钴钒水滑石纳米片用于电催化尿素氧化
2023-03-15刘瑶钰王宇辰刘碧莹MahmoudAmer严凯
刘瑶钰,王宇辰,*,刘碧莹,Mahmoud Amer ,严凯,*
1中山大学环境科学与工程学院,广州 510275
2 Mechanical Engineering Department, Faculty of Engineering, Alexandria University, Alexandria 21544, Egypt
1 Introduction
Hydrogen is considered to be a promising candidate to substitute traditional fossil fuel, which stems from its environmental compatibility and high energy density1-3.Electrochemical water splitting, as a green pathway for hydrogen manufacture, has gained increasing attention. However, water electrolysis technology is suffered from high theoretical splitting voltage (1.23 V)owing to the anodic oxygen evolution reaction(OER)4,5. Urea oxidation reaction (UOR)with low thermodynamic equilibrium potential (0.37 Vvs. reversible hydrogen electrode, RHE)is considered a potential nucleophile electrooxidation reaction to replace OER6. Thereby, making hydrogen from urea electrolysis is deemed a wide foreground route. Based on this, the dedicated construction of highperformance UOR electrocatalysts is crucial for hydrogen production.
Among the reported UOR electrocatalysts, transition metal based layered double hydroxides (TM-LDHs)nanosheets have drawn much attention owing to their abundant exposed active sites, controllable layered structure and tunable interlayer anions7,8. To achieve the ideal UOR performance of TM-LDHs nanosheets, researchers have endeavored to adjusted their structure and morphology by heteroatom doping9and/or defect engineering10. For instance, Wanget al.11successfully fabricated ultrathin NiMoV-LDHs nanosheets with excellent UOR catalytic activity (1.40 Vvs.RHE@100 mA·cm-2), which was attributed to the rational doping of Mo and V for modulating the electronic structure of the as-fabricated electrocatalyst. Sunet al.12utilized a one-pot hydrothermal method to incorporate Rh into NiFe-LDHs nanosheets to realize outstanding UOR performance, which enabled 10 mA·cm-2with a low potential of 1.35 Vvs.RHE. Density functional theory (DFT)calculations unveiled that improved UOR kinetics originated from the positive effect of oxygen vacancies by Rh-doping. Therefore, the adjustment of electronic structure of TM-LDHs nanosheets is essential for promoting their electrocatalytic activity towards UOR.
Recently, ad-electron compensation effect was proposed to construct TM-LDHs nanosheets with high OER performance13-15.Considering the similar electrocatalytic mechanism of TMLDHs, this miraculous effect was expected to be validated in the application of UOR. On the basis of the Brewer-Engel valencebond theory, the presence of pairedd-electrons and semi-d-orbits was beneficial for the adsorption/desorption of intermediate during electrocatalytic process16. Since V was an early transition metal element (VB group element)with a vacantd-orbital, the combination of V and late transition metals in TM-LDHs nanosheets was prone to generate the electronic configuration with paired electrons and semi-d-orbits17, which was a prerequisite to realize high UOR activity. Therefore, our present work developed CoV-LDHs nanosheets as high-performance UOR electrocatalystsviaa facile one-step method. This research affords a worthy strategy for designing high-efficient UOR electrocatalysts.
2 Experimental
2.1 Preparation of CoV-LDHs
All of chemical substances remained the same as received and were conducted without any retreatment. The CoV-LDHs electrocatalyst was fabricatedviathe coprecipitation method.1.50 mmol of cobalt chloride hexahydrate (CoCl2·6H2O,Macklin, AR)and 1.50 mmol of vanadium chloride (VCl3,Macklin, AR, 99%)were added into 250 mL beaker containing 150 mL ultrapure water (18.25 MΩ·cm). Subsequently, the aqueous solution of 1.00 mol·L-1sodium hydroxide (NaOH,Aladdin, AR, 96%)was slowly dropped into above mixed solution under mechanical stirring (500 r·min-1), until the pH value of the liquid solution reached 10.50 and remained constant.Afterwards, the above solution was standing at room temperature for 1 h, centrifuged twice with ultrapure water and ethanol. To obtain the final products, the centrifuged sample was dried overnight in vacuum oven at 60 °C.
2.2 Preparation of Co(OH)2
Co(OH)2was prepared for the comparison with the UOR performance of CoV-LDHs. Co(OH)2powders were derived following the same procedure as CoV-LDHs without adding VCl3. In the Supporting Information, the details of structure,morphology and electrochemical characterizations are expressed.
3 Results and discussion
The CoV-LDHs was preparedviaa simple one-step coprecipitation method (Fig. 1). The structure of obtained samples was analyzed by X-ray diffraction (XRD), which is presented in Fig. 2a. For the Co(OH)2sample, the diffraction peaks at 11.0°,22.2°, 34.0°, 45.1° and 59.3° are ascribed to (003), (006), (100),(108)and (110)crystal planes ofα-Co(OH)2(JCPDS No. 46-0605). The appearance of diffraction peaks at 19.0°, 32.5°, 37.9°,51.4° and 57.9° indicated thatβ-Co(OH)2was also formed in the coprecipitation process18. After the incorporation of V, most characteristic diffraction peaks ofα-Co(OH)2and all characteristic diffraction peaks ofβ-Co(OH)2disappeared,revealing the successful fabrication of hydrotalcite-like structure19.Meanwhile, the negligible peaks related to (003)and (006)crystal planes confirmed the formation of LDHs nanosheet structure20. Furthermore, the wettability of as-prepared samples was investigatedviacontact angle measurements. As illustrated in Fig. 2b, the contact angles of Co(OH)2and CoV-LDHs were calibrated as 22.1° and 14.6°, respectively. Compared with Co(OH)2, the enhanced wettability of CoV-LDHs was beneficial for the contact between the electrode and electrolyte, which increases the utilization of electrochemical active sites for UOR21,22. Additionally, the atomic ratio of CoV-LDHs was measured by inductively coupled plasma mass spectrometry(ICP-MS), which was consistent with the feed metal ratio (Table S1).
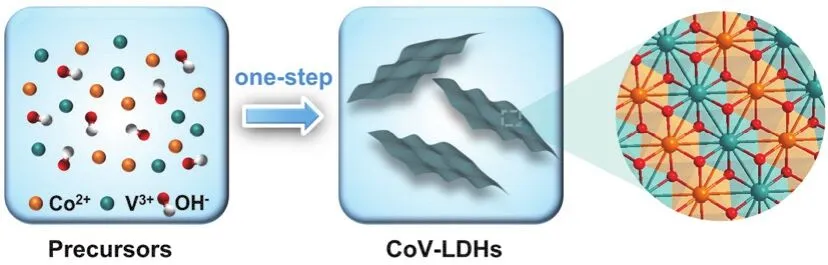
Fig. 1 The fabrication procedure of CoV-LDHs.
The morphologies of Co(OH)2and CoV-LDHs were studied by transmission electron microscopy (TEM)technique. As viewed in Fig. 3a, Co(OH)2showed the intact lamellar structure with the size of few hundreds of nanometers. With the introduction of V, CoV-LDHs displayed the irregular nanosheet structure with relatively small size (Fig. 3b). High resolution TEM (HRTEM)images and selected area electron diffraction(SAED)patterns also confirmed the crystal structure of LDHs.For CoV-LDHs in Fig. 3c, a clear lattice fringe with 0.156 nm spacing was indexed to the (110)crystal plane of CoV-LDHs in XRD results. Similarly, the lattice fringe of 0.199 nm related to the (108)plane of Co(OH)2was also observed in Fig. S1(Supporting Information). Additionally, the HAADF image and energy dispersive spectroscopy (EDS)mapping images illustrate that the homogeneous distribution of Co, V and O on the CoVLDHs (Fig. S2).
X-ray photoelectron spectroscopy (XPS)was applied to penetrate electronic structure and valence state of as-synthesized samples. The XPS survey spectra (Fig. S3)showed feature elements of Co, V and O. As displayed in Fig. 4a, the binding energy of Co(OH)2at 782.08 eV corresponded to Co2+. For CoVLDHs, the characteristic peak shifted to low binding energy,indicating that the introduction of V (3d34s2)perturbs the electron environment around Co (3d74s2)atom and thus increases the valence state of Co23,24. Meanwhile, V 2pspectra of CoV-LDHs in Fig. 4b were fitted into two peaks at 517.2 eV and 518.3 eV, which were assigned to V3+and V4+, respectively25.It is reported that the electrocatalytic performance of LDHs is greatly affected by the electronic configuration of central transition metal atoms26. Fig. 4c is plotted to explain the cooperative electronic interaction between Co and V cations. For Co(OH)2, the fully occupiedd-orbitals of Co cations generated a repulsive electron-electron interaction between Co-Co unit16.After introducing V cations with half-filledd-orbitals,d-electron compensation between Co and V decreased the repulsive interaction of Co-Co unit, thus facilitating the adsorption of urea13,27. Therefore CoV-LDHs were expected to possess high UOR activity.
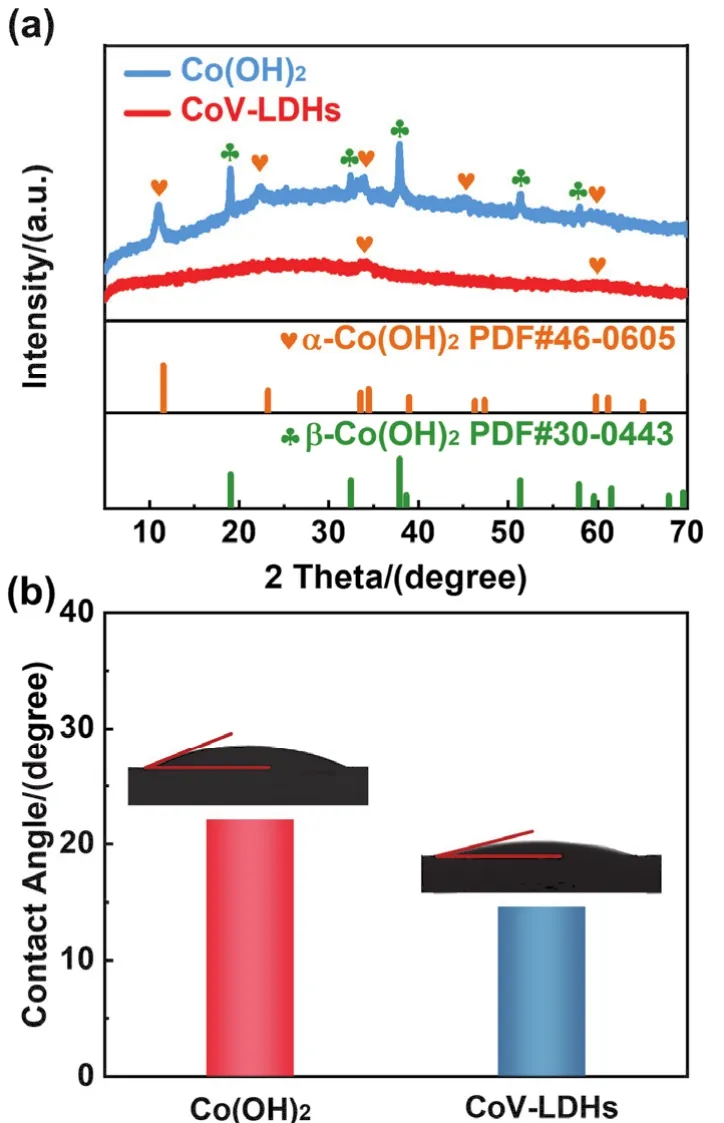
Fig. 2 (a)XRD patterns of Co(OH)2 and CoV-LDHs. (b)Contact angles of Co(OH)2 and CoV-LDHs with ultrapure water.

Fig. 3 TEM images of (a)Co(OH)2 and (b)CoV-LDHs, (c)HRTEM image (inset:SAED pattern)of CoV-LDHs.
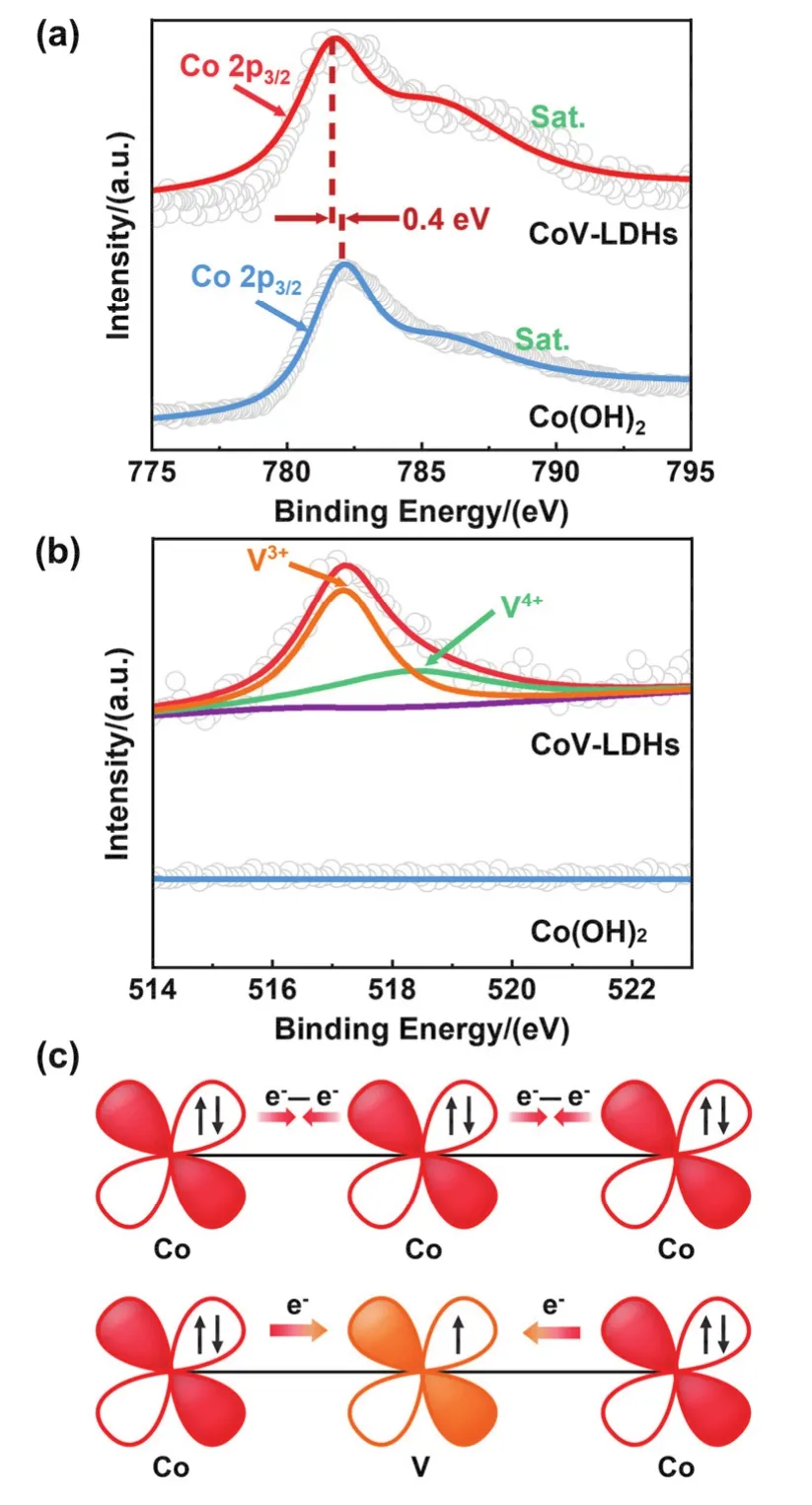
Fig. 4 XPS spectra of (a)Co 2p, (b)V 2p of Co(OH)2 and CoV-LDHs. (c)The electronic coupling diagram between Co and V in Co(OH)2 and CoV-LDHs.
In order to assess the UOR performance of CoV-LDHs, a series of electrochemical measurements were performed. The concentration of urea was chosen as 0.33 mol·L-1since 2%-2.5% (w)urea (equal to ~0.33 mol·L-1)was naturally existed in human urine28. Fig. S4 shows linear sweep voltammetry (LSV)curves of CoV-LDHs in the absence and presence of urea at 5 mV·s-1. To arrive a current density of 10 mA·cm-2, the required potential for UOR was 60 mV smaller than that for OER since urea with a nucleophile amino group plays a vital role in reducing the anodic oxidation potential29,30. Fig. 5a displays the comparison of UOR activities of Co(OH)2, CoV-LDHs and pure carbon fiber paper (CFP). Apparently, the current density of both Co(OH)2and CoV-LDHs outperformed that of CFP with the increasing potential. While the current density was elevated to 10 mA·cm-2, the potential for Co(OH)2and CoV-LDHs were realized as 1.59 and 1.52 Vvs.RHE, respectively, demonstrating that the redistributed electronic structure evoked by the introduction of V notably enhance the UOR performance14.Also, the relatively low Tafel slope of 99.9 mV·dec-1(Fig. 5b)reflected a rapid UOR kinetics for CoV-LDHs. Afterwards, the electrochemical active surface area (ECSA)was elaborated by the double-layer capacitance (Cdl)to further evaluate the UOR catalytic performance. Fig. S5 presents the CV curves of Co(OH)2and CoV-LDHs with scan rates from 2 to 8 mV·s-1. By fitting the slope of current density at 1.10 Vvs.RHE with scan rates (Fig. 5c),Cdlvalues of Co(OH)2and CoV-LDHs were derived as 24.5 and 34.6 mF·cm-2, respectively. TheseCdlvalues suggested the exposure of more catalytic active sites, which could originate from the beneficial effect of V11.
The electrochemical impedance spectroscopy (EIS)test was performed to gain an insight into the interface dynamics of asprepared samples. By fitting the Nyquist plots with a classical equivalent circuit (Figs. 5d and S6), the equivalent series resistance (RESR)and charge transfer resistance (Rct)values are summarized in Table S2. On the basis of these results, the relatively lowRESR(2.09 Ω)andRct(1.75 Ω)of CoV-LDHs demonstrated the superior electronic and ionic charge transfer ability for UOR31. Furthermore, the durability of the CoV-LDHs electrocatalyst was also investigatedviaa chronopotentiometry(CP)test at 10 mA·cm-2for 10 h (Fig. 5e). After 10 h, a trivial potential increase was observed owing to the change of urea concentration in the galvanostatic process. Besides, the LSV curve after CP test was almost unchanged in comparison with the initial state (Fig. S7), further indicating the excellent durability of CoV-LDHs towards UOR. These electrochemical results demonstrated the excellent durability of CoV-LDHs towards UOR, which was also proved by unvaried morphology and structure of post-UOR electrocatalyst (inset of Figs. 5e, S8 and S9).
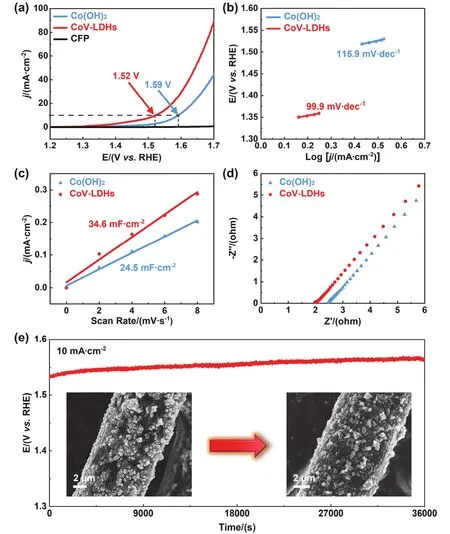
Fig. 5 (a)LSV curves of Co(OH)2, CoV-LDHs and CFP for UOR. (b)Tafel plots, (c)calculated Cdl values and(d)Nyquist plots of Co(OH)2 and CoV-LDHs. (e)The chronopotentiometric curve of CoV-LDHs for UOR at 10 mA·cm-2.Inset:SEM images of CoV-LDHs electrode before and after durability test.
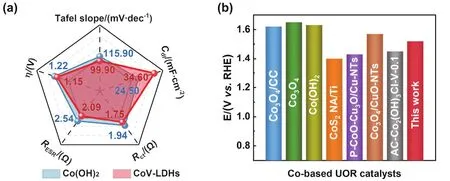
Fig. 6 (a)Comparison of Tafel slope, overpotential (ŋ), RESR, Rct, and Cdl of Co(OH)2 and CoV-LDHs.(b)Comparison of potential values of recently reported Co-based UOR catalysts at 10 mA·cm-2.
Based on above material and electrochemical characterizations of Co(OH)2and CoV-LDHs, CoV-LDHs nanosheets exhibited outstanding UOR performance (Fig. 6a),which is mainly attributed to thed-electron compensation effect,the enhanced wettability and the large electrochemical surface area for UOR. Concretely, after combining the early transition metal of V with vacant/semi-filled 3dorbitals and the late transition metal of Co with partially full-filled 3dorbitals, the overlap ofd-orbitals is beneficial for the absorption/activation of urea32. Meanwhile, the hydrophilic character of CoV-LDHs accelerates the charge transfer between the electrocatalyst and urea. Consequently, the UOR performance of CoV-LDHs is superior or comparable to that of recently reported Co-based electrocatalysts (Table S3 and Fig. 6b), such as Co3O4/CC33,Co3O434, Co(OH)234, CoS2NA/Ti35, P-CoO-Cu2O/Cu-NTs36,Co3O4/CuO-NTs36, AC-Co2(OH)3Cl-V-0.127.
4 Conclusions
In summary, CoV-LDHs nanosheets were successfully prepared through a one-step method. In comparison with Co(OH)2, CoV-LDHs nanosheets not only exposed more active sites, but also improved the interaction between the electrocatalyst and electrolyte. More importantly, the rational combination of Co and V elements weakened the repulsive Co-Co interactions to facilitate the adsorption of urea molecules onto the surface of CoV-LDHs nanosheets. Thus, CoV-LDHs nanosheets exhibited superior UOR electrocatalytic activity.Specifically, CoV-LDHs required only 1.52 Vvs. RHE to obtain 10 mA·cm-2current density and derived a low Tafel slope of 99.9 mV·dec-1. Besides, CoV-LDHs presented a favorable stability over 10 h with an inconspicuous potential decline. This work offers an advanced design protocol for UOR electrocatalysts, which can be extensively expanded to other transition metal based electrocatalysts.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
