Phosphorus and nitrogen co-doped graphene for catalytic dehydrochlorination of 1,2-dichloroethane
2023-02-28HaonanYuXiaofengYangHongbinYangJinmingXuYanqiangHuang
Haonan Yu, Xiaofeng Yang, Hongbin Yang, Jinming Xu,, Yanqiang Huang,
1 CAS Key Laboratory of Science and Technology on Applied Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China 2 University of Chinese Academy of Sciences, Beijing 100049, China
Keywords:Catalysis Nanomaterials Fixed-bed Dehydrochlorination Doped graphene
ABSTRACT A phosphorus and nitrogen co-doped graphene (PNG) was developed via a two-step pyrolysis approach through the intermedium of g-C3N4 template and glyphosate as the phosphorus source,and was used for the catalytic dehydrochlorination of 1,2-dichloroethane(EDC)to vinyl chloride monomer(VCM)production.The characterization results indicate that a volcano relationship of surface area and surface properties with the usage of phosphorus precursor was observed, and the sample of PNG-900-6 possesses not only the thin film structure with enhanced surface area but also the smaller grain size of PNG attachments.Accordingly, such PNGs show a great improvement of catalytic performance in the dehydrochlorination of EDC,and the PNG-900-6 catalyst behaves the best with a 4-times higher activity than that on the nitrogen doped graphene (NG).It was also proved that the synergetic effect of the unique P-C coordination on the graphene to generate more quaternary nitrogen species was crucial in determining the catalytic performance of EDC conversion.Our results demonstrate that the phosphorus and nitrogen co-doped graphene offers many advantages in physical structure and chemical property, and are also great potential on the catalytic application in the dehydrochlorination of 1,2-dichloroethane.
1.Introduction
Vinyl chloride monomer (VCM) is one of the most important bulk chemicals which serves as a homo-polymer to produce thermoplastic polyvinyl chloride (PVC) polymers.It is manufactured by either the dehydrochlorination of 1,2-dichloroethane (ethylene dichloride, EDC) or the hydrochlorination of acetylene [1-4].Among them, the dehydrochlorination of EDC has been widely used in commercial applications owing to the convenient acquisition of EDC from petrochemical industry, and the direct thermopyrolysis of EDC is usually employed through an industrial cracking furnace at a reaction temperature as high as 500 °C.Although the conversion of EDC can be achieved approximately 50% with the selectivity of VCM as high as 95%-99% [3,5], it suffers from the severe coking and a serious waste of energy consumption due to its high operation temperature [6].Therefore, the development of catalytic dehydrochlorination instead of the conventional thermo-pyrolysis of EDC is highly desirable at a lower conversion temperature, and it has also attracted great recent interests [7].
Previous studies have demonstrated that basic zeolites [8],metal oxides[9-12],and carbon materials[13-17]offer a high catalytic activity of dehydrochlorination.However, considering the corrosiveness of the by-product of HCl, carbon materials should be the best candidate for the catalytic dehydrochlorination of EDC.It has been proved that the catalytic performances of carbon materials are governed not only by their pore structures but also by their surface functionalities [18-23].Particularly, the nitrogen species covalently bonding into the carbon framework could import basic functional groups(e.g.,pyridine-like sites)on the surface, which thus greatly improve their activity in EDC conversion[24].For example, Mochida and Sotowa et al.[4] reported that polyacrylonitrile-based active carbon fiber and pyridine deposited pitch-based active carbon fiber showed excellent catalytic activity and stability for dehydrochlorination of EDC, with a reaction temperature at 360°C.More recently,we have also demonstrated that the mesopores in nitrogen-doped ordered mesoporous carbons facilitated the mass transportation and greatly improved the stability of catalysts, and both the quaternary nitrogen species and pyridinic nitrogen species are confirmed to serve as catalytic active sites during reaction [15,25].As a result, the nitrogen-doped carbon provides an effective method to improve the catalytic performances of EDC dehydrochlorination.
Nevertheless,it has been established that the dehydrochlorination of EDC can also be promoted by an acid-catalyzed mechanism.As such, the incorporation of acidic functional groups into the nitrogen-doped carbon was considered in EDC dehydrochlorination.In this regard, the co-doped acid heteroatoms such as N, P,O,S,or B with the basic nitrogen-carbon catalysts has been widely explored,and was proved to exhibit unusual catalytic behaviors in various reactions[14,17,18].Moreover,the N,B-co-doped AC(activated carbon)was found to deliver excellent catalytic performance,and the pyridinic-N with the internal B3N3was suggested to play significant roles in the catalysis of EDC dehydrochlorination [26].On the other hand,the phosphorus element is the other most popular co-doped element, and the covalence of P elements into the graphene framework was always suggested to be concerned with its excellent catalytic performance [27], while the graphene was generally suffering from a great decrease of surface area after the co-doping of heteroatoms such as phosphorus.Thereby,the development of an effective synthesis method for the phosphorus and nitrogen co-doped graphene is highly required for the improved activity toward EDC dehydrochlorination.
In this research, we have explored the idea of synthesis of N,Pco-doped carbon catalysts for the dehydrochlorination of EDC and enhancing catalytic activity through a synergistic catalysis.A phosphorus and nitrogen co-doped graphene(PNG)was developed via a two-step pyrolysis approach through the intermedium of g-C3N4template and glyphosate as the phosphorus source.A series of phosphorus and nitrogen co-doped graphenes (PNGs) were prepared by pyrolyzing the precursor of melamine and glyphosate.It was found that the obtained PNGs showed remarkable activities in the catalytic dehydrochlorination of EDC.The conversion of EDC shows a volcano relationship along with the glyphosate content increasing and the selectivity of VCM was all above 99%.It was confirmed that the synergetic effect of phosphorus with an increased quaternary nitrogen species was crucial in determining the catalytic performance of EDC conversion.
2.Experimental
2.1.Chemicals
Melamine (AR), L-alanine (AR), hydrochloric acid (37%), and ethanol (AR) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.Glyphosate (AR) was bought from Shanghai Macklin Biochemical Technology Co., Ltd.All chemicals were used as received without further purification.Deionized water from Millipore Q water purification system was used in all experiments.
2.2.Synthesis of PNGs
In a typical synthesis,12.00 g of melamine and 6.00 g of glyphosate were mixed by ball milling for 4 h in a ZrO2tank to produce a homogeneous composite.Subsequently, 15 ml of hydrochloric acid-ethanol solution (volume ratio, 1:5) was added and mixed evenly in a mortar.After being dried in an oven at 60 °C for 8 h,the mixture was ball milled once again for 2 h.Then,the white fine powder was pyrolyzed at 600 °C for 1.5 h and 900 °C for 2 h in a quartz tube under argon atmosphere.The heating rate was 2.5 °C·min-1below 600 °C and increased to 2 °C·min-1above 600 °C.After cooling to room temperature, a black product was obtained,and named as PNG-900-6.A series of samples were synthesized by this method.They were denoted as PNG-900-X,where X represents the amount of glyphosate.
For comparison, the reference sample (nitrogen doped graphene,NG)was prepared via the same route just replacing glyphosate with the same amount of L-alanine.
2.3.Characterization
Scanning electron microscope (SEM, Japan Electronics Co., Ltd.,JSM-7800F) was selected to observe the surface topography of the carbon material.The elemental composition and chemical state of the samples were analyzed by X-ray photoelectron spectroscopy(XPS, Escalab 250 Xi+, Thermo Fisher Scientific, USA) using a monochromatic aluminum Kα beam (1486.6 eV).The binding energy was calibrated with reference to the C 1s peak (284.8 eV).Nitrogen sorption analysis was performed on a Quadrasorb EVO apparatus (Quantachrome Instruments, USA) of at -196 °C.Prior to the measurements, the samples were degassed at 300 °C for about 4 h.The Brunauer-Emmett-Teller (BET) method was used to calculate the specific surface areas.The pore size distributions(PSD) were calculated from the adsorption branches of the isotherms using the Barrett-Joyner-Halenda (BJH) method.Raman was used to analyze the degree of graphitization before and after phosphorus and nitrogen doping of the samples(Raman,Renishaw and JPK, Nano Wizard, UK).Thermogravimetric analysis was used to analyze the pyrolysis process of catalysts under N2atmosphere(TG, NETZSCH (Shanghai) Machinery and Instruments Co., Ltd.,STA449F5-Thermostar).
2.4.Catalyst evaluation
Dehydrochlorination of EDC was carried out with a fixed bed reactor made of a quartz tube.Before reaction, 0.05 g of catalyst was packed in the quartz tube, and the reactor was purged with nitrogen to remove water and air in the reaction system.The EDC vapor was fed into the reactor by flowing nitrogen into an EDC saturator at 5°C,and the flow rate of nitrogen was 5 ml·min-1.When the temperature of reactor reached 250°C,the EDC gas was fed into the reactor.During our activity evaluation, the catalysts were tested on the time of stream for more than 4 h and the conversion of EDC was maintained at a nearly constant value to acquire a stable value over time.The reactant and products were analyzed by a gas chromatograph (Agilent Technologies 8890 ,USA) with flame-ionization detector (FID).
3.Results and Discussion
3.1.Synthesis and textural features of PNGs
It is well-known that the layered carbon nitride(g-C3N4)can be conveniently synthesized by the polymerization of melamine at 600 °C while the as-obtained g-C3N4can be decomposed completely above 750°C,which thus provides us the chance to develop a graphene-like carbon material with hierarchical pore structure[27-32].Accordingly, the preparation of PNGs in our research was achieved by the pyrolysis of the mixture of melamine and glyphosate in a two-step heating process.Briefly,the template g-C3N4from melamine polymerization inserted with the N-P-C hybrid deriving from the co-pyrolysis of melamine and glyphosate was first synthesized at a relative low temperature (600 °C), and the graphene-like PNG sheets with pore formation was then obtained after the decomposition of the g-C3N4template in a temperature of 900°C.Indeed,a primary mass loss was observed in a temperature range of 750-900°C by the thermogravimetric analysis of the pyrolysis process, while it keeps nearly constant after 900 °C(Fig.S1 in Supplementary Material).The phosphorous content and textual structure of PNGs can then be regulated by changing the amount of glyphosate, with a varied mass ratio of melamine and glyphosate of 12: 2, 12: 4, 12: 6 and 12: 8, and the samples were denoted as PNG-900-2, PNG-900-4, PNG-900-6 and PNG-900-8,respectively.For comparison,reference sample NG was also prepared via the same route by replacing of glyphosate as the L-alanine, which was referred as the NG-900-6 sample.
As shown in Fig.1, a particle morphology comprised of the thin film structure can be clearly observed for PNGs and NG from the scanning electron microscopy (SEM).However, such thin film varied with the phosphorous usage in the preparation process,and the samples of PNG-900-2 and PNG-900-6 show a more distinctive thin film structure compared with PNG-900-4, PNG-900-8 and NG-900-6.Moreover, the average grain size of PNGs was found to become smaller as the increased usage of glyphosate.As a comparison, the PNG-900-6 sample (Fig.1(g) and 1 (h)) features of not only the thin film structure but also the smaller grain size, indicative of a unique texture and properties of this sample.
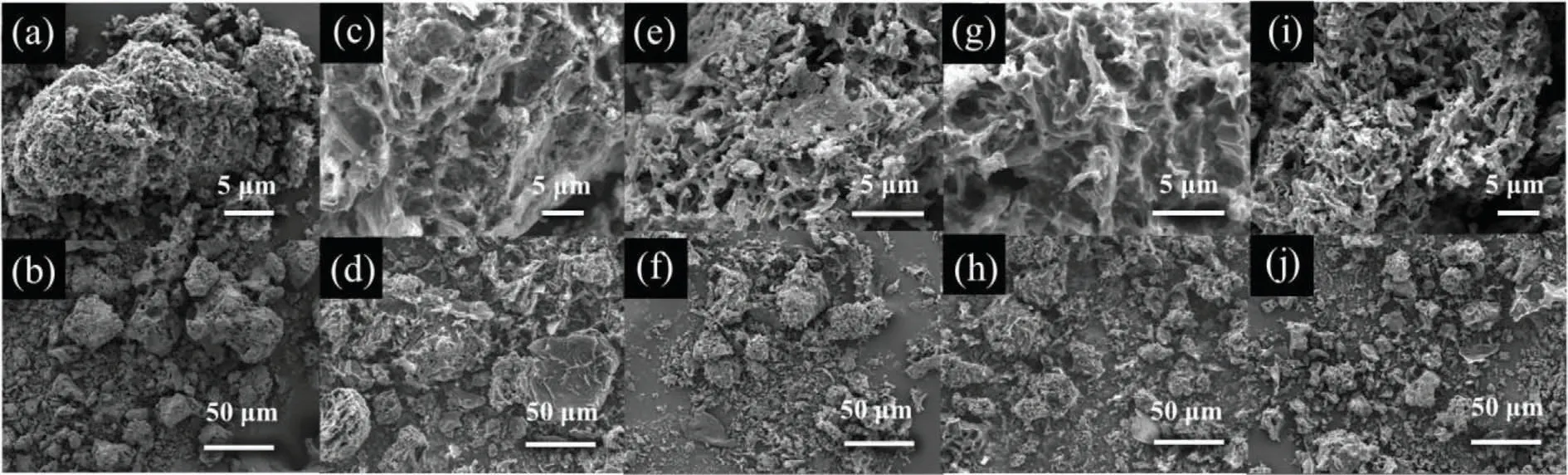
Fig.1.SEM images of PNGs and NG.(a), (b) NG-900-6, (c), (d) PNG-900-2, (e), (f) PNG-900-4, (g), (h) PNG-900-6, (i), (j) PNG-900-8.
The N2sorption isotherms and pore size distributions of PNGs and NG are shown in Fig.2, with the corresponding textural parameters listed in Table 1.The N2adsorption and desorption isotherms of PNGs and NG possess the type IV curve and the H4-type hysteresis loop, indicating that there are irregular mesoporous pores and slit pores in the samples.In terms of pore volume versus pore size, PNGs had an average pore size of ~3.4 nm, which was mainly comprised by the micropores (0-2 nm) and mesopores(3-8 nm), while the pores in NG-900-6 are the only mesoporous pores with average pore size of about 17.3 nm.As a result,the pore volumes (total pore volume and micropore volume) of PNGs are much larger than that of NG-900-6.One possible explanation for this might be that the size of the phosphorus containing function groups embedded in the graphene layer are larger than that of nitrogen containing function groups,and they can enlarge the distance between the graphene layers and generate more micropores in the carbon materials due to the decomposition of the g-C3N4template during the second stage of pyrolysis.Correspondingly,the specific surface area of PNG has a sharp increase,with a nearly 10-fold increase as compared with the NG sample.Interestingly,the surface area of PNG-900-6 sample was determined to be 1129 m2·g-1, which was also larger than that of PNG-900-2,PNG-900-4 and PNG-900-8 samples and showed a volcano relationship with the amount of glyphosate.This can be explained as that the glyphosate to co-pyrolysis with melamine was the main source for the N,P-co-doped framework of carbon material, while the excessive glyphosate makes the less availability of template g-C3N4to generate graphene-like pore structure in PNG-900-8(Fig.1(i) and 1(j)).
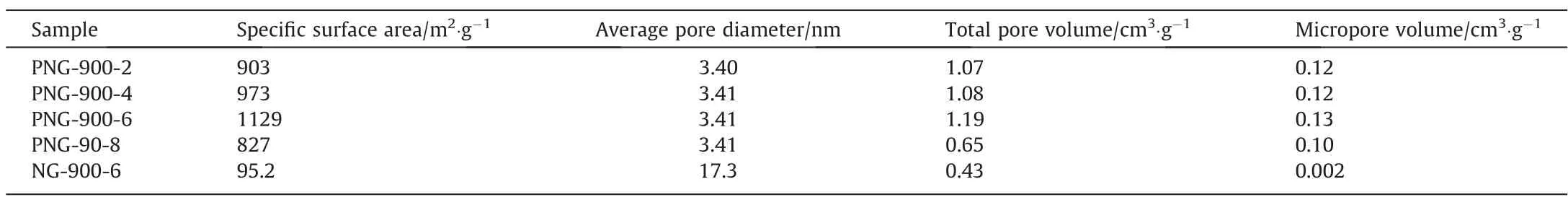
Table 1 Textual parameters of PNGs and NG
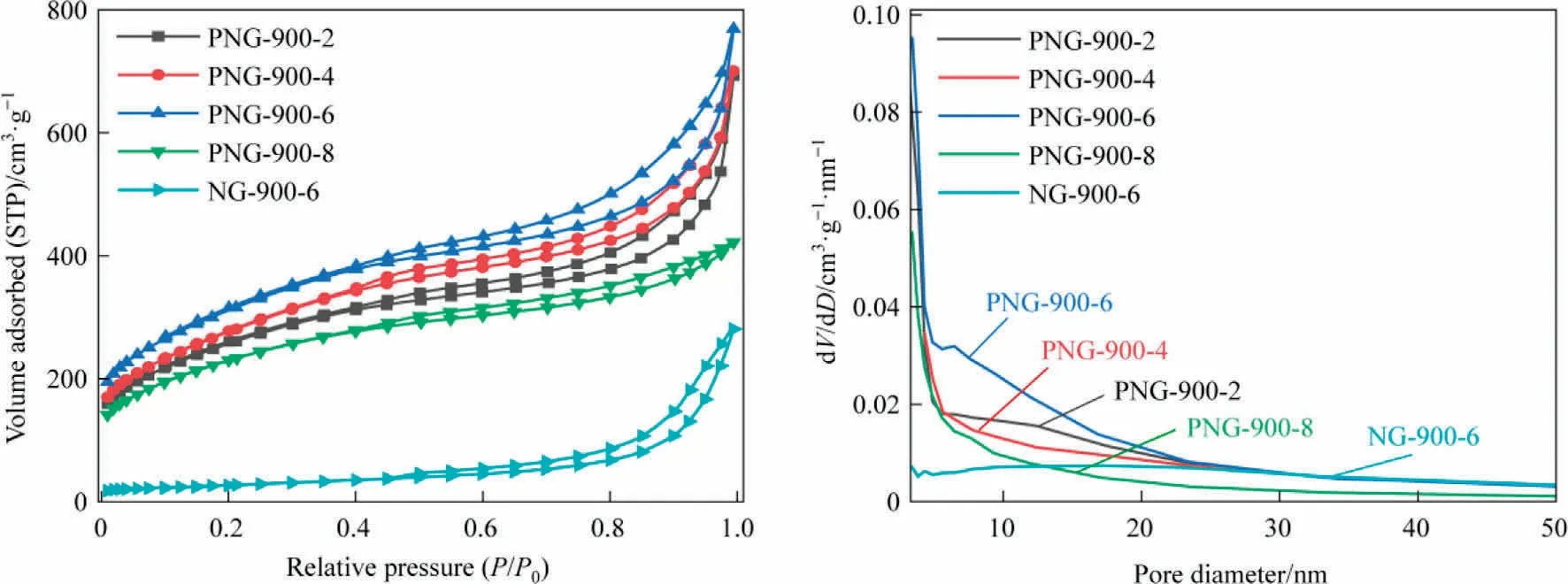
Fig.2.N2 sorption isotherms (a) and Pore size distributions (b) of PNGs and NG.
The carbon defects in the N,P-co-doped framework of PNGs was then explored by the Raman spectrum analysis, in terms of the characteristic peaks for different chemical states of carbon atoms in graphene-like materials.As seen in Fig.3, the so-called D and G bands were observed at 1350 and 1580 cm-1, which were ascribed to the disordered carbon and the sp2hybridized graphitic carbon, respectively.Therefore, the ratio of ID/IGrepresents the degree of graphitization in carbon materials.As compared, the ID/IGof the PNGs samples shows a comparable value of ~0.93,indicative of almost the same degree of graphitization for all the PNGs,while the NG sample has a lower graphitization with a value of ~1.03 for ID/IG.It suggests that the co-doped N,P in our developed PNGs would promote the graphitization of carbon material,in accordance with the SEM observations of the larger thin film structure for PNGs.
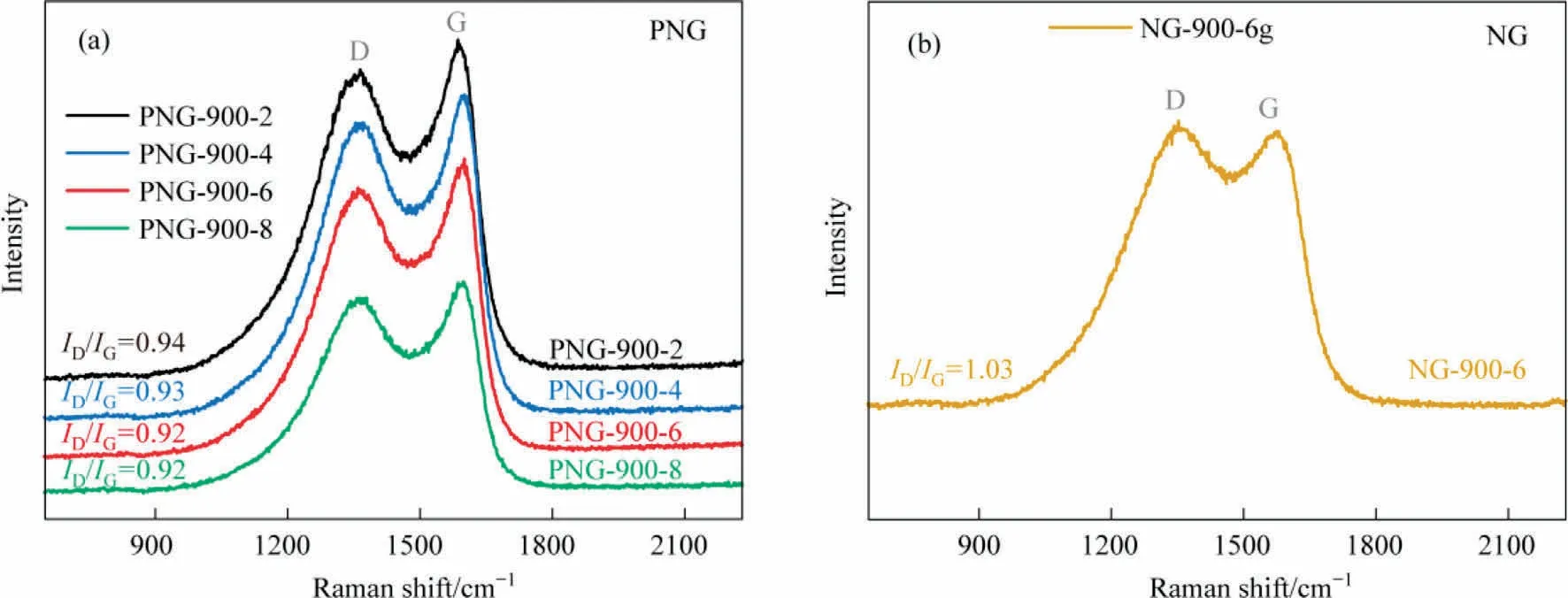
Fig.3.Raman spectra of (a) PNGs and (b) NG.
3.2.Catalytic dehydrochlorination of 1,2-dichloroethane
The as-prepared PNG catalysts was then tested in the catalytic dehydrochlorination of 1,2-dichloroethane for VCM production,under a reaction temperature of 250 °C at the atmosphere condition, with the final results summarized in Table 2 and schematically shown in Fig.4.As we can see, all the catalysts showed an excellent selectivity by maintaining a nearly complete VCM selectivity (>99%), while the catalytic activities varied with different PNGs and showed an improved activity toward EDC dehydrochlorination as compared with the NG-900-6 sample.Alike to the observations in the texture of PNGs, the volcano relationship was also observed in the catalytic performance of PNGs, and the PNG-900-6 catalyst exhibited the highest activity, with a conversion of 34% in the EDC dehydrochlorination even for a reaction time exceeding 300 h (Fig.S2).
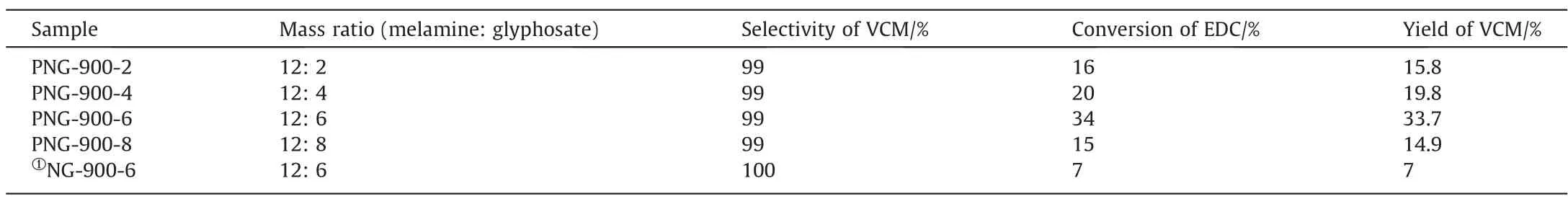
Table 2 EDC conversion, VCM selectivity, and yield of PNGs and NG
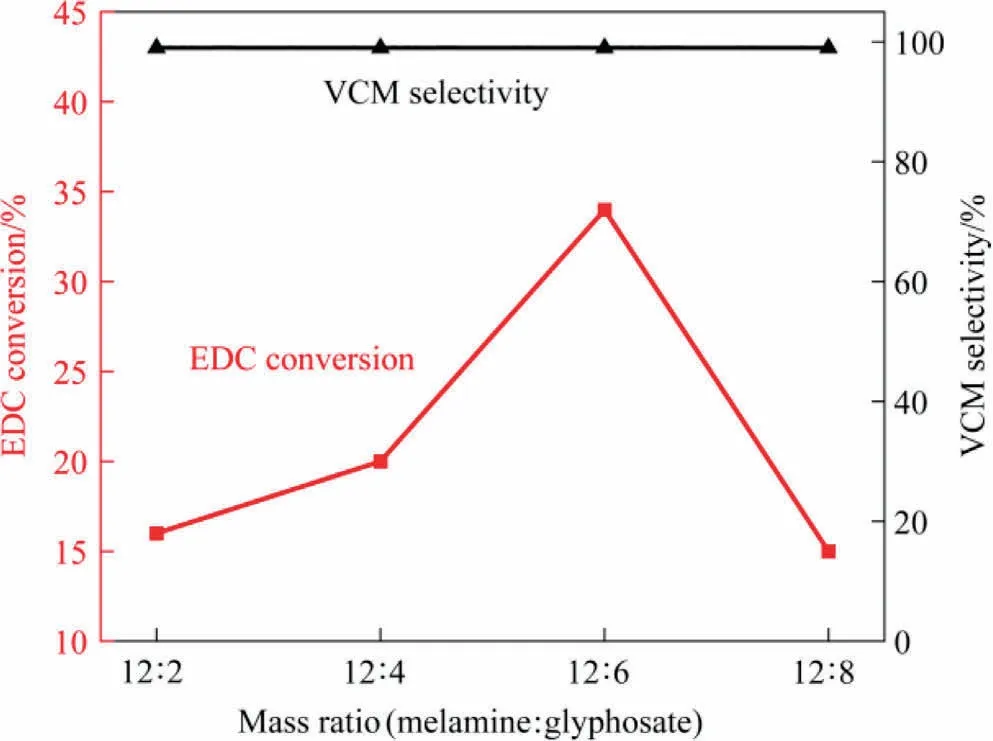
Fig.4.EDC conversion and VCM selectivity of PNGs.
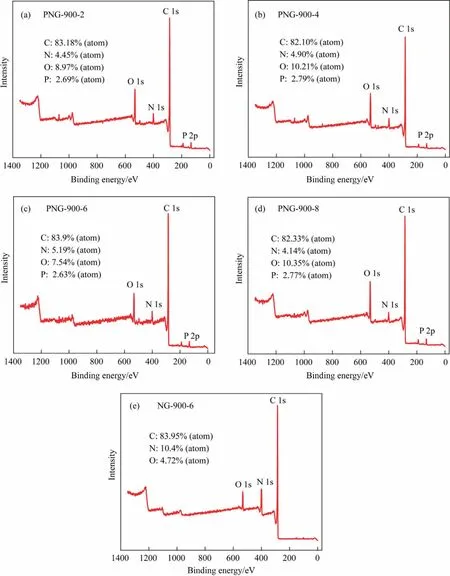
Fig.5.XPS survey spectra of (a) PNG-900-2, (b) PNG-900-4, (c) PNG-900-6, (d) PNG-900-8, (e) NG-900-6.
To understand the unusual catalytic behavior of the PNG-900-6 catalyst in EDC dehydrochlorination, the chemical compositions and elemental states of the co-doped graphene, was then performed for the PNGs and NGs samples with XPS analysis (Figs.5 and 6).It was found that a characteristic peak at 133 eV which was attributed to the 2p state of phosphorus, can be observed in all the PNGs, demonstrating the successful introduction of the phosphorus species into the nitrogen-carbon materials.However,even though a varied feeding of phosphorus precursor was performed for different PNGs during the preparation process, the residual P for all the PNGs was nearly the same with an atomic percentage of ~2.7 at%, it suggests the possible formation of the only N,P co-doped carbon material with a more specific composition.Meanwhile,the nitrogen content in PNGs has a significant decrease as compared with that of NG.It means that the decomposition of the g-C3N4template in the second stage of pyrolysis process.On the other hand,the presence of P also makes an improved content of O element in the PNGs,suggesting the possible formation of P-O species for PNG.As a compared,a relative higher ratio of P:O was found for the PNG-900-6 sample, which suggests that a varied coordination of P might be involved for the synthesis conditions of PNG-900-6 sample(Table 3).Indeed,by quantitatively analyzing the P peak of PNG samples,a significant peak derived from P-C species can be distinguished at 132.5 eV, which indicated that a large amount of P is anchored in the carbon framework by bonding with C after the co-pyrolysis of melamine and glyphosate during the preparation of PNG-900-6 (Fig.6(a)-(e)).
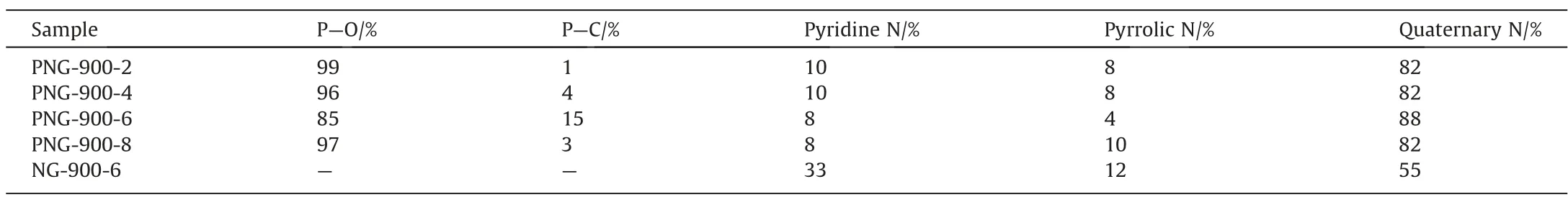
Table 3 Percentage content of P species and N species

Fig.6.High-resolution P 2p XPS spectra of(a)PNG-900-2,(b)PNG-900-4,(c)PNG-900-6,and(d)PNG-900-8.High-resolution N 1s XPS spectra of spectra of,(e)PNG-900-2,(f) PNG-900-4, (g) PNG-900-6, (h) PNG-900-8, and (i) NG-900-6.
According to the previous reports,pyridine can react with chlorinated hydrocarbons to generate the quaternary nitrogen species[15,25].It is believed that such newly formed quaternary nitrogen species together with the original quaternary nitrogen in the carbon-based catalysts are concerned with the catalytic dehydrochlorination.Accordingly, the active sites of nitrogen species in the PNGs was then determined by the decomposition of N peak in XPS according to the possible forms of N species, including pyridine nitrogen, pyrrolic nitrogen, and quaternary nitrogen(Fig.6(e)-(i)).It demonstrates that various nitrogen species are containing in the PNGs and NGs samples, among which the nitrogen-positive species represented by pyridine nitrogen and quaternary nitrogen account for the majority and play the primary role of basic sites (Table 3).Apparently, the PNG-900-6 sample possesses a higher fraction of the quaternary nitrogen species,implying that it behaves a much higher activity in EDC dehydrochlorination than other catalysts.
From our results,it is believed that the participation of the pyridine nitrogen together with the quaternary nitrogen governs the catalytic performance in EDC dehydrochlorination, and the codoped phosphorus would greatly improve the quaternary nitrogen in the PNGs through the coordination of P-C into the carbon framework.Moreover,the PNG-900-6 sample with the optimized ratio of melamine and glyphosate for co-pyrolysis in the preparation process, makes the most availability of quaternary nitrogen in carbon surface with a promoted surface area,and thus leads to an increase of the activity for EDC dehydrochlorination.
4.Conclusions
In this work, a phosphorus and nitrogen co-doped graphene(PNG) was developed via a two-step pyrolysis approach through the intermedium of g-C3N4template and glyphosate as the phosphorus source.A optimized ratio of melamine and glyphosate for co-pyrolysis in the preparation process was obtained,and the sample of PNG-900-6 possesses not only the thin film structure with enhanced surface area but also the smaller grain size of PNG attachments.As a result, a volcano relationship of physical structure and catalytic performance in the dehydrochlorination of EDC with the usage of phosphorus precursor was observed.The synergetic effect of the unique P-C coordination on the graphene to produce more quaternary nitrogen species was suggested to contribute its promoted catalytic performance.Our results demonstrate that the co-doped of phosphorus would be an effective method to regulate the reactivity of N-graphene,and they are also great potential on the catalytic application in the dehydrochlorination of 1,2-dichloroethane.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is financially supported by the Chinese Academy of Sciences Project for Young Scientists in Basic Research, China(YSBR-022), the National Natural Science Foundation of China,China (21925803), and the Youth Innovation Promotion Association CAS, China.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.05.012.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Intrinsic kinetics of catalytic hydrogenation of 2-nitro-4-acetylamino anisole to 2-amino-4-acetylamino anisole over Raney nickel catalyst
- Experiments and model development of p-nitrochlorobenzene and naphthalene purification in a continuous tower melting crystallizer
- α-Synuclein: A fusion chaperone significantly boosting the enzymatic performance of PET hydrolase
- Influence of water vapor on the separation of volatile organic compound/nitrogen mixture by polydimethylsiloxane membrane
- Mass transfer mechanism and relationship of gas-liquid annular flow in a microfluidic cross-junction device
- Enhanced photocatalytic activity of methylene blue using heterojunction Ag@TiO2 nanocomposite: Mechanistic and optimization study
