多房棘球蚴源性外泌体对巨噬细胞极化的影响
2023-04-29冶赓博陈功富崔紫烟吴俊杰黄登亮尹凤娇王志鑫于文昊孔繁玉樊海宁任利
冶赓博 陈功富 崔紫烟 吴俊杰 黄登亮 尹凤娇 王志鑫 于文昊 孔繁玉 樊海宁 任利
摘要:目的 探究不同時间及浓度多房棘球蚴源性外泌体对巨噬细胞极化的影响。方法 从60只造模BALB/c小鼠中随机选取4只,应用7.0T MRI观察腹腔病灶生长情况;解剖造模小鼠,通过腹腔病灶提取原头节进行体外培养,超速离心法从培养上清中提取外泌体,透射电镜及蛋白质免疫印迹法鉴定外泌体表征。将未加外泌体处理的巨噬细胞单独培养组设为对照组,不同浓度多房棘球蚴来源外泌体与巨噬细胞共培养组设为实验组(10 μg/mL组、50 μg/mL组),分别共培养48 h和72 h,显微镜下观察巨噬细胞形态变化。通过流式细胞术和酶联免疫吸附实验(ELISA)检测极化状态。符合正态分布的计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。结果 7.0T MRI显示小鼠腹腔内弥漫分布、大小不等的病灶形成;多房棘球蚴源性外泌体直径100 nm左右,呈杯型或茶托型,其表面标志物CD9、TSG101和CD63表达阳性。共培养后,实验组大部分细胞拉长,形态不规则,主要呈多角形;流式细胞术检测发现,共培养48 h,对照组CD16/32、CD206、CD369阳性率分别为(99.53±0.06)%、(90.27±0.21)%、(2.40±0.20)%;与对照组相比,除10 μg/mL外泌体组CD369阳性率[(0.80±0.00)%]降低(P<0.05),其余组别CD16/32、CD206、CD369阳性率均明显升高(P值均<0.000 1);共培养72 h,对照组CD16/32、CD206、CD369阳性率分别为(99.67±0.06)%、(85.47±0.55)%、(6.60±0.20)%,实验组CD16/32、CD206、CD369阳性率较对照组均明显升高(P值均<0.05)。ELISA结果示:共培养48 h,对照组IL-6及TNFα水平分别为(58.53±15.52) pg/mL、(320.70±5.30)pg/mL,实验组外泌体浓度为50 μg/mL时IL-6[(98.81±15.55) pg/mL]较对照组升高(P<0.05);共培养72 h,对照组IL-6及TNFα水平分别为(76.22±9.68)pg/mL、(323.90±87.37)pg/mL,当外泌体浓度为10 μg/mL时TNFα水平[(164.20±14.17)pg/mL]较对照组明显下降(P<0.05);当外泌体浓度为50 μg/mL时IL-6水平[(99.52±8.35)pg/mL]较对照组升高(P<0.05)。结论 多房棘球蚴源性外泌体可调控巨噬细胞极化,且在浓度为10 μg/mL,共培养72 h后,可导致巨噬细胞M2样极化,具体方式有待进一步研究。
关键词:棘球蚴病, 肝; 外泌体; 巨噬细胞; 小鼠, 近交BALB C
基金项目:青海省科技厅项目(2019-ZJ-7031)
Effect of exosomes derived from Echinococcus multilocularis on macrophage polarization: A preliminary study
YE Gengbo1,2, CHEN Gongfu1,2, CUI Ziyan1,2, WU Junjie1,2, HUANG Dengliang2, YIN Fengjiao1,2, WANG Zhixin1,2, YU Wenhao1,2, KONG Fanyu1,2, FAN Haining1,2, REN Li1,2. (1. Department of Hepatopancreatobiliary Surgery, The Affiliated Hospital of Qinghai University, Xining 810001, China; 2. Qinghai Province Key Laboratory of Hydatid Disease Research, Xining 810001, China)
Corresponding author:
REN Li, renliweimin_xn@126.com (ORCID:0000-0001-6306-3533)
Abstract:
Objective To investigate the effect of exosomes derived from Echinococcus multilocularis on macrophage polarization after treatment for different durations and concentrations. Methods A total of 60 BALB/c mice were used for modeling, among which 4 mice were selected to observe the growth of abdominal lesions on 7.0T MRI. The mice for modeling were dissected, and the protoscoleces was taken from the abdominal lesion and cultured in vitro; ultracentrifugation was used to extract the exosomes from the supernatant, and transmission electron microscopy and Western blotting were used for the characterization of exosomes. The macrophages without exosome treatment were established as control group, and the macrophages co-cultured with different concentrations of exosomes derived from Echinococcus multilocularis were established as experimental group (10 μg/mL group and 50 μg/mL group) and were cultured for 48 and 72 hours. The morphological changes of macrophages were observed under a microscope, and flow cytometry and ELISA were used to observe polarization state. A one-way analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results The results of 7.0T MRI showed the formation of diffuse lesions with different sizes in the abdominal cavity of mice, and the exosomes derived from Echinococcus multilocularis were approximately 100 nm in diameter and were cup-shaped or saucer-shaped, with the positive expression of the surface markers CD9, TSG101, and CD63. After co-culture, most of the cells in the experimental group were elongated with an irregular and polygonal shape. Flow cytometry showed that after 48 hours of co-culture, the positive rates of CD16/32, CD206, and CD369 in the control group were 99.53%±0.06%, 90.27%±0.21%, and 2.40%±0.20%, respectively; compared with the control group, except that the 10 μg/mL exosome group had a significant reduction in the positive rate of CD369 (0.80%±0.00%) (P<0.05), all the other groups had a significant increase in the positive rates of CD16/32, CD206, and CD369 (all P<0.000 1); after 72 hours of co-culture, the positive rates of CD16/32, CD206, and CD369 in the control group were 99.67%±0.06%, 85.47%±0.55%, and 6.60%±0.20%, respectively, and compared with the control group, the experimental group had significant increases in the positive rates of CD16/32, CD206, and CD369 (all P<0.05). ELISA showed that after 48 hours of co-culture, the levels of IL-6 and TNFα in the control group were 58.53±15.52 pg/mL and 320.70±5.30 pg/mL, respectively, and when the exosome concentration was 50 μg/mL, the level of IL-6 in the experimental group was 98.81±15.55 pg/mL, which was higher than that in the control group (P<0.05); after 72 hours of co-culture, the levels of IL-6 and TNFα in the control group were 76.22±9.68 pg/mL and 323.90±87.37 pg/mL, respectively, and when the exosome concentration was 10 μg/mL, the level of TNFα was 164.20±14.17 pg/mL, which was significantly lower than that in the control group (P<0.05); when the exosome concentration was 50 μg/mL, the level of IL-6 was 99.52±8.35 pg/mL, which was significantly higher than that in the control group (P<0.05). Conclusion Exosomes derived from Echinococcus multilocularis can regulate macrophage polarization and induce M2-like polarization of macrophages after co-culture at a concentration of 10 μg /mL for 72 hours, and further studies are needed to clarify the specific method.
Key words:
Echinococcosis, Hepatic; Exosomes; Macrophages; Mice, Inbred BALB C
Research funding:
Project of Qinghai Provincial Department of Science and Technology (2019-ZJ-7031)
棘球蚴病俗称包虫病,分为细粒棘球蚴病和多房棘球蚴病(alveolar echinococcosis,AE)。AE由于浸润性生长和转移特点,故有“虫癌”之称,对人类健康和社会经济威胁更为严重[1]。巨噬细胞是先天免疫的重要成员,M1型巨噬细胞可分泌如TNFα、IL-6及低水平IL-10等促炎因子,还可通过上调MHC-Ⅱ类分子和共刺激分子CD40、CD80及CD86,帮助机体抵御多种病原体的侵害[2];M2型可表达IL-10、TGFβ等抗炎分子,并通过高表达CD206、CD301和CD369等受体发挥吞噬作用[3-4]。现研究[5-6]认为M2型巨噬细胞极化与维持慢性炎症、免疫抑制及肿瘤生长有关。巨噬细胞在受到不同微环境刺激后,通过M1/M2表型的转化,发挥不同的功能。外泌体是由多种细胞分泌的纳米级囊泡外小体,被视为细胞间“通讯特工”,在调节细胞功能中起着重要作用[7]。
当多房棘球蚴侵入机体后,与宿主建立的复杂免疫抑制机制能成功使其躲避宿主免疫系统的杀伤并长期存活[8-9],多项研究表明,血吸虫[10]、马来丝虫[11]、弓形虫[12]等寄生虫源性外泌体可通过调节巨噬细胞极化从而协助免疫逃逸,然而,多房棘球蚴来源的外泌体影响巨噬细胞表型和功能的研究较少见。本研究拟采用多房棘球蚴源性外泌体体外刺激巨噬细胞,利用流式和ELISA检测巨噬细胞表面分子标志物的动态变化,旨在探讨多房棘球蚴源性外泌体是否具有促巨噬细胞极化的能力,为进一步研究多房棘球蚴感染免疫逃逸新机制奠定基础。
1 材料与方法
1.1 实验动物 SPF级BALB/c小鼠60只,雌性,体质量16~18 g,10周龄,购于北京华阜康生物科技股份有限公司[生产许可证编号:SCXK(京)2019-0008];种鼠为长爪沙鼠1只,体质量65 g,由青海大学包虫病重点实验室提供[生产许可证编号:SCXK(浙)2019-0002];实验动物使用许可证号均为:SYXK(青)2020-0001。
1.2 主要试剂及设备 J774A.1小鼠单核巨噬细胞(上海泽叶生物有限公司);无外泌体胎牛血清(上海逍鹏生物科技有限公司);IL-6(美国Abcam公司);TNFα(南京森贝伽生物科技有限公司);CD369、CD206、CD16/CD32(美国eBioscience 公司);BCA 蛋白浓度测定试剂盒(上海碧云天生物技术有限公司);小鼠麻醉剂为异氟烷(深圳瑞沃德生命有限公司,试剂编号:R510-22-8)和氧气的混合气体;超净工作台(苏州净化设备厂);Bruker Biospin 70/16小动物专用7.0TMRI扫描系统(德国Bruker公司);透射电子显微镜(日本TEM,Joel 公司);冷冻高速离心机(美国Thermo 公司);高速离心机(德国 Eppendorf 公司);流式细胞仪(美国BD公司); SDS-PAGE蛋白电泳仪(上海天能科技有限公司)。
1.3 方法
1.3.1 7.0T MRI扫描 BALB/c小鼠腹腔感染原头节4个月后,随机选取4只置于密闭麻醉箱内给予异氟烷与氧气混合气体麻醉,以俯卧位置于扫描床上,Bruker Biospin 70/16小动物专用7.0TMRI扫描系统进行扫描,了解腹腔病灶生长情况以确定造模成功。
1.3.2 原头节的提取 颈椎脱臼法处死感染多房棘球蚴的BALB/c模型小鼠,无菌条件下打开腹腔,分离出腹腔中多房棘球蚴病灶组织,无菌PBS液冲洗3次,刀片切碎至糊状,80目及300目滤网分别反复过滤,获得乳白色原头节,置于含10%无外泌体胎牛血清及1 mL 5%双抗的培养基中,于37 ℃的二氧化碳培养箱中培养3 d,装入50 mL离心管,800×g离心5 min,吸取培养上清,-80 ℃冻存备用。
1.3.3 外泌体提取及BCA定量 超速离心法提取上清中外泌体:4 ℃、300×g离心10 min,取上清;2 000×g离心10 min、去除死细胞及细胞碎片,取上清;0.22 μm无菌滤器滤过以进一步去除细胞碎片;将滤过液在4 ℃ 140 000×g离心2 h,弃上清,PBS重悬沉淀,获得的沉淀即为外泌体。鉴定:将沉淀重悬于100 μL PBS中,TEM觀察形态,Western Blot检测特异性表面标志物CD63、CD9和TSG101的表达,纳米颗粒追踪分析仪分析其直径。为避免降解于-80 ℃保存备用。将标准品用PBS稀释为2 mg/mL、1 mg/mL、0.5 mg/mL、0.25 mg/mL、0.125 mg/mL、0.062 5 mg/mL,外泌体稀释为20 μL作为待测样本,以PBS作空白对照,每个反应设置3个重复;将200 μL BCA工作液和25 μL样品加在96孔细胞培养板中并充分混匀,37 ℃静置30 min;用酶标仪测定并记录OD562,根据标准曲线和使用的上清液体积计算出上清液的蛋白浓度,以外泌体样品的蛋白浓度作为外泌体浓度进行后续实验。
1.3.4 细胞培养 对照组:未加外泌体的巨噬细胞单独培养;实验组:多房棘球蚴来源外泌体与巨噬细胞共培养(10 μg/mL组、50 μg/mL组)。分别培养48 h、72 h后,流式和ELISA检测巨噬细胞标志物(M1:IL-6、TNFα、CD16/32;M2:CD206、CD369)的表达情况,并在显微镜下观察巨噬细胞形态变化。
1.3.5 流式细胞术检测细胞比例变化 刺激巨噬细胞48 h、72 h后,当各实验组细胞覆盖率达70%~80%时,收集细胞;1 300 r/min、离心3 min,弃上清;PBS 洗涤细胞沉淀,1 300 r/min、离心3 min,弃上清;重复上一步骤;100 μL PBS溶液配成混悬细胞沉淀并加入荧光抗体10 μL,4 ℃、避光条件下孵化0.5 h;离心并收集细胞,PBS 清洗细胞后置于离心机中,以1 300 r/min离心3 min,吸除上清;200 μL PBS溶液配置成混悬细胞沉淀,混匀并转移至96孔板中,每孔滴入200 μL,上机检测巨噬细胞极化指标。
1.3.6 极化相关因子的检测 用ELISA法检测IL-6及TNFα:收集各组上清液,参考操作说明书,检测各组细胞因子,据OD值绘制出标准曲线,计算各样本浓度,所有检测重复3次。
1.4 统计学方法 应用SPSS 19.0软件分析数据,并用GraphPad Prism 8.3.0软件绘图。符合正态分布的计量资料以x±s表示,多组间比較采用单因素方差分析,进一步两两比较采用LSD-t检验。P<0.05为差异有统计学意义。
2 结果
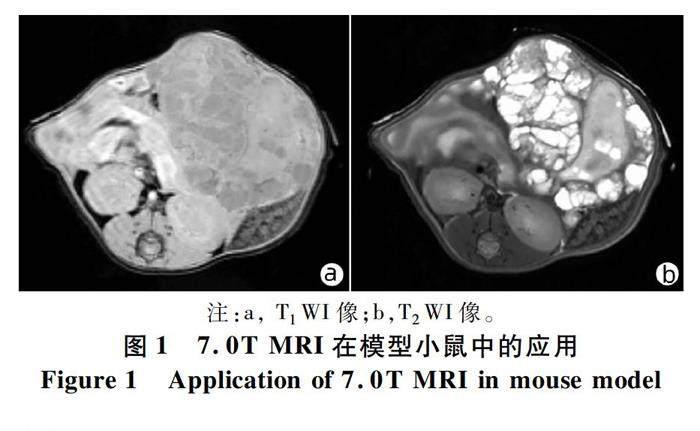
2.1 7.0T MRI扫描结果 与正常小鼠相比,实验组:腹腔内可见弥漫分布、大小不等的多房囊性和少量囊实性病灶;T1WI:呈多房囊状,部分较大的病灶内可见等信号的分隔;边界显示清楚(图1a)。T2WI:呈高信号及混杂信号,部分可见囊实性改变,实性成分周边见多发小囊泡结构,部分肝脏受侵(图1b)。
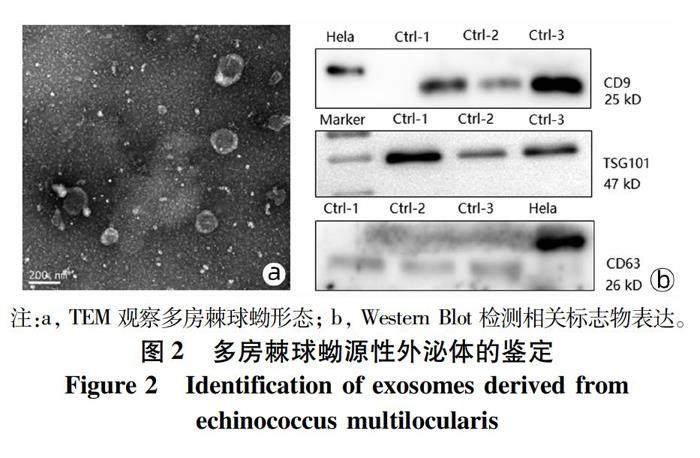
2.2 多房棘球蚴源性外泌体鉴定 观察到多房棘球蚴源性外泌体呈杯型或茶托型,双层膜结构,直径100 nm左右,背景清晰,无污染(图2a);Western Blot结果显示,标志物CD9和TSG101表达阳性,CD63表达为弱阳性(图2b);本实验使用超速离心法提取的外泌体,结构、大小、浓度及表面标志物均符合要求。
2.3 巨噬细胞形态观察 共培养48 h、72 h后,显微镜下观察巨噬细胞形态,对照组细胞多呈椭圆形或圆形,而经过不同浓度外泌体处理后,共培养48 h和72 h实验组大部分细胞拉长,形态不规则,主要呈多角形(图3)。
2.4 流式细胞术检测结果 共培养48 h:与对照组相比,除10 μg/mL外泌体组CD369阳性率降低,其余组别CD16/32、CD206、CD369阳性率均明显升高(P值均<0.000 1);共培养72 h:与对照组相比,实验组CD16/32、CD206、CD369阳性率较对照组均明显升高(P值均<0.05)(表1)。随共培养时间及外泌体浓度变化,相关分子呈现不同变化趋势(图4、5)。
2.5 ELISA检测结果 共培养48 h:与对照组相比,50 μg/mL外泌体组IL-6水平升高(P<0.05);共培养72 h:与对照组相比,当外泌体浓度为10 μg/mL时TNFα水平明显下降(P<0.05);当外泌体浓度为50 μg/mL时IL-6水平较对照组升高(P<0.05)(表2)。TNFα的浓度呈现先下降后升高趋势,在浓度为10 μg/mL 时,下降显著(图6)。
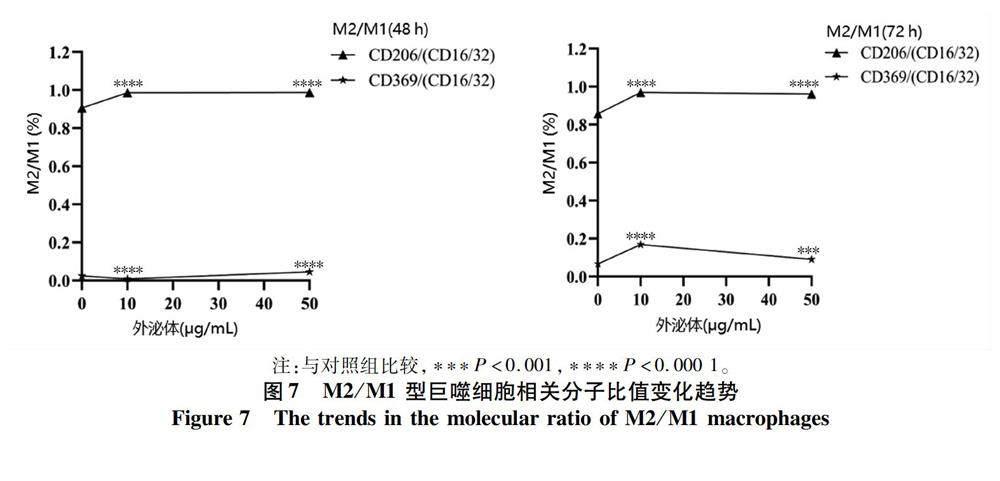
2.6 M2/M1型巨噬细胞相关分子比值 将未予外泌体刺激的巨噬细胞设为对照组,对M2/M1型巨噬细胞的比值进一步行统计分析,结果显示,浓度为10 μg/mL 刺激72 h后,M2型巨噬细胞相关分子占比显著增加,差异具有统计学意义(P值均<0.000 1),再增加外泌体浓度时,其比值出现下降趋势(表3,图7)。
3 讨论
1983年,Pan和Johnstone两位学者在实验时意外发现了直径30~150 nm 的小囊泡[13],以此开启了外泌体世界的大门。从最初被视为细胞排出废物的途径[14],到Raposo等[15]在1996年,提出外泌体参与抗原呈递和适应性免疫反应,促使其重新受到研究者们的关注。近年来,已证实寄生虫也能分泌外泌体或者刺激宿主细胞分泌外泌体[9,16-17],与宿主细胞进行交流,进而调节多种病理生理反应,对寄生虫的致病力、繁殖力以及免疫逃逸等均产生重要作用。巨噬细胞因极高的可塑性和异质性而被视为免疫系统的精灵,起初以原始巨噬细胞(M0型)处于静止状态,当受到外界不同环境因素诱导时,引起表型和功能的变化,称为巨噬细胞极化[18]。其中M1型和M2型功能迥异,在不同环境下相互转化、相互制衡。
有研究[19]证实,AE患者在行肝脏切除手术后,巨噬细胞的数量增加,说明巨噬细胞在AE的发展过程中起了作用。也有研究[20]表明,AE患者的肝组织和外周血中,M2/M1比值较健康人群明显升高。课题组在前期研究[21]中也发现,当多房棘球蚴感染小鼠后,宿主可分泌IL-6、TNFα等炎性细胞因子,上调M1型巨噬细胞的表达,帮助宿主清除病原体;同时,感染多房棘球蚴小鼠的巨噬细胞也可向M2型极化,再次证实多房棘球蚴致病过程中存在巨噬细胞表型的转变,使其逃避机体内免疫杀伤作用,继而发生一系列病理损伤。然而,在AE致病过程中,介导巨噬细胞表型转换的物质尚不明确。前期在AE患者外周血、病灶囊液中分离获得外泌体发现,从病灶囊液上清中提取的外泌体蛋白含量显著高于外周血;也在棘球蚴体外培养上清中分离得到外泌体,并发现外泌体相关标志物表达阳性,故推测多房棘球蚴分泌的外泌体在感染机体和在发生免疫逃逸的过程中可能扮演重要角色。
本研究通过构建多房棘球蚴腹腔感染小鼠模型,将7.0T MRI扫描应用于无创了解模型鼠腹腔病灶生长情况后,适时提取多房棘球蚴原头节,用无外泌体的胎牛血清培养原头节并分离上清,成功获取并鉴定多房棘球蚴源性外泌体。在后续进行外泌体与巨噬细胞分组共培养时,观察到巨噬细胞发生形态的变化,提示外泌体使巨噬细胞发生极化。再利用流式细胞技术和ELISA分别检测M1和M2型相关指标,结果表明多房棘球蚴源性外泌体可使巨噬细胞发生M1及M2样极化,这与前期研究结果相一致,即感染多房棘球蚴小鼠病灶中出现 M1型和M2型巨噬细胞双重浸润。通过进一步观察,当外泌体浓度为10 μg/mL培养72 h后,M1型巨噬细胞有关的细胞因子TNFα明显下降,同时,M2型巨噬细胞有关表面分子CD206及CD369表达水平显著增加(P值均<0.05),这表明多房棘球蚴源性外泌体使巨噬细胞向M2型转化。随后又进行 M2/M1 型巨噬细胞相关表面分子比值分析,结果显示以外泌体浓度为10 μg/mL时培养72 h,与对照组相比,CD369/(CD16/32)和CD206/(CD16/32)的比值明显升高,差异均有统计学意义(P值均<0.000 1),而且随着外泌体浓度的增加,M2/M1型巨噬细胞相关指标的比值呈下降趋势,说明外泌体浓度为10 μg/mL 时,M2 型巨噬细胞明显增多。因此,体外研究实验认为多房棘球蚴源性外泌体在浓度为10 μg/mL时是促进M2型巨噬细胞转化最佳浓度。
综上所述,在多房棘球蚴感染过程中,存在巨噬细胞表型的转换;而本研究肯定了多房棘球蚴源性外泌体在巨噬细胞表型转换中的作用;当外泌体浓度为10 μg/mL,共培养72 h后能有效促进巨噬细胞M2型极化,这可能是多房棘球蚴通过重塑宿主免疫状态,帮助多房棘球蚴逃脱宿主免疫杀伤作用的新机制。但外泌体中何种物质以及通过何种信号通路发挥作用,还需在此基础上结合体内实验进一步进行生物信息学及蛋白组学等方面的研究,进而阐明AE致病过程中,宿主-宿主、宿主-寄生虫间复杂的分子调控机制,寻找新型治疗靶点,为AE诊断的新型生物标志物、临床药物开发研究方面奠定基础。
伦理学声明:本研究于2019年7月3日经青海大学附属医院科研伦理委员会审批,批号为P-SL-2019042,符合实验室动物管理与使用准则。
利益冲突声明:本研究不存在研究者、伦理委员会成员以及与公开研究成果有关的利益冲突。
作者贡献声明:冶賡博、陈功富负责课题设计,撰写论文;黄登亮、崔紫烟、吴俊杰负责实验实施,数据整理;孔繁玉、于文昊、尹凤娇查阅文献,分析数据;任利、樊海宁、王志鑫负责课题设计,指导撰写论文并最后定稿。
参考文献:
[1]
Sichuan Hydatid Disease Clinical Medical Research Center, Hydatid Disease Professional Committee of Sichuan Medical Association. Expert consensus on diagnosis and treatment of alveolar hepatic echinococcosis(2020 version)[J]. Chin J Bases Clin Gen Surg, 2020, 27(1): 13-17. DOI: 10.7507/1007-9424.201911105.
四川省包虫病临床医学研究中心, 四川省医师协会包虫病专业委员会. 泡型肝包虫病诊疗专家共识(2020版)[J]. 中国普外基础与临床杂志,2020, 27(1): 13-17. DOI: 10.7507/1007-9424.201911105.
[2]SCHULTZE JL, SCHMIDT SV. Molecular features of macrophage activation[J]. Semin Immunol, 2015, 27(6): 416-423. DOI: 10.1016/j.smim.2016.03.009.
[3]SCHULTZE JL, FREEMAN T, HUME DA, et al. A transcriptional perspective on human macrophage biology[J]. Semin Immunol, 2015, 27(1): 44-50. DOI: 10.1016/j.smim.2015.02.001.
[4]LI WH, ZHANG YX, ZHAO D, et al. Dectin-1 affects heart remodeling after myocardial infarction by regulating macrophage polarization[J]. Immunol J, 2021, 37(8): 692-697. DOI: 10.13431/j.cnki.immunol.j.20210096.
李文华, 张一馨, 赵迪, 等. Dectin-1通过调节巨噬细胞极化影响心肌梗死后的心脏重塑[J]. 免疫学杂志, 2021, 37(8): 692-697. DOI: 10.13431/j.cnki.immunol.j.20210096.
[5]MOSSER DM, EDWARDS JP. Exploring the full spectrum of macrophage activation[J]. Nat Rev Immunol, 2008, 8(12): 958-969. DOI: 10.1038/nri2448.
[6]YAO T, XU ZH, YAO JY, et al. Effect of hepatocellular carcinoma cell-derived exosomes on M2 polarization of tumor-associated macrophages[J]. J Clin Hepatol, 2022, 38(3): 558-562. DOI: 10.3969/j.issn.1001-5256.2022.03.013.
姚涛, 徐植红, 姚纪友, 等. 肝癌细胞来源外泌体对肿瘤相关M2型巨噬细胞极化的影响[J]. 临床肝胆病杂志, 2022, 38(3): 558-562. DOI: 10.3969/j.issn.1001-5256.2022.03.013.
[7]van NIEL G, DANGELO G, RAPOSO G. Shedding light on the cell biology of extracellular vesicles[J]. Nat Rev Mol Cell Biol, 2018, 19(4): 213-228. DOI: 10.1038/nrm.2017.125.
[8]GRUBOR NM, JOVANOVA-NESIC KD, SHOENFELD Y. Liver cystic echinococcosis and human host immune and autoimmune follow-up: A review[J]. World J Hepatol, 2017, 9(30): 1176-1189. DOI: 10.4254/wjh.v9.i30.1176.
[9]WU Z, WANG L, LI J, et al. Extracellular vesicle-mediated communication within host-parasite interactions[J]. Front Immunol, 2018, 9: 3066. DOI: 10.3389/fimmu.2018.03066.
[10]WANG L, LI Z, SHEN J, et al. Exosome-like vesicles derived by Schistosoma japonicum adult worms mediates M1 type immune- activity of macrophage[J]. Parasitol Res, 2015, 114(5): 1865-1873. DOI: 10.1007/s00436-015-4373-7.
[11]ZAMANIAN M, FRASER LM, AGBEDANU PN, et al. Release of small RNA-containing exosome-like vesicles from the human filarial parasite brugia malayi[J]. PLoS Negl Trop Dis, 2015, 9(9): e0004069. DOI: 10.1371/journal.pntd.0004069.
[12]LI Y, LIU Y, XIU F, et al. Characterization of exosomes derived from Toxoplasma gondii and their functions in modulating immune responses[J]. Int J Nanomedicine, 2018, 13: 467-477. DOI: 10.2147/IJN.S151110.
[13]PAN BT, JOHNSTONE RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor[J]. Cell, 1983, 33(3): 967-978. DOI: 10.1016/0092-8674(83)90040-5.
[14]HESSVIK NP, LLORENTE A. Current knowledge on exosome biogenesis and release[J]. Cell Mol Life Sci, 2018, 75(2): 193-208. DOI: 10.1007/s00018-017-2595-9.
[15]RAPOSO G, NIJMAN HW, STOORVOGEL W, et al. B lymphocytes secrete
antigen-presenting vesicles[J]. J Exp Med, 1996, 183(3): 1161-1172. DOI: 10.1084/jem.183.3.1161.
[16]COAKLEY G, MAIZELS RM, BUCK AH. Exosomes and other extracellular vesicles: The new communicators in parasite infections[J]. Trends Parasitol, 2015, 31(10): 477-489. DOI: 10.1016/j.pt.2015.06.009.
[17]COAKLEY G, BUCK AH, MAIZELS RM. Host parasite communications-Messages from helminths for the immune system: Parasite communication and cell-cell interactions[J]. Mol Biochem Parasitol, 2016, 208(1): 33-40. DOI: 10.1016/j.molbiopara.2016.06.003.
[18]SHAPOURI-MOGHADDAM A, MOHAMMADIAN S, VAZINI H, et al. Macrophage plasticity, polarization, and function in health and disease[J]. J Cell Physiol, 2018, 233(9): 6425-6440. DOI: 10.1002/jcp.26429.
[19]ZHANG C, LIN R, LI Z, et al. Immune exhaustion of T cells in alveolar echinococcosis patients and its reversal by blocking checkpoint receptor TIGIT in a murine model[J]. Hepatology, 2020, 71(4): 1297-1315. DOI: 10.1002/hep.30896.
[20]GAO YS, ZHU MB, GUO YZ, et al. Clinical analysis on hepatic hydatid disease in Yili River Valley[J]. Chin J Parasitol Parasitic Dis, 2005, 23(1): 3-13. DOI: 10.3969/j.issn.1000-7423.2005.01.003.
高永盛, 朱马拜, 郭永忠, 等. 新疆伊犁河谷肝棘球蚴病临床资料分析[J]. 中国寄生虫学与寄生虫病杂志, 2005, 23(1): 3-13. DOI: 10.3969/j.issn.1000-7423.2005.01.003.
[21]WANG DX, WANG H, FAN HN, et al. Study on the role of macrophage polarization during E.multilocularis-infection in mice[J]. Chin High Altitude Med Biology, 2018, 39(2): 118-122. DOI: 10.13452/j.cnki.jqmc.2018.02.010.
王东旭, 王虎, 樊海宁, 等. 巨噬细胞极化在小鼠泡型包虫病中的作用[J]. 中国高原医学与生物学杂志, 2018, 39(2): 118-122. DOI: 10.13452/j.cnki.jqmc.2018.02.010.
收稿日期:
2022-09-27;录用日期:2022-11-22
本文编輯:林姣
