免疫检查点抑制剂在肝细胞癌肝移植中的应用
2023-04-29解有成陈顺李初谊郑英贾栋张久聪于晓辉
解有成 陈顺 李初谊 郑英 贾栋 张久聪 于晓辉


摘要:肝移植作為肝细胞癌的根治性治疗策略之一,对米兰标准之内的患者有较好的临床效果。然而,术后高的复发率、转移率使得患者长期生存仍然面临挑战。因此,如何提高远期生存率,降低术后肿瘤转移成为亟待解决的关键难题。近年来,免疫检查点抑制剂(ICI)以其良好的安全性和客观反应性为晚期肝癌患者治疗提供了新的机遇,也成为了提高肝移植治疗效果的潜在方法。当前,早期临床研究已经报道了ICI单一及联合治疗在肝细胞癌肝移植术前降期或桥接治疗以及术后辅助治疗中的独特优势。本文旨在通过对近年来ICI在肝细胞癌肝移植中的临床试验及应用进展进行综述,并对其安全性和有效性进行探讨,以期为临床用药提供一定参考。
关键词:癌, 肝细胞; 肝移植; 免疫抑制剂
基金项目:甘肃省重点研发计划(20YF8FA099); 甘肃省青年科技基金(20JR10RA016); 甘肃省非感染性肝病临床医学研究中心(21JR7RA017); 甘肃省卫健委科研计划(GSWSKY2021-047)
Application of immune checkpoint inhibitors in liver transplantation for patients with hepatocellular carcinoma
XIE Youcheng1,2, CHEN Shun1, LI Chuyi1, ZHENG Ying1, JIA Dong1,2, ZHANG Jiucong1, YU Xiaohui1. (1. Department of Gastroenterology, The 940 Hospital of Joint Service Support Force of the Chinese Peoples Liberation Army, Lanzhou 730050, China; 2. The First Clinical Medical School, Gansu University of Chinese Medicine, Lanzhou 730000, China)
Corresponding author:YU Xiaohui, yuxiaohui@126.com (ORCID:0000-0002-8633-3281)
Abstract:
Liver transplantation, as one of the radical treatment strategies for hepatocellular carcinoma, has a good clinical effect in patients meeting the Milan criteria; however, the high recurrence rate and metastasis rate after surgery bring great challenges to the long-term survival of such patients. Therefore, how to improve long-term survival rate and reduce postoperative tumor metastasis has become a key problem that needs to be solved urgently. In recent years, immune checkpoint inhibitors (ICIs), with their good safety and objective reactivity, have provided a new opportunity for the treatment of patients with advanced liver cancer and have become potential candidates for improving the therapeutic effect of liver transplantation. At present, early clinical studies have reported the unique advantages of ICIs used alone or in combination in downstaging or bridging therapy before liver transplantation for hepatocellular carcinoma and adjuvant therapy after liver transplantation. Therefore, this article reviews the clinical trials of ICIs in liver transplantation for hepatocellular carcinoma and the advances in the application of ICIs in recent years and discuss its safety and efficacy, in order to provide a certain reference for clinical medication.
Key words:
Carcinoma, Hepatocellular; Liver Transplantation; Immunosuppressive Agents
Research funding:
Gansu Provincial Key R&D Program (20YF8FA099); Gansu Provincial Youth Science and Technology Fund (20JR10RA016); Gansu Provincial Clinical Medical Research Centre for Non-infectious Liver Diseases (21JR7RA017); Scientific Research Plan of Gansu Provincial Health Commission (GSWSKY2021-047)
肝细胞癌(hepatocellular carcinoma, HCC)是我国常见恶性肿瘤之一,其发病率居于第四位,死亡率居于第二位[1]。当前肝移植(liver transplantation, LT)仍然是HCC根治性治疗主要方式之一,但鉴于米兰分期对于肿瘤大小、个数和肝储备功能的严格限制,仅有少数患者能从中获益,且LT术后高达30%复发率[2],进一步限制了LT临床应用。故寻找能扩大LT适应证,提升治疗效果及预防术后复发成为目前研究的热点。
近年来,免疫检查点抑制剂(immune checkpoint inhibitors, ICI)对不同肝病类型、不同阶段、不同地区的晚期HCC患者,均显示出较好的临床疗效。鉴于其良好的客觀反应性和安全性[3-5],推动研究者进一步探索其在LT中的应用。近期研究显示,ICI在黑色素瘤[6]、肺癌[7]、肾癌[8]等移植术中的治疗可使患者有更好的生存益处,这也使得ICI在LT中的应用备受瞩目。本文通过已有文献,对ICI在LT中的临床试验和应用进展进行综述,阐明LT中应用ICI的有效性和安全性,以期为临床用药提供参考。
1 ICI的抗肿瘤机制
免疫检查点(immune checkpoints, IC)是指在免疫细胞上表达、能调节免疫激活程度的一系列分子,ICI则是通过阻断IC与其配体之间的相互作用来防止T淋巴细胞失活,从而发挥抗肿瘤作用[9]。
目前,ICI已被FDA批准作为HCC系统治疗的一线治疗方案(表1),常见的ICI包括抗程序化细胞死亡-1(programmed cell death-1,PD-1)/程序性死亡受体配体-1(programmed death ligand-1,PD-L1),以及抗细胞毒性T淋巴细胞相关蛋白4(cytotoxic T lymphocyte-associated antigen-4,CTLA-4)。除上述抗体外,还有更多的靶点抗体如LAG-3(lymphocyte-activation gene-3,又称CD223)抗体等仍在进一步研究中[10]。
2 ICI在HCC患者LT术前降期或桥接治疗
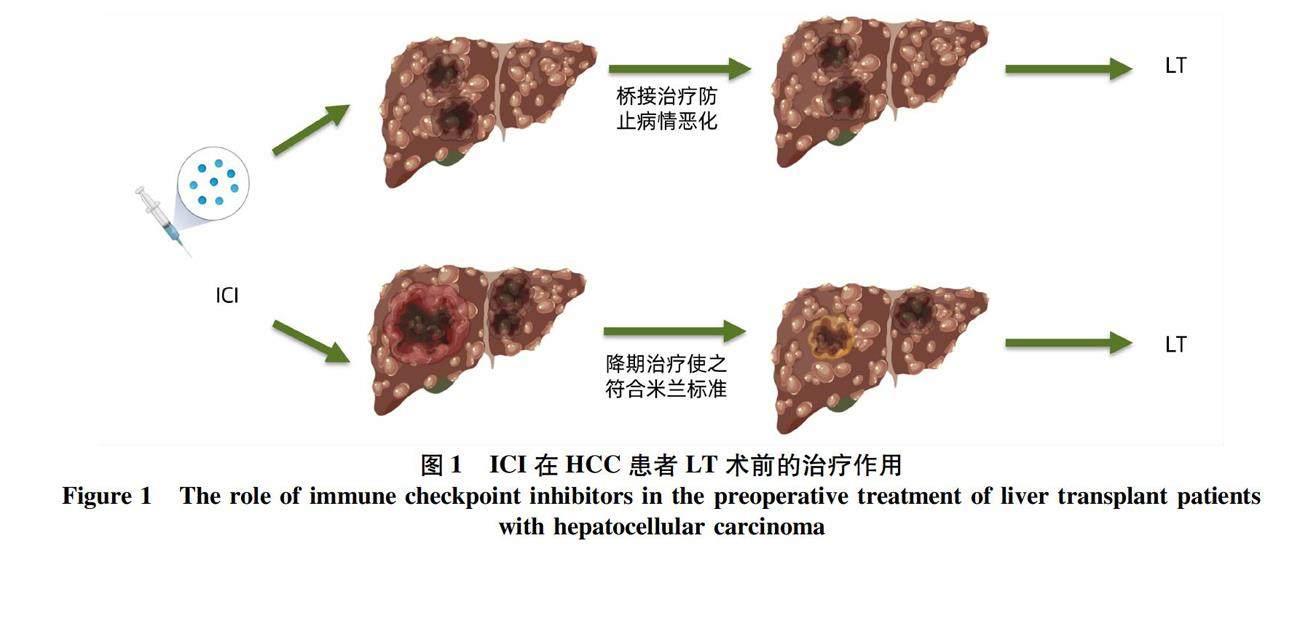
LT是根治HCC的理想治疗方法,但供体的稀缺限制了LT的应用,为防止患者失去LT机会,桥接、降期治疗是HCC患者在LT前的关键[11]。桥接治疗是指LT等待期间通过辅助治疗控制肿瘤进展[12],降期治疗是指通过新辅助治疗手段,减轻肿瘤负荷,降低分期,使超出标准的患者能重新被纳入LT标准[13](图1)。
局部区域治疗是最常见的桥接、降期治疗方式,经导管动脉化疗栓塞术(transarterial chemoembolization, TACE)作为局部区域治疗标准治疗手段[14],虽能一定程度缓解疾病进展,但一些学者认为部分患者对TACE疗效不佳,易出现TACE治疗抵抗,或治疗后肝功能受损[15]。此外,研究[16]表明TACE可增强PD-1和PD-L1的表达,若联合ICI,可能进一步改善预后。
Schwacha-Eipper等[17]首次报道了在LT术前使用ICI,使1例肝内多发转移灶的HCC患者成功降级到米兰标准之内,顺利进行LT手术。之后,越来越多的患者开始在LT前使用ICI进行桥接或降期治疗。迄今为止,共有22例病例报道了在LT前使用ICI,大多数患者预后良好(90%)。
虽然上述研究的疗效和安全性的结果令人振奋,但仅是Ⅰ或Ⅱ期临床试验,且样本量较小,很难确定ICI与致命排斥反应相关的危险因素,故临床使用ICI作为桥接或降期治疗仍十分谨慎,正在进行的进一步大规模Ⅱ、Ⅲ期临床试验将进一步评估免疫治疗在可切除的HCC新辅助治疗中的作用。
3 ICI在LT术后辅助治疗
3.1 LT后预防HCC复发的辅助治疗 尽管LT切除了原发肿瘤及肝内潜在病变,但5年复发率达30%[2],一旦复发转移,疾病将迅速进展。目前认为循环肿瘤细胞或肝外未检测到的病变是HCC复发的主要原因[18]。因此及时、精准、规范地进行术后辅助治疗,才能降低术后复发风险,延长总体生存期。目前尚无证据支持化疗或索拉非尼辅助治疗能降低LT后HCC复发风险[19]。
目前尚缺乏关于ICI预防移植后复发的研究,正在进行的临床试验(NCT03383458)探究了肝脏切除或消融术后高风险复发患者使用ICI的安全性和有效性,虽不包括LT受者但可能对LT患者提供借鉴。由于排斥反应的风险不可预测,因此应谨慎使用ICI作为LT后预防性复发辅助治疗。
3.2 LT后HCC复发的免疫治疗 由于LT术后长期免疫抑制,HCC复发风险增加。目前对于复发患者治疗手段有限,临床仍以放化疗和局部消融、栓塞为主,但效果有限。近年来,随着移植患者中免疫治疗的深入,ICI越来越多地被用作复发后的治疗选择。
近期研究结果显示,ICI在移植术后复发治疗中存在极大的优势,使患者获得更好的生存益处,一项针对8例接受ICI的LT患者的回顾性研究[20]结果显示,尽管排斥反应风险增加,但总生存期明显改善。近来文献[21-22]报道LT术后免疫治疗取得良好效果:1例LT术后HCC复发并多发转移患者,间断使用ICI治疗2年后康复,且移植物肝功能也维持在正常水平。另1例LT术后复发伴双肺转移患者,接受ICI
治疗后,无进展生存期与总生存期均延长,虽然患者最终因脑转移去世,但治疗期间未出现任何排斥反应。有学者探究终末期HCC在各种治疗(包括放化疗、手术以及分子靶向药物)无效的情况下实施ICI后发现,在ICI亚组中客观缓解率为25%,与非移植患者相比,移植患者的抗肿瘤效应更好[23-24]。
尽管目前个案报道,复发后使用免疫治疗LT后HCC复发具有较好的疗效,但LT患者需终身免疫抑制可能会影响ICI的抗肿瘤疗效。同时,ICI通过调控免疫应答杀伤肿瘤,可能免疫细胞过度活化导致排斥反应。虽然文献[25]报道免疫抑制剂与ICI同时使用时排斥病死率远低于肿瘤特异性病死率,但存在部分患者使用ICI方案导致移植物排斥反应与抗肿瘤疗效下降。此外,推荐于非移植HCC的ICI方案可能并不完全适用于LT后复发患者。
如何平衡移植物保护性免疫抑制和抗肿瘤免疫增强是一个关键问题,尽管最新试验[26]表明,肝移植物中PD-1淋巴细胞表达水平与排斥反应风险呈正相关,但在大规模Ⅱ、Ⅲ期临床试验预测和证实移植物排斥风险之前,ICI须谨慎使用。
4 ICI应用于LT患者的局限性与挑战
目前已有2例报道LT围手术期使用ICI发生致命性排斥反应[27-28],由于样本量较小,很难确定ICI与致命排斥反应相关的危险因素。不少学者认为与ICI剂量以及LT前停药时间有关[29]:最后一剂ICI未被及时清除,使移植肝表达PD-L1实现“免疫逃逸”,而发生排斥反应[30]。所以使用ICI患者LT术前应考虑逐渐减量。由于ICI与其靶點的结合是可逆的,因此可以使用血浆置换,但是过早停用 ICI后易出现疾病进展,可能无法从LT中获益。此外,专家建议[31]LT受者在ICI之前进行移植肝穿刺活检,PD-L1高表达提示易出现相关移植损伤。
综上所述,当前早期临床试验研究和个案报道已经初步证实了在严密监测下,ICI在LT患者中应用的安全性和有效性[32]。但是如何选择最佳的治疗时间和剂量、明确排斥反应的危险因素还需要更深入研究。此外,由于每种免疫疗法的疗效和排斥风险各不相同,因此对特定患者实施个体化评估、治疗仍是当务之急。目前正在进行的临床试验可能进一步回答这些问题(表2)。
5 小结及展望
ICI已成为中晚期HCC的选择,但对于LT患者来说,免疫疗法的应用仍需谨慎探索,避免ICI相关的排斥反应风险。总体而言,在新辅助治疗中使用ICI对HCC和移植肿瘤学具有重大意义,目前正在进行的临床试验将提供关于ICI的安全性和有效性见解,并有可能改变当前的治疗模式,使更多LT患者获益。
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:解有成负责课题设计,资料分析,撰写论文;陈顺、贾栋、郑英参与收集数据,修改论文;于晓辉、张久聪、李初谊负责拟定写作思路,指导撰写文章并最后定稿。
参考文献:
[1]SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660.
[2]Committee of Liver Transplantation, Chinese College of Transplant Doctors, Chinese Medical Doctor Association; Section of Liver Transplantation, Chinese Society of Organ Transplantation, Chinese Medical Association. Chinese expert consensus on application of sirolimus in liver transplantation for hepatocellular carcinoma(2020 edition)[J]. J Clin Hepatol, 2020, 36(11): 2429-2434. DOI: 10.3969/j.issn.1001-5256.2020.11.007.
中国医师协会器官移植医师分会肝移植学组, 中华医学会器官移植学分会肝移植学组. 西罗莫司在肝癌肝移植中应用的中国专家共识(2020版)[J]. 临床肝胆病杂志, 2020, 36(11): 2429-2434. DOI: 10.3969/j.issn.1001-5256.2020.11.007.
[3]EL-KHOUEIRY AB, SANGRO B, YAU T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet, 2017, 389(10088): 2492-2502. DOI: 10.1016/S0140-6736(17)31046-2.
[4]ZENG L, SU J, QIU W, et al. Survival outcomes and safety of programmed cell death/programmed cell death ligand 1 inhibitors for unresectable hepatocellular carcinoma: result from phase Ⅲ trials[J]. Cancer Control, 2022, 29: 10732748221092924. DOI: 10.1177/10732748221092924.
[5]YAU T, KANG YK, KIM TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial[J]. JAMA Oncol, 2020, 6(11): e204564. DOI: 10.1001/jamaoncol.2020.4564.
[6]TSUNG I, WORDEN FP, FONTANA RJ. A pilot study of checkpoint inhibitors in solid organ transplant recipients with metastatic cutaneous squamous cell carcinoma[J]. Oncologist, 2021, 26(2): 133-138. DOI: 10.1002/onco.13539.
[7]LEONETTI A, WEVER B, MAZZASCHI G, et al. Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer[J]. Drug Resist Updat, 2019, 46: 100644. DOI: 10.1016/j.drup.2019.100644.
[8]MURAKAMI N, MULVANEY P, DANESH M, et al. A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant[J]. Kidney Int, 2021, 100(1): 196-205. DOI: 10.1016/j.kint.2020.12.015.
[9]DOROSHOW DB, BHALLA S, BEASLEY MB, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors[J]. Nat Rev Clin Oncol, 2021, 18(6): 345-362. DOI: 10.1038/s41571-021-00473-5.
[10]SHARMA P, SIDDIQUI BA, ANANDHAN S, et al. The next decade of immune checkpoint therapy[J]. Cancer Discov, 2021, 11(4): 838-857. DOI: 10.1158/2159-8290.CD-20-1680.
[11]LI X, WANG Y, YANG H, et al. Liver and hepatocyte transplantation: What can pigs contribute?[J]. Front Immunol, 2021, 12: 802692. DOI: 10.3389/fimmu.2021.802692.
[12]HAN CZ, WEI Q, XU X. Transplant oncology creates a new era of liver transplantation for the treatment of liver cancer[J]. J Clin Hepatol, 2021, 37(2): 253-256. DOI: 10.3969/j.issn.1001-5256.2021.02.002.
韓承祚, 卫强, 徐骁. 移植肿瘤学开创肝移植治疗肝癌新时代[J]. 临床肝胆病杂志, 2021, 37(2): 253-256. DOI: 10.3969/j.issn.1001-5256.2021.02.002.
[13]JI R, DOU KF, XU H. Prevention and management of recurrence and metastasis of hepatocellular carcinoma after liver transplantation[J]. J Clin Hepatol, 2014, 30(1): 7-10. DOI: 10.3969/j.issn.1001-5256.2014.01.003.
季茹, 窦科峰, 许辉. 肝细胞癌患者肝移植术后肿瘤复发转移的防治[J]. 临床肝胆病杂志, 2014, 30(1): 7-10. DOI: 10.3969/j.issn.1001-5256.2014.01.003.
[14]JIANG C, JING S, ZHOU H, et al. Efficacy and prognostic factors of trans-arterial chemoembolization combined with stereotactic body radiation therapy for BCLC stage B hepatocellular carcinoma[J]. Front Oncol, 2021, 11: 640461. DOI: 10.3389/fonc.2021.640461.
[15]KUDO M. A new treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subsequent selective TACE[J]. Liver Cancer, 2019, 8(5): 299-311. DOI: 10.1159/000502905.
[16]LAWAL G, XIAO Y, RAHNEMAI-AZAR AA, et al. The immunology of hepatocellular carcinoma[J]. Vaccines (Basel), 2021, 9(10): 1184. DOI: 10.3390/vaccines9101184.
[17]SCHWACHA-EIPPER B, MINCIUNA I, BANZ V, et al. Immunotherapy as a downstaging therapy for liver transplantation[J]. Hepatology, 2020, 72(4): 1488-1490. DOI: 10.1002/hep.31234.
[18]YE Q, LING S, ZHENG S, et al. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA[J]. Mol Cancer, 2019, 18(1): 114. DOI: 10.1186/s12943-019-1043-x.
[19]
PELIZZARO F, GAMBATO M, GRINGERI E, et al Management of hepatocellular carcinoma recurrence after liver transplantation[J]. Cancers (Basel), 2021, 13(19): 4882. DOI: 10.3390/cancers13194882.
[20]OWOYEMI I, VAUGHAN LE, COSTELLO CM, et al. Clinical outcomes of solid organ transplant recipients with metastatic cancers who are treated with immune checkpoint inhibitors: A single-center analysis[J]. Cancer, 2020, 126(21): 4780-4787. DOI: 10.1002/cncr.33134.
[21]AMJAD W, KOTIAH S, GUPTA A, et al. Successful treatment of disseminated hepatocellular carcinoma after liver transplantation with nivolumab[J]. J Clin Exp Hepatol, 2020, 10(2): 185-187. DOI: 10.1016/j.jceh.2019.11.009.
[22]ZHUANG L, MOU HB, YU LF, et al. Immune checkpoint inhibitor for hepatocellular carcinoma recurrence after liver transplantation[J]. Hepatobiliary Pancreat Dis Int, 2020, 19(1): 91-93. DOI: 10.1016/j.hbpd.2019.09.011.
[23]FINN RS, RYOO BY, MERLE P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase iii trial[J]. J Clin Oncol, 2020, 38(3): 193-202. DOI: 10.1200/JCO.19.01307.
[24]YAU T, PARK JW, FINN RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial[J]. Lancet Oncol, 2022, 23(1): 77-90. DOI: 10.1016/S1470-2045(21)00604-5.
[25]DELYON J, ZUBER J, DORENT R, et al. Immune checkpoint inhibitors in transplantation-a case series and comprehensive review of current knowledge[J]. Transplantation, 2021, 105(1): 67-78. DOI: 10.1097/TP.0000000000003292.
[26]SHI GM, WANG J, HUANG XW, et al. Graft programmed death ligand 1 expression as a marker for transplant rejection following anti-programmed death 1 immunotherapy for recurrent liver tumors[J]. Liver Transpl, 2021, 27(3): 444-449. DOI: 10.1002/lt.25887.
[27]NORDNESS MF, HAMEL S, GODFREY CM, et al. Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for HCC: Are checkpoint inhibitors safe for the pretransplant patient?[J]. Am J Transplant, 2020, 20(3): 879-883. DOI: 10.1111/ajt.15617.
[28]CHEN GH, WANG GB, HUANG F, et al. Pretransplant use of toripalimab for hepatocellular carcinoma resulting in fatal acute hepatic necrosis in the immediate postoperative period[J]. Transpl Immunol, 2021, 66: 101386. DOI: 10.1016/j.trim.2021.101386.
[29]BRAHMER JR, DRAKE CG, WOLLNER I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates[J]. J Clin Oncol, 2010, 28(19): 3167-3175. DOI: 10.1200/JCO.2009.26.7609.
[30]
GAO Q, ANWAR IJ, ABRAHAM N, et al. Liver transplantation for hepatocellular carcinoma after downstaging or bridging therapy with immune checkpoint inhibitors[J]. Cancers (Basel), 2021, 13(24): 6307. DOI: 10.3390/cancers13246307.
[31]MORITA M, FUJINO M, JIANG G, et al. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft[J]. Am J Transplant, 2010, 10(1): 40-46. DOI: 10.1111/j.1600-6143.2009.02859.x.
[32]LIU ZB, WU JS, LIN DD, et al. Safety of PD-1 inhibitor in preoperative treatment of liver transplantation for liver cancer[J]. Ogran Transplant, 2021, 12(4): 445-449. DOI: 10.3969/j.issn.1674-7445.2021.04.011.
劉召波, 武聚山, 林栋栋, 等. PD-1抑制剂用于肝癌肝移植术前治疗的安全性探讨[J]. 器官移植, 2021, 12(4): 445-449. DOI: 10.3969/j.issn.1674-7445.2021.04.011.
[33]ClinicalTrials.gov. Combination camrelizumab (SHR-1210) and apatinib for downstaging/bridging of HCC before liver transplant[EB/OL]. (2019-07-29) [2022-07-28]. https://clinicaltrials.gov/ct2/show/ NCT04035876.
[34]ClinicalTrials.gov. Pembrolizumab and LENvatinib in participants with hepatocellular carcinoma (HCC) before liver transplant (PLENTY202001) [EB/OL]. (2020-06-11) [2022-07-28]. https: //clinicaltrials.gov/ct2/show/ NCT04425226.
[35]ClinicalTrials.gov. Atezolizumab and bevacizumab pre-liver transplantation for patients with hepatocellular carcinoma beyond milan criteria [EB/OL]. (2022-01-11) [2022-07-28]. https://clinicaltrials.gov/ct2/show/ NCT05185505.
[36]ClinicalTrials.gov. Durvalumab (MEDI4736) and tremelimumab for hepatocellular carcinoma in patients listed for a liver transplant [EB/OL]. (2021-08-30) [2022-07-28]. https://clinicaltrials.gov/ct2/show/ NCT05027425.
[37]ClinicalTrials.gov. Durvalumab and lenvatinib in participants with locally advanced and metastatic hepatocellular carcinoma ( Dulect2020-1 ) (Dulect2020-1) [EB/OL]. (2020-06-23) [2022-07-28]. https: //clinicaltrials.gov/ct2/show/ NCT04443322.
[38]ClinicalTrials.gov. Safety and efficacy of camrelizumab (Anti-PD-1 Antibody) in recurrent HCC after liver transplantation [EB/OL]. (2020-09-25) [2022-07-28]. https://clinicaltrials.gov/ct2/show/ NCT04564313.
[39]ClinicalTrials.gov. IMRT plus PD-1 blockade and lenvatinib for HCC with PVTT (Vp3) before liver transplantation (iPLENTY-pvtt) [EB/OL]. (2022-04-21) [2022-07-28]. https://clinicaltrials.gov/ct2/show/ NCT05339581.
[40]ClinicalTrials.gov. Sequential PD-1/PD-L1 inhibitor and LENvatinib in TLCT and refractory hepatoblastoma after chemotherapy (sPLENTY-pc) [EB/OL]. (2022-04-11) [2022-07-28]. https://clinicaltrials.gov/ct2/show/ NCT05322187.
收稿日期:
2022-07-31;录用日期:2022-09-18
本文编辑:林姣
