视黄醇结合蛋白1通过激活TAK1促进大鼠乳鼠原代心肌细胞肥大*
2023-03-10谢菁周艳丽陈思思王继春江洪
谢菁, 周艳丽, 陈思思, 王继春, 江洪
视黄醇结合蛋白1通过激活TAK1促进大鼠乳鼠原代心肌细胞肥大*
谢菁, 周艳丽, 陈思思, 王继春, 江洪△
(武汉大学人民医院心血管内科,武汉大学心血管病研究所,心血管病湖北省重点实验室,湖北 武汉 430000)
探究视黄醇结合蛋白1(retinol binding protein 1, Rbp1)在大鼠乳鼠原代心肌细胞肥大中的功能和机制。本研究通过联合分析基因表达数据库(Gene Expression Omnibus, GEO)中的小鼠心肌肥厚基因芯片数据,寻找在心肌肥厚发生过程中的关键调控因子,构建该基因过表达及敲减腺病毒并感染大鼠乳鼠原代心肌细胞,利用血管紧张素II(angiotensin II, Ang II)诱导大鼠乳鼠原代心肌细胞肥大模型,研究目的基因对大鼠乳鼠原代心肌细胞肥大的影响,应用Western blot及RT-qPCR探究目的基因调控大鼠乳鼠原代心肌细胞肥大的分子机制。在小鼠心肌肥厚心脏组织中的表达显著上调(<0.01),体外细胞实验显示与对照组相比,过表达组的大鼠乳鼠原代心肌细胞面积显著增大,心肌肥厚标志基因心钠肽(atrial natriuretic peptide,)和肌球蛋白重链7(myosin heavy chain 7,)的表达显著升高(<0.01),证实过表达能促进Ang II诱导的大鼠乳鼠原代心肌细胞肥大;相反,敲减则能显著抑制Ang II诱导的大鼠乳鼠原代心肌细胞面积增加和心肌肥厚标志物表达。机制研究显示,过表达显著激活转化生长因子β激活激酶1(transforming growth factor-β-activated kinase 1, TAK1)和P38(<0.01),应用TAK1抑制剂可阻断过表达对Ang II诱导大鼠乳鼠原代心肌细胞肥大的促进作用。Rbp1可通过激活TAK1信号通路促进Ang II诱导的大鼠乳鼠原代心肌细胞肥大。
视黄醇结合蛋白1;大鼠乳鼠原代心肌细胞;心肌肥大;TAK1/P38信号通路
心力衰竭是心室充盈和(或)射血功能受损,心脏排血量不能满足机体组织代谢需要的临床综合征,是临床上心肌肥厚等多种心血管疾病的最终结局[1]。心肌肥厚是心力衰竭发生发展的一个基本病理过程。众多基础研究提示,心肌肥厚是一个复杂的、众多基因和信号通路参与的病理过程[2],且目前临床上缺乏有效药物。因此,阐明心肌肥厚发生发展的分子机制,寻找调节心肌肥厚的关键靶点具有重要意义。
视黄醇结合蛋白(retinol binding protein, Rbp)是人体内视黄醇转运蛋白,主要帮助肝脏中视黄醇的转移,并协助视黄醇进行代谢、存储、发挥作用,半衰期较短,能敏感的反映机体的疾病状态[3]。既往研究提示Rbp1可驱动维生素A为活性代谢产物全反视黄酸(all--retinoic acid, ATRA),ATRA可调节细胞的增殖、凋亡、分化及迁移[4-5]。此外,Rbp1还参与生物体内多个生理过程如肥胖及血脂代谢异常等[6],但Rbp1在心肌肥厚中的作用还未见报道。本项工作通过腺病毒感染大鼠乳鼠原代心肌细胞和PE诱导的大鼠乳鼠原代心肌细胞肥大模型,探讨Rbp1对大鼠乳鼠原代心肌细胞肥大的影响。
材料和方法
1 主要试剂
BCA蛋白试剂盒(Thermo Scientific); PVDF膜(Millipore);抗Rbp1抗体(ABclonal);抗TAK1单克隆抗体(Abcam);p-P38单克隆抗体、P38多克隆抗体、p-TAK1单克隆抗体及GAPDH单克隆抗体(Cell Signaling Technology);化学发光底物试剂(Bio-Rad);Trizol总RNA提取试剂(Invitrogen); cDNA转录合成试剂盒和SYBR Green PCR Master Mix(Roche);DMEM/F12培养液(Gibco);胎牛血清(Gibco);抗辅肌动蛋白α(α-actinin)抗体(Merck Millipore);TAK1抑制剂NG52(MedChemExpress);其它生化试剂均为进口分装或国产分析纯;所用引物均由生工生物工程(上海)股份有限公司合成。
2 主要方法
2.1生物信息学分析借助R包GEOquery (Davis and Meltzer, 2007)从美国国家生物技术信息中心(National Center of Biotechnology Information, NCBI)的GEO数据库下载对应数据集的原始数据及芯片探针对应的基因名称注释文件,随后使用R包寡核苷酸(Carvalho and Irizarry, 2010)中的函数(read.celfiles)读取芯片原始数据,并使用RMA(Robust Multiarray Average)算法对原始数据进行背景校正,使用分位数方法进行样本间的标准化,运用统计学方法将前面得到的荧光强度值从探针水平汇总到探针组水平,再将汇总后的数据转换为以2为底的对数,经过RMA函数运算得到标准化后的探针表达量矩阵,根据得到的注释文件,对探针组名称进行基因名称注释,最终得到基因的表达量矩阵。计算不同组别之间基因的表达差异,根据基因表达量差异,去除重复的基因,保留重复基因中差异最大的一个。使用检验对不同组进行统计学检验,依据倍数多少和值确定差异基因。
2.2大鼠乳鼠原代心肌细胞的分离和培养选1~2日龄SD大鼠乳鼠[湖北省实验动物研究中心,许可证号:SCXK(鄂)2020-0018],大鼠乳鼠原代心肌细胞分离培养方法参照如前所述[8],具体方法简述如下:取SD大鼠乳鼠心脏剥离血管成分,剪成1 mm×1 mm×1 mm的组织块,加入0.125%胰蛋白酶进行消化,加入DMEM/F12培养液[含有10%胎牛血清、1%青霉素/链霉素和0.1 mmol/L 5-溴脱氧尿苷(抑制成纤维细胞生长)]培养24 h差时贴壁,分离大鼠乳鼠原代心肌细胞后,使用腺病毒感染大鼠乳鼠原代心肌细胞,6 h后更换为无血清培养液进行饥饿处理12 h,然后加入1 μmol/L Ang II刺激48 h,对照加入等量的磷酸缓冲盐溶液(phosphate-buffered saline, PBS),整个细胞培养在37.0 ℃、5% CO2条件下进行。
2.3大鼠乳鼠原代心肌细胞α-actinin免疫荧光染色大鼠乳鼠原代心肌细胞免疫荧光染色方法参照如前所述[7],具体方法简述如下:大鼠乳鼠原代心肌细胞经Ang II刺激培养48 h后,加入4%甲醛固定30 min,再加入0.1% Triton X-100透化后加入10%牛血清白蛋白室温下封闭,加入α-actinin抗体(1∶100稀释)孵育,随后加入Ⅱ抗[驴抗鼠IgG (H+L), Invitrogen, 1∶200稀释)及DAPI(染核),使用Image-Pro Plus 6.0软件测量大鼠乳鼠原代心肌细胞的表面积。
2.4RT-qPCR检测RT-qPCR的方法参照文献[7],具体简述如下:取小鼠心脏左心室组织(来自武汉大学李红良教授馈赠)或大鼠乳鼠原代心肌细胞样本加入Trizol试剂提取总RNA,使用cDNA转录合成试剂盒将RNA反转成cDNA,随后在一定体系下加入SYBR Green PCR Master Mix在RT-qPCR仪中检测待测基因的表达量,同时以甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase,)为内参基因,所使用的引物序列见表1。
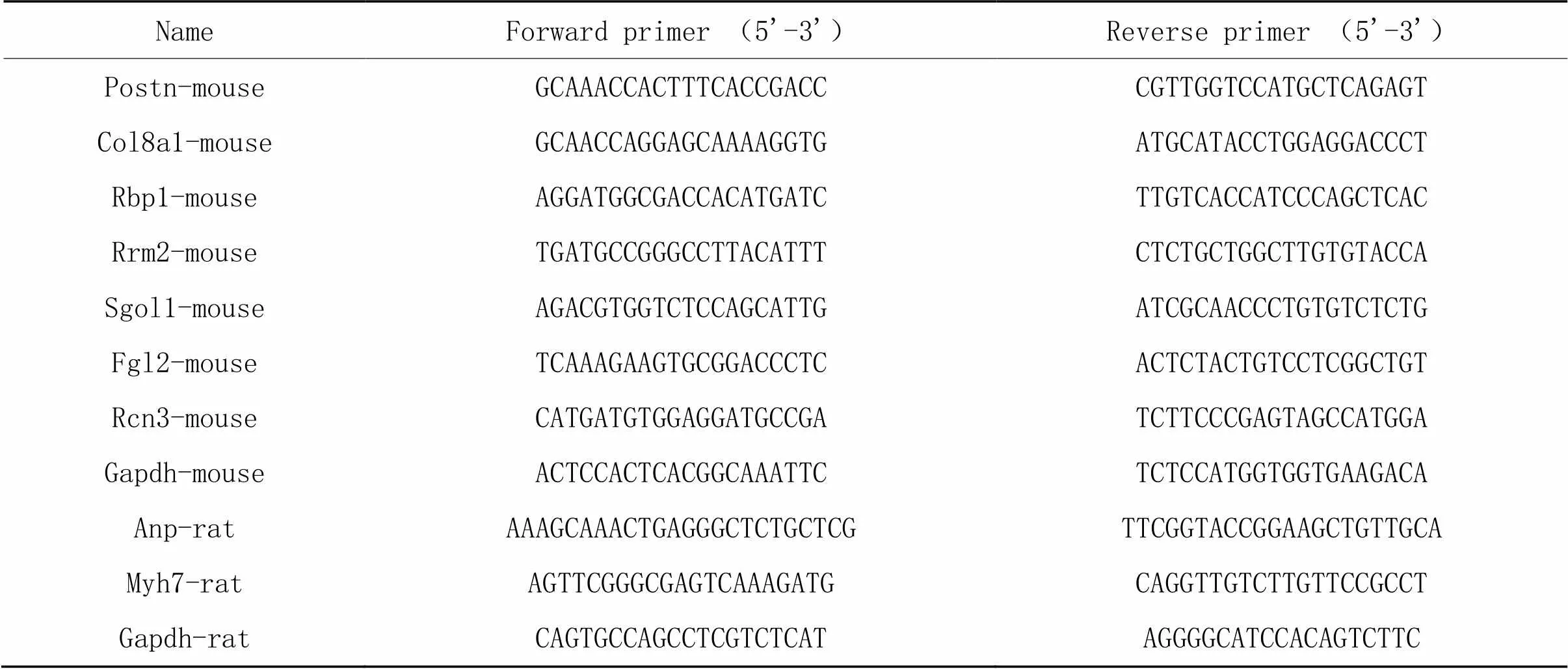
表1 RT-qPCR引物序列
2.5Western blot实验Western blot的方法参照如前所述[8],具体简述如下:收集处理过的大鼠乳鼠原代心肌细胞样本并加入RIPA裂解液进行裂解,充分裂解后离心,取上清为总蛋白,使用BCA蛋白试剂盒进行定量,上样相同质量总蛋白于10% SDS-PAGE分离蛋白并转移至PVDF膜,随后放入5%脱脂奶粉室温下封闭1 h,TBST清洗PVDF膜3次,每次5 min,加入Ⅰ抗4 ℃孵育过夜。TBST清洗,加入对应种属的Ⅱ抗(Jackson ImmunoResearch)室温孵育1 h。用化学发光底物显色,伯乐凝胶成像系统(ChemiDoc XRS+)收集信号。使用Image Lab(Version5.1)软件分析结果。
2.6载体构建载体构建的方法参照如前所述[7],具体简述如下:将基因亚克隆到巨细胞病毒启动子控制下的复制缺陷型腺病毒(adenovirus,Ad)载体中并用于过表达,以表达绿色荧光蛋白(green fluorescent protein,GFP)为对照。携带靶向的短发夹RNA(short-hairpin RNA,shRNA)的复制缺陷型腺病毒载体用于敲减(sh)表达,同时以对照shRNA腺病毒作为对照(shCT)。腺病毒以50个颗粒/细胞的感染复数(multiplicity of infection, MOI)感染大鼠乳鼠原代心肌细胞24 h,随后进行检测鉴定。过表达及敲减引物为:
Ad-Rat-F:GGCTAGCGATATCGGATCCGCCA-CCATGCCTGTGGACTTCAACGG;Ad-Rat-R: CGT-CCTTGTAATCACTAGTGTGTACTTTCTTAAACACTTG-CTTGCAG;Adsh-Rat-F: CCGGGGTACTGGAAGATGCTGAGCACTCGAGTGCTCAGCATCTTCCAGTACCTTTTTG;Adsh-Rat-R:AATTCAAAAAGGTACTGGAAGATGCTGAGCACTCGAGTGCTCAGCA-TCTTCCAGTACC。
2.7TAK1的抑制剂(inhibitor of TAK1, iTAK1)处理大鼠乳鼠原代心肌细胞对应的腺病毒分别感染大鼠乳鼠原代心肌细胞后,加入2.5×10-6mol/L二甲基亚砜(DMSO)溶解的iTAK1-NG52,对照组加入等量DMSO,与此同时再加入1×10-6mol/L Ang II刺激48 h后收集大鼠乳鼠原代心肌细胞样本进行检测。
3 统计学处理
本课题所有数据统计均采用均数±标准差(mean±SD)的形式,两组间数据比较使用双尾检验,多组间数据比较使用单因素方差分析,并使用检验(假定方差齐)或(假定方差不齐)校正。SPSS(Statistical Package for the Social Sciences)25.0 软件分析数据,<0.05为有统计学差异。
结果
1 Rbp1表达水平在小鼠心肌肥厚中显著上调
本研究通过生物信息学分析5个小鼠主动脉缩窄手术诱导的小鼠心肌肥厚模型基因芯片数据库,样本情况详见表2,根据<0.05与Fold change>1.5筛选上调差异基因,<0.05与Fold change<0.667筛选下调差异基因,分别获得102、218、293、410和98个差异表达基因,见图1A;联合分析后,获得11个在4个及以上芯片数据库结果中均显著改变的差异基因,见图1B;其中和在心肌肥厚中的功能未知,见图1C;随后,应用RT-qPCR检测对照假手术(sham)和主动脉结扎(aortic banding, AB)手术4周的小鼠心脏组织中上述差异基因的表达,结果显示与sham组比较,在AB组中的表达改变最为显著(<0.01),见图1D。
表2 小鼠心肌肥厚相关GEO数据库
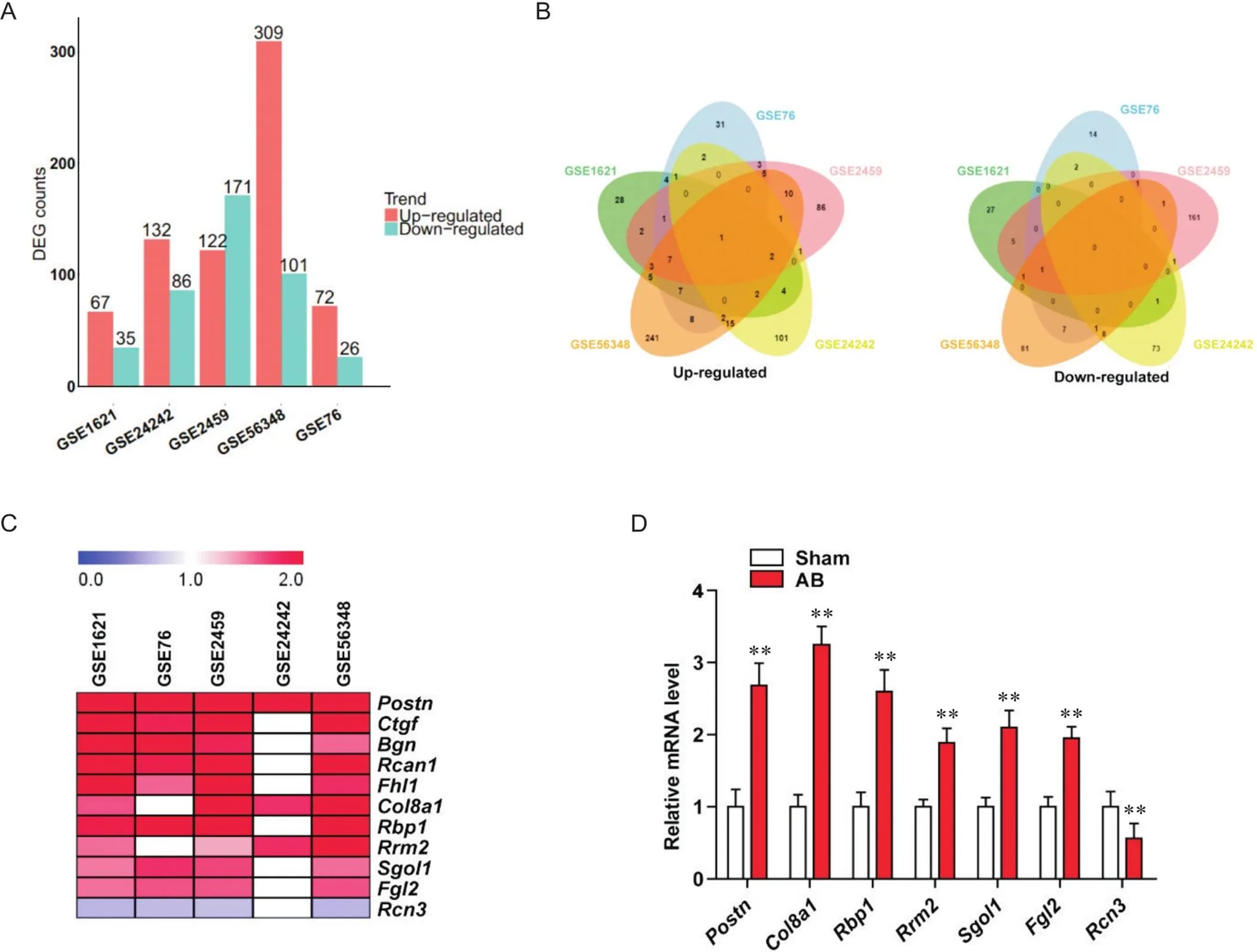
Figure 1. Combined analysis of mouse cardiac hypertrophy microarray data to search for the key regulatory genes in the process of cardiac hypertrophy. A: the number of differentially expressed genes in each cardiac hypertrophy microarray data from GEO database; B: Venn diagram of 5 cardiac hypertrophy microarray data from GEO database; C: heat map of genes significantly changed in more than 4 cardiac hypertrophy microarray data; D: relative mRNA expression of target genes in heart of cardiac hypertrophy mice after sham or aortic banding (AB) surgery for 4 weeks. Mean±SD. n=5. **P<0.01 vs sham group.
2 Rbp1敲减抑制Ang II诱导的大鼠乳鼠原代心肌细胞肥大
Western blot检测结果显示,与对照组相比,敲减组Rbp1蛋白水平显著下调,证实敲减成功,见图2A;α-actinin免疫荧光染色及RT-qPCR检测结果显示,敲减能显著抑制大鼠乳鼠原代心肌细胞表面积增大(<0.01),并显著下调肥大标志基因和的表达(<0.01),见图2B、2C。
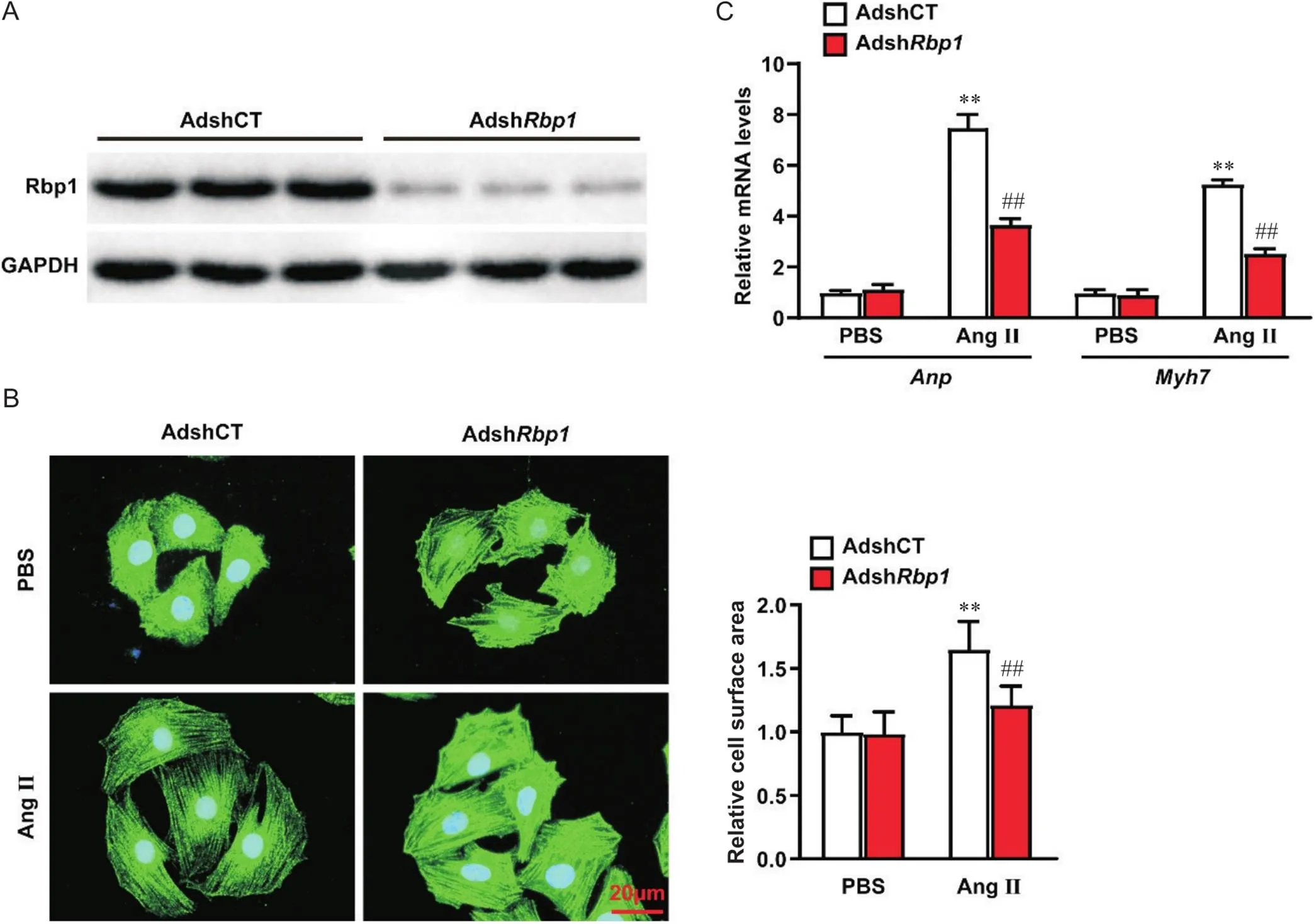
Figure 2. Knockdown of Rbp1 inhibited Ang II-induced primary neonatal rat cardiomyocyte hypertrophy. A: the protein level of Rbp1 in primary neonatal rat cardiomyocytes with knockdown of Rbp1(AdshRbp1) and control primary neonatal rat cardiomyocytes (AdshCT) groups; B: representative immunofluorescence images of anti-α-actinin staining and its statistical results in the indicated groups (scale bar=20 μm); C: relative mRNA levels of Anp and Myh7 in the indicated groups. Mean±SD. n=3. **P<0.01 vs AdshCT PBS group; ##P<0.01 vs AdshCT Ang II group.
3 Rbp1过表达促进Ang II诱导的大鼠乳鼠原代心肌细胞的肥大
Western blot检测结果显示,与对照组相比,过表达组Rbp1蛋白表达水平显著上调,证实Rbp1过表达成功,见图3A;α-actinin免疫荧光染色及RT-qPCR检测结果表明,过表达显著促进了大鼠乳鼠原代心肌细胞表面积大小及肥大标志基因和的表达(<0.01),见图3B、C。
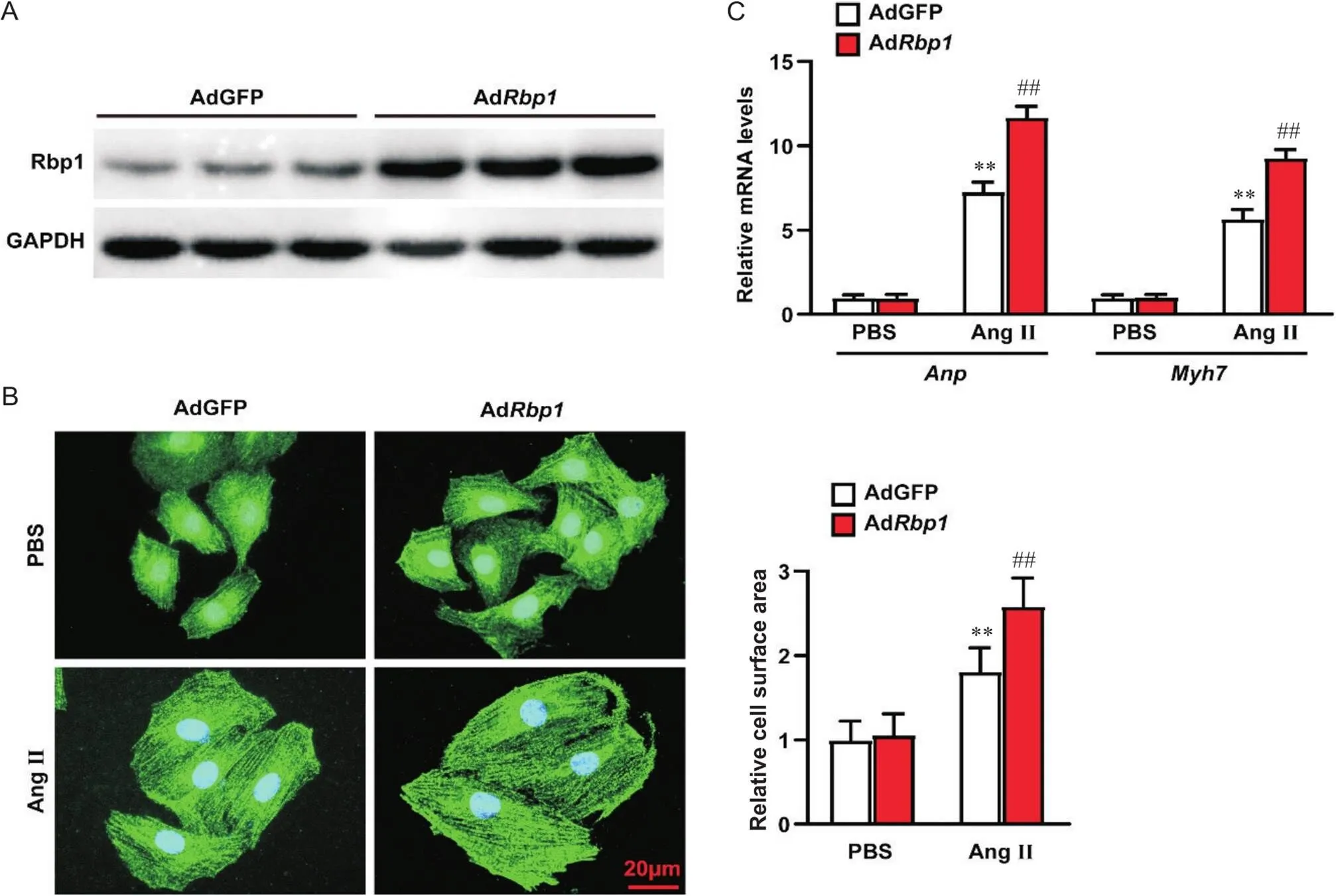
Figure 3. Overexpression of Rbp1 promoted Ang II-induced primary neonatal rat cardiomyocyte hypertrophy. A: the protein level of Rbp1 in primary neonatal rat cardiomyocytes overexpressing Rbp1 (AdRbp1) and control primary neonatal rat cardiomyocytes (AdGFP) groups; B: representative immunofluorescence images of anti-α-actinin staining and its statistical results in the indicated groups (scale bar=20 μm); C: relative mRNA levels of Anp and Myh7 in the indicated groups. Mean±SD. n=3. **P<0.01 vs AdGFP PBS group; ##P<0.01 vs AdGFP Ang II group.
4 Rbp1显著激活TAK1-P38信号通路
Western blot结果显示,与对照组相比,敲减显著抑制了AngII诱导引起的TAK1和P38的活性升高(<0.01),而过表达则显著促进了TAK1和P38活性(<0.01),见图4。
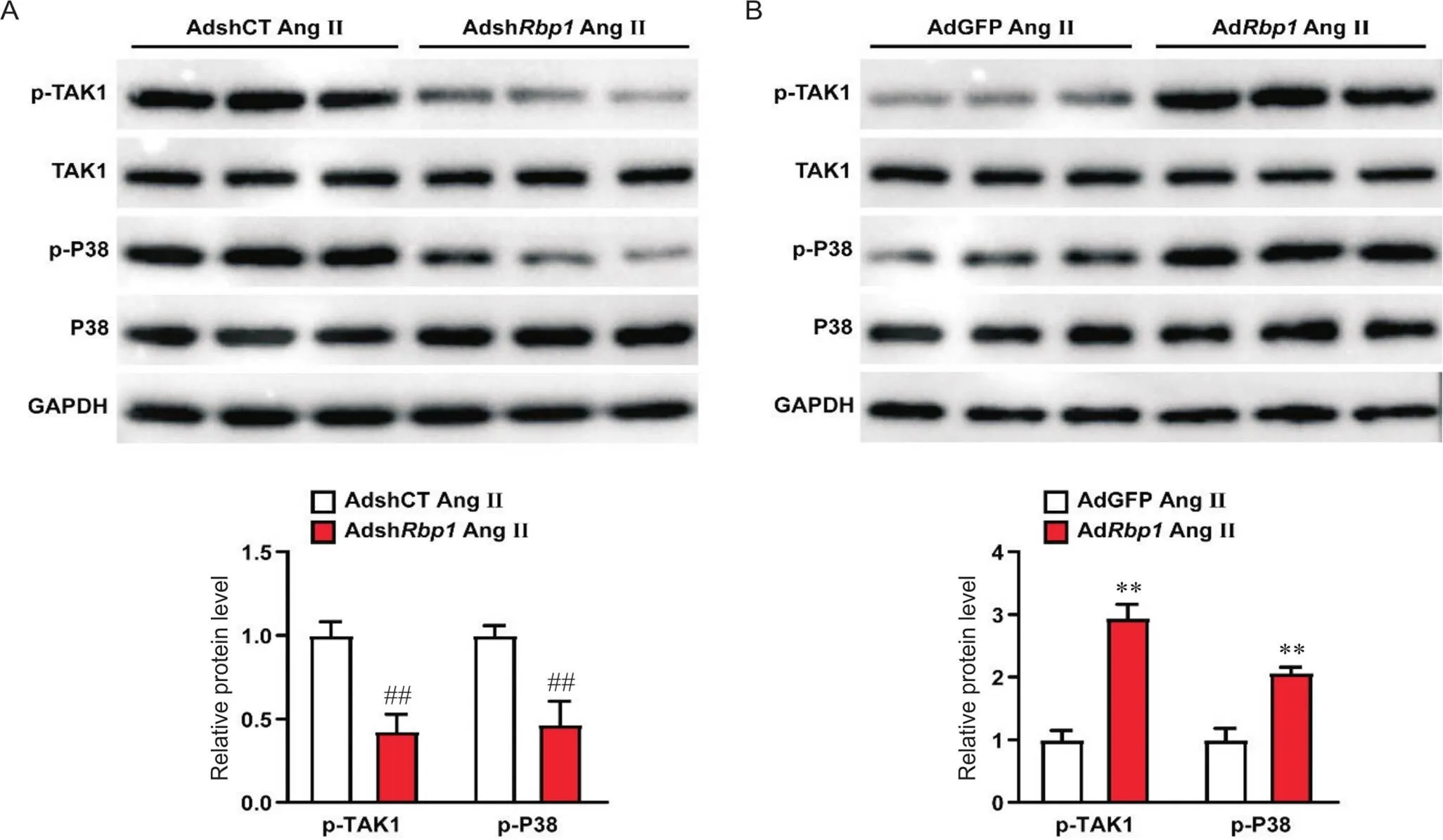
Figure 4. Rbp1 activated TAK1/P38 signaling pathway in primary neonatal rat cardiomyocytes. The protein levels of p-TAK1, TAK1, p-P38 and P38 were detected after Rbp1 knockdown (A) or overexpression (B). Mean±SD. n=3. ##P<0.01 vs AdshCT Ang II group; **P<0.01 vs AdGFP Ang II group.
5 TAK1抑制剂阻断Rbp1对大鼠乳鼠原代心肌细胞肥大的促进作用
Western blot检测、α-actinin免疫荧光染色和RT-qPCR检测结果显示,与对照组相比,NG52显著抑制TAK1和下游P38活性,见图5A;并且NG52显著抑制了过表达诱导的大鼠乳鼠原代心肌细胞表面积增加(<0.01),同时还显著下调肥大标志基因和的表达(<0.01),见图5B、C。

Figure 5. Inhibition of TAK1 activity blocked the promotional effect of Rbp1 overexpression on Ang II-induced primary neonatal rat cardiomyocytes hypertrophy. A: the protein levels of Rbp1, p-TAK1, TAK1, p-P38 and P38 in Ang II-stimulated AdRbp1 and AdGFP with TAK1 inhibitor (iTAK1) or DMSO as control (CT) in primary neonatal rat cardiomyocytes; B: representative immunofluorescence images of anti-α-actinin staining and its statistical results in the indicated groups (scale bar=20 μm); C: relative mRNA levels of Anp and Myh7 in the indicated groups. Mean±SD. n=3. **P<0.01 vs AdGFP CT Ang II group; ##P<0.01 vs AdRbp1 CT Ang II group.
讨论
据报道,细胞内的多种信号通路如MAPK、磷脂酰肌醇3-激酶/蛋白激酶B(phosphatidylinositol 3-kinase/protein kinase B, PI3K/AKT)、钙调磷酸酶(calcineurin)/活化T细胞核因子(nuclear factor of activated T-cells, NFAT)等参与心肌肥厚的调控[10-12]。这些信号通路在刺激信号的传导下,调控细胞核中的基因转录、线粒体功能、内质网应激和炎症应答等大量分子事件,进而调控心肌细胞中大量蛋白质合成和成纤维细胞表型转换[13-14]。Rbp是人体内视黄醇转运蛋白家族,包含6个家族成员,以往研究表明Rbp4通过激活Toll样受体4(toll-like receptor 4, TLR4)/髓样分化因子88(myeloid differentiation factor 88, MyD88)炎症通路可以促进胰岛素抵抗和心肌细胞肥大,血清中Rbp4的增加与高血压、动脉粥样硬化等心血管疾病有密切联系[15],提示Rbp家族可能参与心肌肥厚的发生发展。研究报道Rbp1影响细胞分化和肿瘤进展[16],但是Rbp1在心肌肥厚中的功能并不清楚。本研究通过生物信息学方法联合分析小鼠心肌肥厚数据库,观察在数据库中的表达显著升高,并在心肌肥厚小鼠心脏组织中得到验证。应用Ang II刺激大鼠乳鼠原代心肌细胞构建体外大鼠乳鼠原代心肌细胞肥大模型[17-18],并通过腺病毒在大鼠乳鼠原代心肌细胞中敲减或过表达后证实Rbp1能促进Ang II诱导的大鼠乳鼠原代心肌细胞肥大。
MAPK信号通路参与病理性心肌肥厚的发生发展,MAPK信号由MAP3K-MAP2K-MAPK级联组成,TAK1是MAP3K家族的成员,广泛参与细胞生命活动[19-20]。在刺激后,TAK1磷酸化并激活MAPKKs(MKK3/6、MKK4/7)和下游底物,包括P38、c-Jun氨基末端激酶(c-Jun N-terminal kinase, JNK)[21],而P38激活可以刺激下游肥大基因的表达,促进心肌细胞肥大[22-23]。为了探讨Rbp1是否通过影响MAPK通路进而调控大鼠乳鼠原代心肌细胞肥大,本研究检测TAK1和下游P38蛋白。结果显示,过表达显著激活TAK1以及下游P38。同时为了进一步验证Rbp1对大鼠乳鼠原代心肌细胞肥大的影响是否依赖于TAK1的激活,本研究使用TAK1的抑制剂NG52处理大鼠乳鼠原代心肌细胞,结果表明NG52可以逆转Ang II刺激下Rbp1对大鼠乳鼠原代心肌细胞肥大的影响,与此前TAK1及P38的相关报道一致[24-25]。本研究也存在不足,未在动物体内研究Rbp1对心肌肥厚的调控作用,同时也未探究Rbp1调控TAK1活性的具体机制,这些科学问题有待在以后的研究中深入探讨。
综上所述,本项工作揭示了Rbp1在Ang II诱导的大鼠乳鼠原代心肌细胞肥大模型中,通过激活MAPK信号通路中的TAK1和下游P38分子促进大鼠乳鼠原代心肌细胞肥大。
[1] McMurray JJ, Pfeffer MA. Heart failure[J]. Lancet, 2005, 365(9474):1877-1889.
[2] Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy[J]. Nat Rev Cardiol, 2018, 15(7):387-407.
[3] Kawaguchi R, Yu J, Honda J, et al. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A[J]. Science, 2007, 315(5813):820-825.
[4] Obrochta KM, Krois CR, Campos B, et al. Insulin regulates retinol dehydrogenase expression and all--retinoic acid biosynthesis through FoxO1[J]. J Biol Chem, 2015, 290(11):7259-7268.
[5] Ferlosio A, Doldo E, Agostinelli S, et al. Cellular retinol binding protein 1 transfection reduces proliferation and AKT-related gene expression in H460 non-small lung cancer cells[J]. Mol Biol Rep, 2020, 47(9):6879-6886.
[6] Choi H, Lee H, Kim TH, et al. G0/G1 switch gene 2 has a critical role in adipocyte differentiation[J]. Cell Death Differ, 2014, 21(7):1071-1080.
[7] Xie J, Zhou X, Hu X, et al. H2O2evokes injury of cardiomyocytes through upregulating HMGB1[J]. Hellenic J Cardiol, 2014, 55(2):101-106.
[8] Wang J, Lu L, Chen S, et al. PERK overexpression-mediated Nrf2/HO-1 pathway alleviates hypoxia/reoxyge-nation-induced injury in neonatal murine cardiomyocytes via improving endoplasmic reticulum stress[J]. Biomed Res Int, 2020, 2020:6458060.
[9] Diedrichs H, Chi M, Boelck B, et al. Increased regulatory activity of the calcineurin/NFAT pathway in human heart failure[J]. Eur J Heart Fail, 2004, 6(1):3-9.
[10] Chen C, Zou LX, Lin QY, et al. Resveratrol as a new inhibitor of immunoproteasome prevents PTEN degradation and attenuates cardiac hypertrophy after pressure overload[J]. Redox Biol, 2019, 20:390-401.
[11] Javadov S, Jang S, Agostini B. Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: therapeutic perspectives[J]. Pharmacol Ther, 2014, 144(2):202-225.
[12] Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways[J]. Nat Rev Mol Cell Biol, 2006, 7(8):589-600.
[13] Gibb AA, Hill BG. Metabolic coordination of physiological and pathological cardiac remodeling[J]. Circ Res, 2018, 123(1):107-128.
[14] Bisserier M, Berthouze-Duquesnes M, Breckler M, et al. Carabin protects against cardiac hypertrophy by blocking calcineurin, Ras, and Ca2+/calmodulin-dependent protein kinase II signaling[J]. Circulation, 2015, 131(4):390-400.
[15] Gao W, Wang H, Zhang L, et al. Retinol-binding protein 4 induces cardiomyocyte hypertrophy by activating TLR4/MyD88 pathway[J]. Endocrinology, 2016, 157(6):2282-2293.
[16] Melis M, Tang XH, Trasino SE, et al. Effects of AM80 compared to AC261066 in a high fat diet mouse model of liver disease[J]. PLoS One, 2019, 14(1):e0211071.
[17] Zhou X, Sun F, Luo S, et al. Let-7a is an antihypertrophic regulator in the heart via targeting calmodulin[J]. Int J Biol Sci, 2017, 13(1):22-31.
[18] Zhang Y, Bloem LJ, Yu L, et al. Protein kinase C beta II activation induces angiotensin converting enzyme expre-ssion in neonatal rat cardiomyocytes[J]. Cardiovasc Res, 2003, 57(1):139-146.
[19] Jadrich JL, O'Connor MB, Coucouvanis E. The TGFβ activated kinase TAK1 regulates vascular development[J]. Development, 2006, 133(8):1529-1541.
[20] Li L, Chen Y, Doan J, et al. Transforming growth factor β-activated kinase 1 signaling pathway critically regulates myocardial survival and remodeling[J]. Circulation, 2014, 130(24):2162-2172.
[21] Sato S, Sanjo H, Takeda K, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses[J]. Nat Immunol, 2005, 6(11):1087-1095.
[22] Wang S, Luo M, Zhang Z, et al. Zinc deficiency exacerbates while zinc supplement attenuates cardiac hypertrophy in high-fat diet-induced obese mice through modulating P38 MAPK-dependent signaling[J]. Toxicol Lett, 2016, 258:134-146.
[23] 彭波辉, 彭昌, 黄丽欣, 等. P38 MAPK信号通路在漆树酸改善苯肾上腺素诱导的小鼠心肌细胞肥大中的作用[J]. 中国病理生理杂志, 2020, 36(2):200-205.
Peng B, Peng C, Huang L, et al. Effects of P38 MAPK signaling pathway on anacardic acid attenuating mouse cardiomyocyte hypertrophy induced by phenylephrine[J]. Chin J Pathophysiol, 2020, 36(2):200-205.
[24] Li J, Yan C, Wang Y, et al. GCN5-mediated regulation of pathological cardiac hypertrophy via activation of the TAK1-JNK/P38 signaling pathway[J]. Cell Death Dis, 2022, 13(4):421.
[25] Zhao J, Jiang X, Liu J, et al. Dual-specificity phosphatase 26 protects against cardiac hypertrophy through TAK1[J]. J Am Heart Assoc, 2021, 10(4):e014311.
Retinol binding protein 1 promotes hypertrophy of primary neonatal rat cardiomyocytes via activating TAK1
XIE Jing, ZHOU Yanli, CHEN Sisi, WANG Jichun, JIANG Hong△
(,,,,,430000,)
To investigate of the function and mechanism of retinol binding protein 1 (Rbp1) in the hypertrophy of primary neonatal rat cardiomyocytes.The microarray data from Gene Expression Omnibus (GEO) database was analyzed to identify the key regulatory factors in the process of cardiac hypertrophy. In order to explore the function of the key gene in the hypertrophy induced by angiotensin II (Ang II), overexpression and knockdown adenoviruses were constructed to infect primary neonatal rat cardiomyocytes. Western blot and RT-qPCR were used to explore the mechanism of the key gene regulating hypertrophy of primary neonatal rat cardiomyocytes.The expression ofwas significantly up-regulated (<0.01) in the heart of mouse cardiac hypertrophy models. Moreover,experimentsshowed that compared with control group, the area of primary neonatal rat cardiomyocytes and the expression of cardiac hypertrophy marker gene atrial natriuretic peptide () and myosin heavy chain 7 () was significantly increased in Rbp1 overexpression group, indicating that overexpression of Rbp1 promotes Ang II-induced primary neonatal rat cardiomyocytes hypertrophy. On the contrary, knockdown ofdramatically inhibited the increase in primary neonatal rat cardiomyocyte area and the expression of cardiac hypertrophy markers induced by Ang II. Overexpression ofactivated transforming growth factor-β-activated kinase 1 (TAK1) and its downstream target P38 (<0.01). Treatment with TAK1 inhibitor blocked the effect ofoverexpression on Ang II-induced primary neonatal rat cardiomyocyte hypertrophy.The Rbp1 promotes Ang II-induced primary neonatal rat cardiomyocyte hypertrophy by activating the TAK1/P38 signaling pathway.
retinol binding protein 1; primary neonatal rat cardiomyocytes; cardiomyocyte hypertrophy; TAK1/P38 signaling pathway
R541.6; R363.2
A
10.3969/j.issn.1000-4718.2023.02.005
1000-4718(2023)02-0233-08
2022-09-02
2022-12-19
[基金项目]中央高校基本科研业务费专项资金青年教师资助项目(No. 2042019kf0093)
Tel: 027-88041911; E-mail: hongj0505@126.com
(责任编辑:宋延君,李淑媛)
