhance the conductive combustion mechanism in flame propagation of Al/Fe2O3/RDX nanocomposites and leads to their faster DDT than RDX.Therefore,the Al/Fe2O3/RDX nanocomposites could be used as DDT primary explosive substitutes or directly applied in the detonators(for example,R-99 and R-95).The addition of RDX to Al/Fe2O3nanothermite may increase the peak combustion velocity of Al/Fe2O3nanothermite because the gas phase produced by the decomposition of RDX enhances the convective combustion mechanism during flame propagation and makes it generate DDT.The Al/Fe2O3/RDX nanocomposites with low RDX contents could be used as ignition composition for the exceptional ignition and energy release qualities of Al/Fe2O3nanothermites.
In the initial stage of combustion after ignition,the heat transfer for Al/Fe2O3/RDX nanocomposites depends on the conductive transmission of heat from point to point in the particles of explosive.Therefore,the thermal conductivity of the nanocomposites has a large effect on the combustion velocity,and so,an Al/Fe2O3/RDX nanocomposite with a small amount of added Al/Fe2O3nanothermite has a higher initial combustion velocity than SFRDX.The addition of a small amount of Al/Fe2O3nanothermite to RDX accelerates the DDT process of RDX,and changes its DDT process,but the DDT mechanism for the RDX does not change.When the content of Al/Fe2O3nanothermite is less than 20 wt%,the DDT mechanism for Al/Fe2O3/RDX nanocomposites belongs to the distinct abrupt mode,as does the DDT mechanism for RDX.Specifically,the compressive burning and shock compressive burning stages are not observed in the flame propagation of the DDT processes of Al/Fe2O3/RDX nanocomposites (for example,R-90 and R-95 in Fig.5).
The addition of RDX to Al/Fe2O3nanothermite makes the latter generate DDT for the gas produced by the decomposition of RDX.Acting together,the conductive combustion and the convective combustion mechanisms have a large effect on the combustion velocity in the DDT process for Al/Fe2O3/RDX nanocomposites with low RDX content,leading to a gradual increase in combustion velocity in flame propagation—i.e.,no sudden change in combustion velocity occurs during the DDT process.Hence,the DDT mechanisms for Al/Fe2O3/RDX nanocomposites fall under the gradual mode with low detonation velocity.The seven stages are all visible in the flame propagation during the DDT process(for example,R-15 and R-30 in Fig.3).
5.Conclusions
This paper describes the properties of combustion and deflagration to detonation transition (DDT) for Al/Fe2O3/RDX nanocomposites in weakly confined conditions.The addition of Al/Fe2O3nanothermite to RDX can improve the firing properties of RDX.The amount of the added Al/Fe2O3nanothermite has a large effect on the DDT properties of the nanocomposites.A small amount of the Al/Fe2O3nanothermite seems to accelerate the DDT process of the nanocomposites.When the amount of the Al/Fe2O3nanothermite is less than 20 wt%,their DDT mechanisms follow to MODE 2,as is the case for pure RDX.The addition of RDX to Al/Fe2O3nanothermite increases the peak combustion velocity of the Al/Fe2O3nanothermite,and an Al/Fe2O3nanothermite with an RDX content of not less than 10 wt% generates DDT.And the DDT mechanisms of Al/Fe2O3/RDX nanocomposites with an RDX content of 10—30 wt%belong to MODE 1.
The gas phase produced by the decomposition of RDX plays a key role in the shock compression combustion stage and in the formation and properties of the DDT in these nanocomposites.The fast DDT of Al/Fe2O3/RDX nanocomposites could be obtained through adjusting their chemical constituents.The data presented in this paper could be used to further improve the DDT properties of Al/Fe2O3/RDX nanocomposites in their future use in primary explosives.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by National Nature Science Foundation of China (No.22075230) and the financial support of the doctoral research foundation (No.19ZX7102) from Southwest University of Science and Technology.
杂志排行
Defence Technology的其它文章
- Dual Attribute Adversarial Camouflage toward camouflaged object detection
- A bi-population immune algorithm for weapon transportation support scheduling problem with pickup and delivery on aircraft carrier deck
- One-step green method to prepare progressive burning gun propellant through gradient denitration strategy
- Benchmark calculations and error cancelations for bond dissociation enthalpies of X—NO2
- GO/HTPB composite liner for anti-migration of small molecules
- Resilient tightly coupled INS/UWB integration method for indoor UAV navigation under challenging scenarios
