Experiments and model development of p-nitrochlorobenzene and naphthalene purification in a continuous tower melting crystallizer
2023-02-28WenlongXiaoYonglinLiZhengmingYiShengYang2HeanLuo
Wenlong Xiao, Yonglin Li, Zhengming Yi,, Sheng Yang2,, He’an Luo
1 School of Chemical Engineering, Xiangtan University, Xiangtan 411105, China
2 School of Energy Science and Engineering, Central South University, Changsha 410083, China
3 College of Materials and Chemical Engineering, Hunan Institute of Engineering, Xiangtan 411105, China
Keywords:Crystallization Separation Mathematical modeling Crystallizer Purification
ABSTRACT With the increasing demand for high-purity products, the industrial application of melt crystallization technology has been highly concerned.In this study,the purification process of nitrochlorobenzene binary eutectic system (NBES) and naphthalene-benzothiophene solid solution system (NBSSS) in tower melting crystallizer is analyzed, and a mathematical model of crystallization process is established.The key parameters in terms of feed concentration,crystal bed height,reflux ratio and stirring speed efficiency on purification effects were discussed by the established model.The results show that the concentration of p-nitrochlorobenzene was purified from 90.85% to 99.99%, when the crystal bed height is 600 mm, the reflux ratio is 2.5, and the stirring speed is 12 r·min-1.The naphthalene concentration is purified from 95.89% to 99.99%, when the crystal bed height is 400 mm, the reflux ratio is 1.43, and the stirring speed is 16 r·min-1.The quality of the model is evaluated by the ARD(average relative deviation).The minimum ARD values of the NBES and NBSSS are 2.39%and 5.22%,respectively,indicating the model satisfactorily explains the purification process.
1.Introduction
There are many systems with isomers and similar molecular structures in the chemical production process.Due to the close boiling point of these substances, more theoretical plates and larger reflux ratio are required for separation in traditional distillation tower,which increases equipment investment and operating costs.Therefore, it is very important to develop an energy-saving and efficient separation technology.
Melt crystallization technology has shown great advantages including solvent free,with simple equipment and low energy consumption[1,2].Compared with the traditional distillation process,the energy consumption of melt crystallization is only 10%-30%[3].It has been widely concerned in the separation and purification of medicine,material intermediates and fine chemical products[4-6].Melt crystallization can be divided into layer crystallization,suspension melting crystallization, and zone smelting according to the different modes of operation.Layer melt crystallization is simple operation,simple device,but the efficiency is low.The crystallization method is suitable for low demand product purification[7].Suspension melting crystallization can be used for large-scale continuous production in industry.However, the crystal growth rate is slower [8].Zone smelting is mainly used in refining metallurgical materials [9].In order to achieve industrial application of this technology,the research of melt crystallizer has never stopped in recent years[10-12].Among them,some crystallizers have been used in industrial production, such as the Kureha crystal purifier(KCP) crystallizer [13], the Brodie crystallizer [14,15], the counter current cooling crystallization(CCCC) crystallizer [16], the Metallwerk Buchs (MWB) crystallizer [15,17], etc.Melt crystallization technique is still requires more in-depth inquiry due to complex mass transfer process and unclear solid-liquid flow state in tower crystallizer[18-20].The binary eutectic system,such as the mixed system of o-nitrochlorobenzene and p-nitrochlorobenzene,and the solid solution system, such as the mixed system of naphthalene and benzothiophene, are the most common types of twocomponent solid-liquid equilibrium relationship [21].The phase diagrams are shown in Fig.1 [22,23].
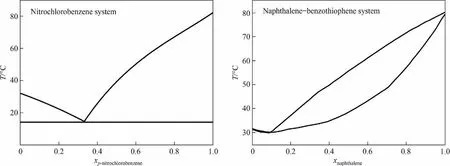
Fig.1.Solid-liquid phase diagram.
The separation of these systems by melt crystallization is rarely reported.The existing tower crystallizer has only some experimental studies and disregards mass transfer process in tower crystallizer [24,25].The annular countercurrent model was proposed by Arkenbout and Smit [26], in 1968, where countercurrent washing and recrystallization was considered.However, many parameters in the model are difficult to obtain directly by calculation, such as the melt flow rate and the recrystallization rate.Thus,the application of the equation is limited.In 1970,the axial diffusion model was developed, where the countercurrent washing mechanism was primarily considered in the purification section [27].The model does not consider the sweating effect and assumes that crystal composition is constant along axis.A packing bed washing model,which based on intermittent experimental data,was developed in 1981 [28].This model can only describe intermittent processes.In 2002,the mathematical model of the purification section of the inclined crystallization tower was proposed with sweating as the dominant mechanism of crystal separation [29,30].The tower crystallizer purification mechanism is a complex theory that includes mass transfer, heat transfer, and the phase equilibrium process [31,32].Column crystallizers are affected by both stirring and gravity, the purification mechanism must take into account additional factors such as melt countercurrent washing and the influence of axial diffusion and mass transfer.Therefore, it is necessary to establish a new model to better guide the crystallization operation process of different systems and describe the mass transfer process between solid and liquid more comprehensively.
In this work, the continuous flow purification process of tower crystallizer was investigated with the nitrochlorobenzene binary eutectic system (NBES) and naphthalene-benzothiophene solid solution system(NBSSS).A general mathematical model for continuous multistage countercurrent fractional crystallization process was established to describe the distribution of mass concentration in the tower crystallizer.The quality of the model is evaluated by the ARD (average relative deviation).The correlations of operational factors on mass transfer were considered.The experimental and calculated results are analyzed.The height of the crystal bed,the reflux ratio and the stirring speed are the key factors that determine the mass transfer rate between the phase interfaces in the crystallizer.After the optimization of the key parameters, the final mass fractions of p-nitrochlorobenzene and naphthalene were purified from 90.85% and 95.89% to over 99.99%, respectively.
2.Methods
2.1.Experimental procedure
The tower crystallizer is shown in the Fig.2.The crystallizer column used a Pyrex tube to direct observe the crystallizer bed.The internal diameter is 50 mm, and the total length is 1350 mm.A stirring motor is installed at the top of the tower to disseminate the crystal bed.A heating coil is installed at the bottom of the crystallizer to regulate the temperature of the tower kettle with hot water as the heat exchange medium.The experimental raw materials were fed from the hopper directly above the tower by a screw feeder to maintain the feed stability.The raw materials for the experiment are provided by Tianjin Industrial and Agricultural Chemical Plan.The important properties of the raw materials are shown in Table 1.
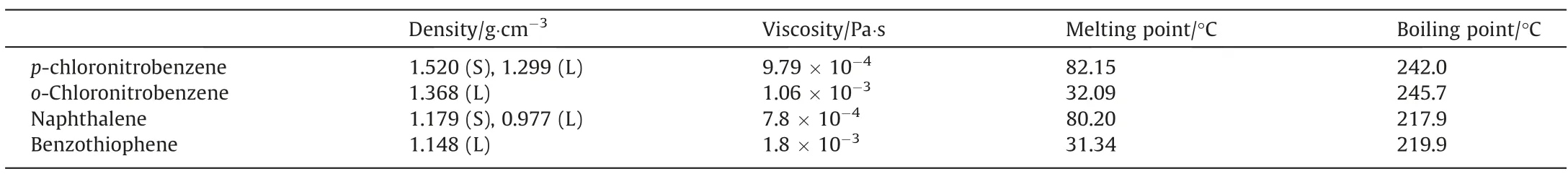
Table 1 Properties of the raw materials

Fig.2.The crystallization experiment equipment.
The melting crystallization experiment was a continuous process.The heating coil was run and kept at 60°C to preheat the column before the experiment began.The material was added from the feed port through the screw pusher.The temperature of the hot water and the stirring rate of the electromotor were controlled.Location away from the heating coil was lower temperature.The crystal was produced at the upper end of the crystallizer andmoved downward under gravity.A certain height of crystal bed suspended in the middle of the crystallizer.The lower valve was subsequently opened to make the product flow out.Impurities overflowed from the residual outlet at the top of the crystallizer.The procedures were stopped until the compositions of the products no longer changed.A syringe was inserted into the sampling port to collect melts.The sample was analyzed by flame ionization detector(FID)gas chromatograph(Varian,USA).The temperatures of the detector and injection port were 250°C.The column temperature was constant at 145°C.Experimental conditions were shown in Table 2.The sample purity for each experiment was added into the Supplementary Material.
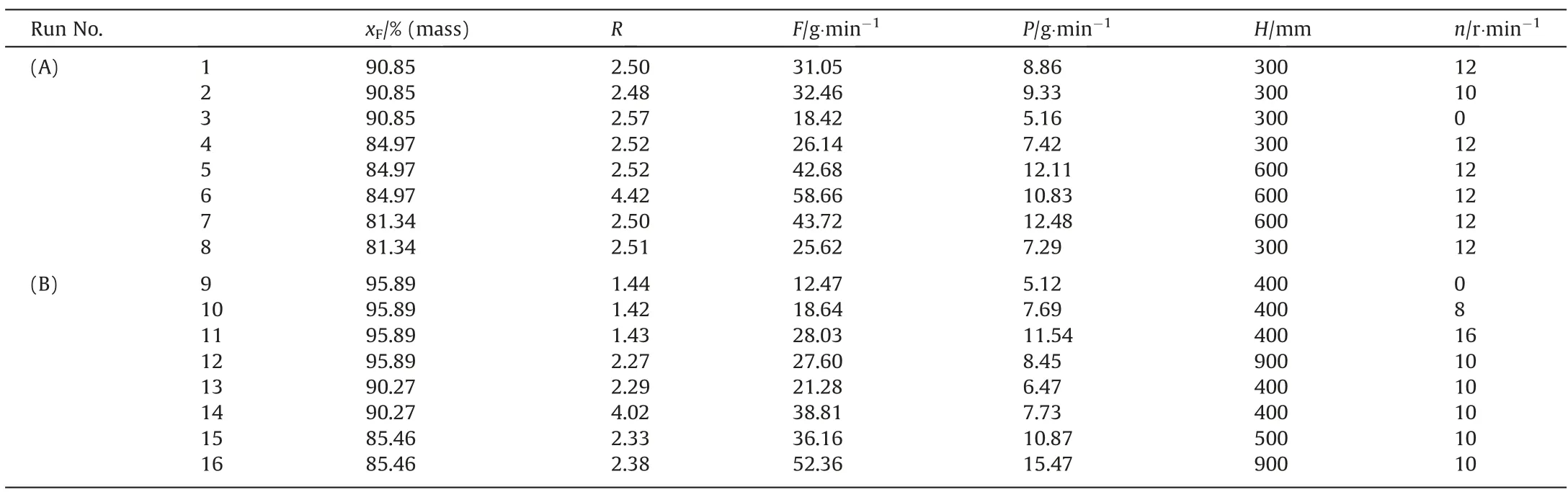
Table 2 Experimental condition parameter table
2.2.Analysis of purification process
Mass transfer process and solid-liquid flow state in tower crystallizer is systematic analyzed.Purification process of crystallizer in steady state is determined.As shown in Fig.3, the crystallizer is divided into three parts: the crystallization section, the crystal bed section and the melting section.The crystallization section is far away from the heater, the temperature is low.The crystals nucleate and grow on the wall.The solid has a higher density than the liquid.The crystals sink due to gravity and stack into a crystal bed in the crystal bed section.The continuously moving crystal bed enters the melting section.The crystal melts with the increase of temperature.Part of the melt is discharged as product.Another part of the melt is squeezed by the falling crystal and rises.The melts will pass through the pores of the crystal bed, which wash the crystal surface and remove the impurities.The rising melt enters the crystallization section and recrystallizes.The melt overflows from the residual outlet.Finally,a continuous stable process is formed.
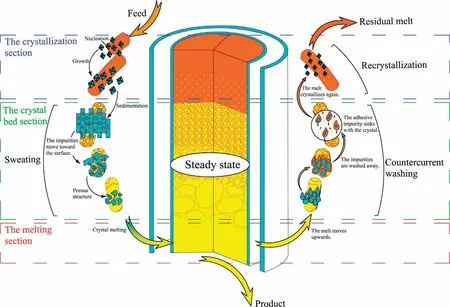
Fig.3.Crystal bed purification mechanism diagram.
2.3.Establishment of mathematical model of crystal bed
The purification mechanism of the crystal bed is shown in Fig.3.In the melting crystallization process, three purification mechanisms can be considered: recrystallization, sweating and countercurrent washing.The crystallizer purification process mainly occurs in the crystal bed section.Therefore, the recrystallization of the crystallization section generally ignored during the analysis of the purification model.The detailed analysis of crystal bed is necessary to study mass transfer process and solid-liquid flow state in tower crystallizer.Fig.4 summarizes the streams in the tower crystallizer.

Fig.4.Nomenclature of streams and their compositions.
Theoretical analysis of the crystal bed section determines the following types: the crystal stream (C), the melt stream (L), the adhesive liquid stream (L’).Where, the C moves downward from the top of the tower under gravity.The C is melted in the melting section to supplement the melt flow L.The L rises with the extrusion of the crystal bed and countercurrent washes the solid phase crystals,which increase the purity of crystal.The L’,two parts:the adhesive liquid flow and the coating liquid flow,moves downward along with C.The adhesive liquid flow is melts adhering to the crystal surface due to the uneven surface of the solid phase and the presence of pores.The coating liquid flow is the impurities that flow out from the inside of the crystal by sweating.
The mass balance of impurity components is
The mass balance over a volume element at z is given by the derivative of Eq.(1).
In which, Mdyis the axial molecular diffusion and can be expressed in a modified form of Fick’s law.
where φ is the suspension density of the crystal bed.z is the height of the purification section, so that the sampling port near the feed port z = 0.
The first term of Eq.(2)represents the change of the impurity in the attachment liquid L′with the height of the tower.Assuming that the density of the attachment liquid is equal to that of the melt, the mass transfer occurs directly between the crystal and the melt.It is expressed as follows:
By combining Eqs.(3) and (4) and substituting into Eq.(2), the following equation can be obtained.
The following assumption can be made
The final mathematical model can be reduced to
The solution of Eq.(6) is
The mass transfer coefficient (Kt) and the liquid-solid interfacial area per unit volume of the column(α)are essential to solve Eq.(7).Firstly,the mass transfer process of crystal bed is analyzed.Assuming that the crystal bed is sufficiently compact and the stirring effect is only to uniformly disperse,the movement of the crystal bed is mainly based on gravity.The melts stream moves upwards from the pores of the crystal bed.Mass transfer occurs between the melt and the wall surface of the pores.Reynolds numbers of melt flow and crystal flow are calculated respectively.The Reynolds number calculation formula is as follows.
where d is the tube diameter,ρ is the density of logistics, u is the countercurrent velocity of molten liquid, μ is logistics viscosity.The flow path through the bed is not straight.The value of d should be multiplied by two.Reynolds number for melts flow in the crystal bed is:
Reynolds number for flow between crystals in the bed is
The Reynolds number <2000 in the above calculation shows that the fluid flow is laminar in the system.Therefore,mass transfer between melt and the wall surface of the pores can be regarded as steady laminar mass transfer in tube.The convection diffusion equation in cylindrical coordinate system can be used to describe the mass transfer [33].When the mass transfer flux is stable, the following equation can be obtained.
where Sh is Sherwood number,r is pipe inner diameter.The crystal bed is a porous in the actual process.Assuming that this crystal bed contains n pore pipes, then
In which,the axial diffusion coefficient Daxof the liquid phase is calculated by the B′
o as follows.
Assuming that the crystal is spherical,if the average ratio of surface area to volume of individual crystal is SC/VC,the α can be given by
2.4.The average relative deviation
The experimental results with the model under various experimental conditions are compared and analyzed in this paper.The ARD is used to describe the deviation of experimental versus modeled data.The ARD is given by
where N is the number of samples, EDjis the experimental data of the j position, SDjis the simulated data of the j position.The ARD calculation results were introduced in the Supplementary Material.
3.Results and Discussion
3.1.Effect of feed mass fraction on Kt
Fig.5 illustrates experimental versus modeled data at different feed mass fraction.Fig.5(a) and (b) are the NBES and the NBSSS,respectively.For both system, the purity of the product increases with the feed mass fraction.This shows different feed mass fraction will affect the concentration distribution in the tower crystallizer.Multi-tower series-operating is an effective way to obtain high purity products.In the actual industrial application process,the number of tower crystallizers can be changed according to different material composition and product purity requirements.On the other hand, the ARD of NBES and NBSSS are increased from 2.39% and 6.81% to 28.75% and 41.74%, respectively, with the increased of the feed mass fraction, which indicated that the feed mass fraction has a great impact on the calculation accuracy of the model.In the calculation of Kt, the mass transfer rate (NA) is assumed to be constant.NAdecreases with the differential concentration in the experiment.The actual mass transfer coefficient will be smaller than the theory as the feed mass fraction increases.Therefore the model can more accurately describe the experimental results at low concentration feeding.

Fig.5.Experimental versus modeled data at different feed mass fractions: (a) nitrochlorobenzene system, (b) naphthalene-benzothiophene system., experimental data.- - -, simulated data.
3.2.Effect of the crystal bed on Kt
The crystal bed, a mass transfer platform, directly affects the separation effect.The height of the crystal bed determines the mass transfer area.The melt flow rate in the pore is reflected by reflux ratio.Fig.6 illustrates the purity of the product increases with the height of the crystal bed.This is because as the height of the crystal bed increases, the mass transfer area between the solid and liquid increases,thereby improving the separation effect.Additionally,the maximum value of ARD is 6.81%.The experimental data can correspond well with the simulated data.The results show that the model can accurately describe the mass concentration distribution in the crystallizer regardless of the height of the crystal bed.It is also worth noting that the time for the reflux melts to pass through the crystal bed increases with H.Thus,the discussion of the model needs to combine the height of crystal bed and reflux ratio.

Fig.6.Experimental versus modeled data at different crystal bed heights:(a)nitrochlorobenzene system,(b)naphthalene-benzothiophene system.,experimental data.- -, simulated data.
Fig.7 shows experimental versus modeled data at different reflux ratios.According to the figures, it is can be obtained that the R is consistent on effect of product purity for both systems.The purity of the product increases with the R.The experimental data can correspond well with the calculated data in small reflux ratios.The calculated data obviously deviates from the experimental with increase of the reflux ratio.In particular the ARD increased from 11.25% to 30.78% in NBSSS.This demonstrates that the flow rate of melt becomes slower under low reflux ratio, which is conducive to molecular diffusion and mass transfer.Theoretical mass transfer coefficient is closer to actual mass transfer coefficient.The model can more accurately describe the experimental results at high crystal bed and low reflux ratio.In the actual industrial application process, the R determines yield and energy consumption.And the H determines equipment cost.These parameters are determined on the basis of economy and production capacity.Consequently, with the high purity and high yield of the product as the standard, a high crystal bed and low reflux ratio are a good choice for operation.
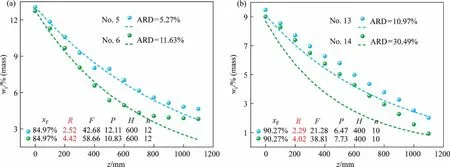
Fig.7.Experimental versus modeled data at different reflux ratios: (a) nitrochlorobenzene system, (b) naphthalene-benzothiophene system., experimental data.- -, simulated data.
3.3.Effect of the stirring on crystal bed structure
Fig.8 shows the experimental versus modeled data at different stirring rates.The crystal is irregularly distributed without stirring.There are faults between the solid and liquid phase, resulting in discontinuity of the liquid phase.This is the main reason for a step change of No.9 in Fig.8(b).The separation effect is significantly increased with the start of stirring device.However, the ARD increased from 3.99% to 28.79% according to Fig.8(a).This indicates that the steady state of crystal bed will be destroyed by excessive stirring speed.And the continuous increase of stirring speed will destroy the structure of crystal bed and make the control process difficult.
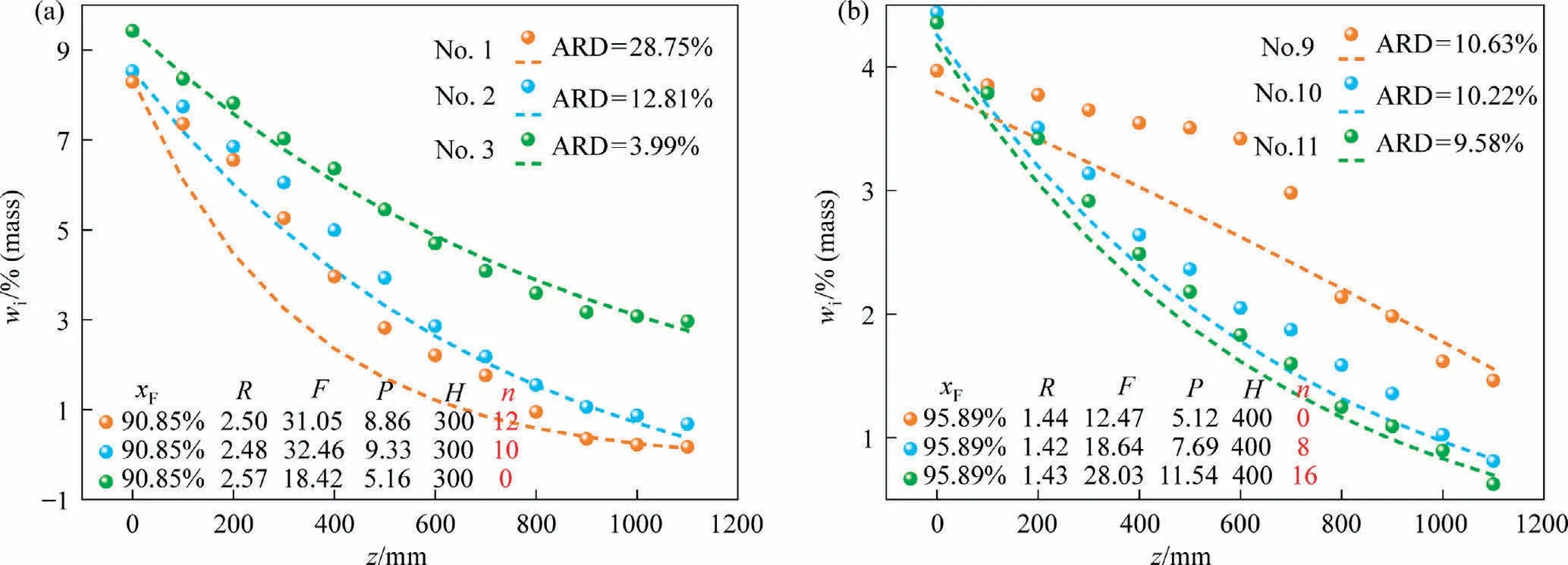
Fig.8.Experimental versus modeled data at different stirring rates: (a) nitrochlorobenzene system, (b) naphthalene-benzothiophene system., experimental data.- - -, simulated data.
4.Conclusions
In this study, the p-nitrochlorobenzene (with o-nitrochlorobenzene as an impurity) and naphthalene (with benzothiophene as an impurity) in the tower crystallizer were investigated as crystallization systems.A mathematical model of continuous multistage countercurrent fractional crystallization process was established by analyzing the principle of crystallizer purification process.The following conclusions are made:
(1) Tower crystallizer purification process experimental data are collected for different feed concentration, crystal bed height,reflux ratio,and stirring speed.The results show that the concentration of p-nitrochlorobenzene was purified from 90.85% to 99.99% when the crystal bed height is 600 mm, the reflux ratio is 2.5, and the stirring speed is 12 r·min-1.The naphthalene concentration is purified from 95.89% to 99.99%, when the crystal bed height is 400 mm,the reflux ratio is 1.43, and the stirring speed is 16 r·min-1.
(2) A mathematical model of continuous multistage countercurrent fractional crystallization process is established.It can be written as
(3) The model calculated data were compared with the experimental data to achieve the best separation state for the internal operation of the crystallizer.The quality of the model is evaluated by the ARD.In the nitrochlorobenzene system and naphthalene-benzothiophene system, the minimum values of ARD were 2.39% and 5.22%, respectively.The result shows the model accurately describes the mass concentration distribution in the crystallizer under different system.Low reflux ratio and high crystal bed are more favorable for mass transfer.In addition, the crystal nucleation,growth and purification process in the tower cannot be detected is the biggest difficulty in the current experiment.Online image and particle size monitoring may be a good solution.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge the financial support by China Hunan Provincial Education Department Innovation Platform Project(20k125),Postgraduate Scientific Research Innovation Project of Hunan Province (CX20210518), and Postgraduate Scientific Research Innovation Project of Xiangtan University(XDCX2021B169).
Supplementary Material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.cjche.2023.06.004.
Nomenclature
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Intrinsic kinetics of catalytic hydrogenation of 2-nitro-4-acetylamino anisole to 2-amino-4-acetylamino anisole over Raney nickel catalyst
- α-Synuclein: A fusion chaperone significantly boosting the enzymatic performance of PET hydrolase
- Influence of water vapor on the separation of volatile organic compound/nitrogen mixture by polydimethylsiloxane membrane
- Mass transfer mechanism and relationship of gas-liquid annular flow in a microfluidic cross-junction device
- Enhanced photocatalytic activity of methylene blue using heterojunction Ag@TiO2 nanocomposite: Mechanistic and optimization study
- Comparative analysis on gas-solid drag models in MFIX-DEM simulations of bubbling fluidized bed
