Influence of water vapor on the separation of volatile organic compound/nitrogen mixture by polydimethylsiloxane membrane
2023-02-28YifanLiangHaiboLeiXinleiHeHaoliZhouWanqinJin
Yifan Liang, Haibo Lei, Xinlei He, Haoli Zhou,4,, Wanqin Jin
1 State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemical Engineering, Nanjing Tech University, Nanjing 210009, China
2 Low-Carbon Technology and Chemical Reaction Engineering Laboratory, School of Chemical Engineering, Sichuan University, Chengdu 610065, China
3 College of Basic Science, Tianjin Agricultural University, Tianjin 300392, China
4 Zhangjiagang Institute of Nanjing Tech University, Suzhou 215699, China
Keywords:Water vapor Ternary mixtures Polydimethylsiloxane Membranes Permeability Selectivity
ABSTRACT In the industrial treatment of waste volatile organic compound(VOC)streams by membrane technology,a third impurity,generally,water vapor,coexists in the mixture of VOC and nitrogen or air,and can affect membrane performance and the design of the industrial process.This study focused on the investigation of the effect of water vapor on the separation performance of the separation of VOC/water/nitrogen mixtures by a polydimethylsiloxane (PDMS) membrane.Three types of VOCs: water-miscible ethanol,water-semi-miscible butanol, and water-immiscible cyclohexane, were selected for the study.Different operating parameters including, concentration of the feed VOC, feed temperature, and concentration of the feed water were compared for the separation of binary and ternary VOC/nitrogen mixtures.The interaction between the VOC and water was analyzed to explain the transportation mechanism after analyzing the difference in the membrane performance for the separation of binary and ternary mixtures.The results indicated that the interaction between the VOC (or nitrogen) and water is the key factor affecting membrane performance.Water can promote the permeation of hydrophilic VOC but prevent hydrophobic VOC through the membrane for the separation of ternary VOC/water/nitrogen mixtures.These results will provide fundamental insights for the design of the recovery application process for industrial membrane-based VOCs, and also guidance for the investigation of the separation mechanism in vapor permeation.
1.Introduction
The term volatile organic compounds(VOCs)generally refer to a class of non-methane organic compounds that easily volatilize in the environment [1].Common VOCs include gasoline, benzene,formaldehyde, and styrene; they can pose a serious threat to the ecological environment and human health if released into the environment [2].Various technologies, including membrane [3], condensation [4], and absorption [3,4], have been investigated to treat the released VOCs.Membrane separation technology has garnered significant attention owing to its unique advantages in terms of raw material adaptability, low energy consumption, and high recovery efficiency [5].S¸ahin et al.[6] fabricated zeolitic imidazolate framework (ZIF) filled polydimethylsiloxane (PDMS) membranes exhibiting a high separation performance.A VOC permeability of 10215 Barrer (1 Barrer = 3.35 ×10-6mol·m·m-2·s-1·Pa-1, STP) and a selectivity of 72 were obtained in the separation of solvent vapors such as ethanol from nitrogen.Guo et al.[7] prepared MIL-101 (metal-organic framework (MOF),chromium (III) terephthalate (MIL-101)) filled PEBA (polyether block amide) membranes for the separation of an ethylbenzene/nitrogen mixture; the results indicated that the ethylbenzene/nitrogen permselectivity increased 10.5 times compared with that of the pristine Pebax membrane.Our group [8] used a labsynthesized microporous polymer membrane to molecularly sieve a nitrogen and VOC mixture; a cyclohexane rejection of >99% could be obtained, with a low energy consumption because a low feed pressure (as low as 10 kPa) was used as the driving force.
Most of the previous works focused on the development of high-performance membrane materials because membrane performance is one of the key factors that can affect the industrial application processes; meanwhile, most all the previous works used binary mixtures to evaluate the membrane performance because of the simple interactions between the separated species and membrane.The researchers focused on the interaction between the species and membrane or the interaction between the two species.Therefore, it was simple for the authors to elucidate the reasons for the high performance of the fabricated membrane.Our previous work used a binary VOC/nitrogen mixture to investigate the effects of the operational parameters on membrane performance[9].Gérardin et al.[10] also used an ideal binary hexane/nitrogen mixture to investigate the original combination of membrane separation with a photocatalytic oxidation process for the removal of trace VOCs from air.
With advancements in research methods and the growing use of membrane materials such as the PDMS membrane [11], it is now possible to study the impact of three or even four species on membrane performance by examining ternary or quaternary mixtures.This is particularly important because in industrial application, an ideal binary mixture is rare, and a separation mixture containing more than two species is generally formed.The existence of a third compound could affect the performance of membrane separation because of the existence of coupling phenomenon between the three species.For example, Lee et al.[12] investigated the transport properties of the ternary mixture of CO2/O2/methanol at 35 ° C in a polyethylene terephthalate(PET) membrane.It was discovered that the incorporation of methanol in the CO2/O2mixture could enhance the permeability of CO2and O2because of the large increase in the mobility of the PET polymer chains induced by methanol.
In the treatment of VOC waste streams, the presence of water vapor is unavoidable owing to incomplete airtightness and could affect the performance of the membrane for the vapor permeation of the VOC waste stream [13].An investigation into the influence of water on the performance of the membrane will provide detailed information for the industrial application of the membrane.However, to the best of our knowledge, no paper has been published on an investigation into the effect of water vapor on the performance of a membrane for the vapor permeation of VOC from its mixtures.In this work, water vapor was added as the third compound to form a ternary mixture with VOC and nitrogen.Three types of VOCs: water-immiscible, watermiscible, and water-semi-miscible based on their degree of solubility in water were used to study the effect of the interaction between water and different types of VOCs or nitrogen on the performance of the membrane; this would provide fundamental data for the industrial design.PDMS was selected as the composite membrane because it has exhibited good separation performance for the separation of a VOC/nitrogen mixture [9].Furthermore, it has already been industrialized by our lab, and various units of industrial equipment have been installed [14].Therefore, an investigation into the influence of water vapor can provide more information for industrial application.To ascertain the impact of water vapor on the separation performance of VOCs, the mixtures of VOC/nitrogen and VOC/water/nitrogen were extensively examined under various experimental conditions.A constant molar composition of water vapor was applied while changing the concentration of the feed VOCs and changing the feed temperature to investigate the difference between the VOC/nitrogen and VOC/water/nitrogen mixtures.Additionally,the effect of water vapor enrichment on the VOC/water/nitrogen mixtures was studied by increasing the molar composition of water vapor in the ternary mixture while keeping the concentration of the feed VOCs and the feed temperature constant.To summarize, in addition to developing membrane materials with high separation performance, the investigation of the separation process involved in the VOC/water/nitrogen ternary mixture is also necessary, and can provide fundamental data for industrial applications of the membrane separation of VOCs.Furthermore, the investigation will provide more guidance for the investigation of the separation mechanism involved in vapor permeation.
2.Experimental
2.1.Materials
Nitrogen, hydrogen, and high-purity air (purity 99.99%) used for gas chromatography were purchased from Jiangsu Tianhong Chemical Co., Ltd.(China).Cyclohexane, ethanol, and butanol that were used as vapor solvents (99%) were purchased from Sino pharm Chemical Reagent Co., Ltd.(China) and used without further purification.
2.2.Preparation and characterization of membranes
The preparation details of the PDMS/polyamide (PA) composite membranes can be found in our previous work [15-17].The microstructure of the membranes was characterized by scanning electron microscopy (SEM, S4800, Hitachi, Japan).In our laboratory, PDMS polymer (RTV 615 A and B) was added to a heptane solution to form a 5% (mass) membrane solution [8,9].This membrane solution was agitated at 70 °C for a predetermined time to achieve a 90 mPa·s viscosity.The membrane solution was then applied using a casting knife to a 0.11 μm polyamide substrate to create a composite membrane, which was then allowed to cure at 80 °C in an oven for more than 8 h after being left overnight to evaporate heptane at room temperature.
2.3.Gas/vapor separation
A schematic of the experimental setup is shown in Fig.1 and the temperatures of two tanks and pipeline systems were controlled by circulating water bath (SLDC-1010, Nanjing Shunliu Instrument Co., Ltd, China).A stream of pure nitrogen was added to the liquid or water tanker of the VOCs to create a saturated stream of the mixture of VOC/nitrogen or VOC/water/nitrogen, which was subsequently combined with another stream of pure nitrogen to reach the required composition of the mixtures [9], as shown in Fig.1(a).The ratio of the flow rates of the two pure N2streams was controlled by a mass flow controller to manage the composition and flow rate of the mixtures (MFC; D08-2F, Sevenstar, China).A membrane cell was placed in a water bath to maintain a constant temperature [15].All double O-ring seals were examined with a gas leak detector (CRC Industries Inc., USA) to ensure the airtightness of the pipe installation before starting the experiment.To reach a steady state, a vacuum pump (D8C, Leybold, Germany) was used to maintain the permeate pressure below 300 Pa for a predetermined time.The gas chromatograph (GC-2014, Shimadzu, Japan)was used to determine the composition of feed and residual streams [15].It was worth noting that water vapor cannot be measured directly by chromatography [16,18], so the VOCs on the permeate side and the water vapor on the feed side were collected via cold trips and weighed using a digital balance, as shown in Fig.1(b), (c).
Owing to the different solubilities of the VOCs in water, the treatment method for the mixture of VOCs and water at the permeate side differed.Water-immiscible cyclohexane was separated from water after more than 1 h in the cold trap; the cyclohexane formed the upper layer and the water formed the bottom layer.The water in the cold trap was removed and weighed with a dropper and an analytical balance.The mass of cyclohexane was obtained by subtracting the mass of water from that of the mixture.The amounts of water-miscible ethanol and water-semimiscible butanol were determined via measuring by GC (456GCMS, Bruker, Germany).
2.4.Membrane performance evaluation
The efficacy of a PDMS/PA composite membrane for the separation of the VOC/nitrogen and VOC/water/nitrogen mixtures was evaluated under different experimental conditions (constant volume/variable pressure) to evaluate the effect of water vapor on the separation of VOCs.The operating temperature range was 25-40 °C and the feed flow rate was 5 L·min-1.The performance of the membrane, expressed in terms of the permeate flux J, was calculated as follows [15,19]:
where W is the mass of the permeate component(g),A is the effective area of the membrane(m2),and t is the time interval for permeation (h).
The intrinsic properties of the membrane can be expressed by the permeability, Pi, which is calculated as follows [15,16]:
where L is the membrane thickness (μm), Xfeedand Xpermare the mole fraction of compound i in the feed and permeate streams,respectively, Jiis the flux of compound i (g·m-2·h-1, and Pfeedand Ppermare the feed and permeate pressure, respectively.
The permeability of the mixtures or single compounds can be determined from the slope of the linear portion of the permeate pressure vs time curve using Eq.(3) [15,20].
where V is the available volume on the permeate side and the other numbers in the formula are scale factors, which have been mentioned several times in the early literature [9,15].The gas permeability unit is Barrer, and 1 Barrer = 10-10cm3·cm·cm-2·s-1-·cmHg-1= 3.35 × 10-16mol·m·m-2·s-1·Pa-1[20-22].
The permeability of each component at the permeate side is calculated from the following relationship [15,18]:
where dpi/dt is the increase in the partial pressure of i over the time interval t on the permeate side.By solving Eqs.(2) and (4), the molar composition and the permeability of the VOCs are calculated at the permeate side.
The selectivity (α) is calculated according to the following expression [15,23]:
where PVOCand PN2are the permeability of VOCs and N2,respectively.
Furthermore, two mixtures were used for the comparison in this paper: the binary VOC/N2mixture and the ternary VOC/N2/H2O mixture.The legend in the figures‘‘VOC with H2O”or‘‘N2with H2O” means that the flux or permebility of VOC or nitrogen in the ternary system, while ‘‘VOC without H2O” or ‘‘N2without H2O”means the flux or permebility of VOC or nitrogen in the binary system.
3.Results and Discussion
3.1.Membrane characterization
Fig.2 presents the SEM images of the PDMS composite membrane.The surface of the PDMS layer is smooth and defect-free.Fig.2(b) shows that the separation layer (PDMS) and the support layer (PA) are tightly bound, and the thickness of the separation layer is approximately 1.2 μm.
3.2.Effect of feed VOC concentrations
Membrane performance can be affected by the membrane materials and by the operating conditions.The effect of the feed concentration of the VOCs on the vapor permeation of the VOC/nitrogen and VOC/water/nitrogen mixtures at a feed flow rate of 5 L·min-1and a temperature of 30 °C was investigated (Fig.3).
Owing to different saturation vapor pressures, the feed molar compositions of cyclohexane, ethanol, butanol, and water of 0.72%-3.60%, 0.36%-1.44%, 0.12%-0.36%, and 1.00%, respectively,were selected.All data were calculated several times,and the error bars are included in all figures.It is observed from Fig.3(a),(c)and(e) that the VOC fluxes increase linearly with an increase in the concentrations of the feed VOC.This is because the VOCs possess a higher driving force at higher concentration,and thus more VOCs can be absorbed and permeate through the membrane, leading to higher fluxes.The linear trend of the fluxes of the VOCs with their feed concentrations indicates that negligible swelling occurs in the membrane under the studied conditions [24,25].When water vapor is added to the mixture,the fluxes of both ethanol and butanol increase, and that of cyclohexane decreases.This is because water molecules have lower kinetic diameters than those of butanol and ethanol molecules, suggesting faster diffusion of water molecules through the membrane than that in butanol and ethanol[26,27].It is reported that the faster molecules can speed up the slower molecules when a coupling effect occurs between the molecules [28,29].Furthermore, in the ethanol/nitrogen/water mixture or butanol/nitrogen/water mixture,hydrogen bonds can be formed between ethanol or butanol and water molecules because of the hydroxyl groups in these molecules [30,31], and a strong coupling effect can be expected.Therefore, the addition of water vapor can enhance the fluxes of ethanol and butanol.While cyclohexane is hydrophobic and has less affinity for water molecules, higher water diffusion cannot enhance the diffusion of cyclohexane through the membrane.Moreover, water molecules can occupy the transporting channels of cyclohexane, leading to a decrease in the flux of cyclohexane with the addition of water vapor.Similar permeabilities trends with and without water vapor can be obtained (Fig.3(b), (d), and (f)).
Fig.3 also shows that a higher concentration of the feed VOC results in a lower nitrogen flux, because nitrogen possesses inert properties and has a low affinity for VOC; moreover, a small coupling effect exists between nitrogen and the VOC.Therefore, a higher VOC flux combined with a higher feed concentration cannot enhance the nitrogen flux.With an increase in the concentration of the feed VOC, the possibilities for the VOC to be solubilized in and permeate through the membrane will be enhanced as discussed above.More transport channels in the membrane are occupied by the VOC and nitrogen transportation is blocked through the membrane [32].This occurs because the limiting step for the permeation of nitrogen through the membrane is the diffusion process in the separation of the VOC/nitrogen mixture [24].A lower nitrogen flux can thus be observed with an increase in the concentration of the VOC.When water vapor is added to the binary VOC/nitrogen mixture, water molecules can occupy the transport channels and hinder the transport of nitrogen through the membrane, leading to a decrease in the nitrogen flux in both the water/ethanol/nitrogen or water/butanol/nitrogen mixtures.Higher selectivity can thus be observed by comparing binary mixtures with and without water (Fig.4(a)-(c)).However, in the ternary water/cyclohexane/nitrogen mixture, hydrophilic water molecules hinder the permeation of hydrophobic cyclohexane through the membrane as discussed above.With the addition of water vapor, more transport channels become available for the permeation of nitrogen through the membrane, and higher nitrogen permeability can thus be observed,leading to a lower selectivity upon the addition of water vapor.These results also indicate that water vapor can enhance the permeation of water-miscible and water-semi-miscible compounds in the gas/vapor separation because of their high affinity and the existence of the coupling effect; however, the existence of water vapor hinders the permeation of immiscible compounds through the membrane because of their low affinity.However,although water vapor can enhance the selectivity of watermiscible and water-semi-miscible compounds over nitrogen, it is still necessary to remove as much water vapor as possible to avoid affecting the concentration of the condensed permeate VOC, as shown in Fig.4(d)-(f).
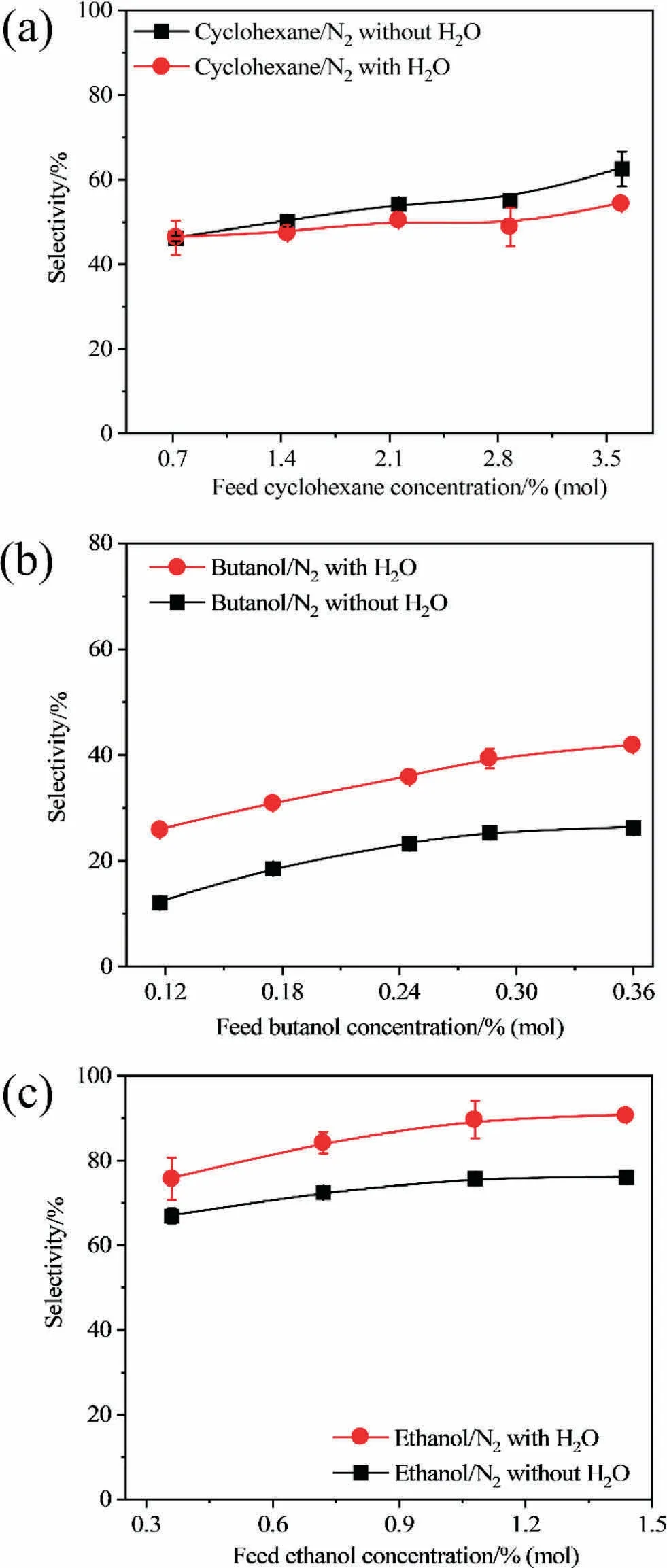
Fig.4.Selectivity of VOC/N2 in different feed VOC concentrations:(a)cyclohexane/N2, (b) butanol/N2, (c) ethanol/N2.
3.3.Effect of feed temperature
In addition to the concentrations of the feed VOC, membrane performance is also a function of temperature.The vapor permeation processes of VOC/N2and VOC/N2/H2O mixtures through the PDMS composite membrane are thus studied with a feed flow rate of 5 L·min-1at temperatures ranging from 25 to 40 °C (Fig.5).
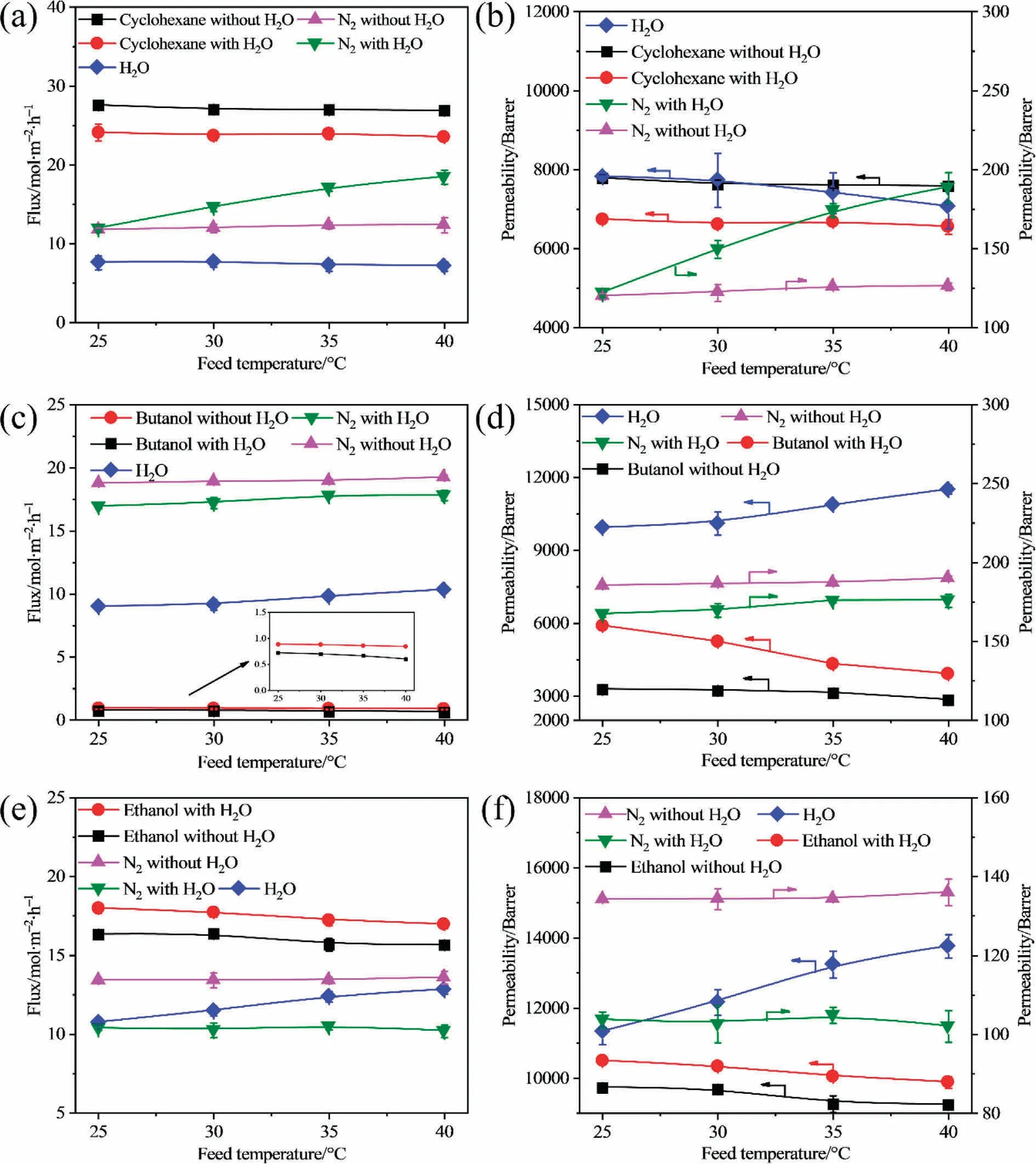
Fig.5.Fluxes (a, c, e) and permeability (b, d, f) of VOCs in separation of various VOCs/N2 and VOCs/N2/H2O mixtures through PDMS composite membrane versus feed temperature.Feed molar compositions of cyclohexane, ethanol, butanol, and water in VOC/nitrogen and VOC/water/nitrogen mixture are 2.16%, 1.08%, 0.18%, and 1%,respectively.
It is observed that both the flux and permeability of the VOC decrease with an increase in temperature.In the vapor/gas separation, the rate-limiting step for VOC vapor permeation is typically the solution step[24].As the temperature increases,the adsorption of VOCs is weakened on the membrane surface because the adsorption process is exothermic [33,34], thereby resulting in reduced fluxes and permeabilities of the VOC.When water vapor is added to binary mixtures, the impact of water on the flux and permeability of the VOC with increasing temperature is similar to that of increasing the concentration of the feed VOC because of the different coupling effects between the different VOCs and water molecules.These results also further indicate that the affinity between the separated species can affect their coupling effect and permeation behavior during the vapor permeation.
Fig.5 shows that the flux and permeability of nitrogen increases slightly with an increase in the temperature of the VOC/nitrogen mixture.Previous works stated that the rate-limiting step for nitrogen in the vapor permeation is the diffusion step [15,35].When the temperature rises, the chain mobility of the polymer segment is enhanced in the rubbery PDMS membrane, resulting in an enlarged free volume, higher flux, and permeability of nitrogen in the vapor permeation of the binary VOC/nitrogen mixture.Finally, the selectivity of the three studied VOCs over nitrogen decreased with an increase in the temperature during the vapor permeation of the binary VOC/nitrogen mixture, as shown in Fig.5(a),(c),(e).When water vapor is added to the binary VOC/nitrogen mixture, the flux and permeability of nitrogen increase in the cyclohexane/water/nitrogen mixture,and decrease in the butanol/water/nitrogen and ethanol/water/nitrogen mixtures, because water vapor obstructs the diffusion of cyclohexane and promotes the diffusion of ethanol and butanol, as discussed in Section 3.2,as shown in Fig.5(d), (f).Fig.5 also shows that a higher temperature can promote the permeation of nitrogen through the membrane in the cyclohexane/water/nitrogen and butanol/water/nitrogen mixtures, but weakens the permeation of nitrogen through the membrane in the ethanol/water/nitrogen mixture because of the different affinities between the VOC and water.Theoretically,a higher temperature leads to lower cyclohexane permeation, and the higher flux and permeability of water can be observed.However, owing to the hydrophobic property of cyclohexane, the low affinity between cyclohexane and water may result in macroscopic phase separation and interfacial voids in the membrane [30,35-37], which can be the transportation channels for the permeation of nitrogen;thus,a higher flux and permeability of nitrogen can be observed at a higher temperature.Therefore, the lower selectivity of cyclohexane over nitrogen in the cyclohexane/water/nitrogen mixture can be observed at higher temperature;the decrease is higher than that in the binary mixture due to the increasing permeability of nitrogen at higher temperatures as shown in Fig.6(a).A higher flux of nitrogen can conversely weaken the permeation of water.Thus,the flux and permeability of water decrease slightly at higher temperatures.However, in the butanol/water/nitrogen mixture, the hydrophobic property of butanol is not as high as that of cyclohexane, thus the increase in the flux and permeability of nitrogen is not as high as that of cyclohexane, resulting in the lower selectivity of butanol over nitrogen in the butanol/water/nitrogen mixture with increasing temperature (Fig.6(b)).However, the selectivity is higher in the butanol/water/nitrogen mixture than that in the butanol/nitrogen mixture because water can promote the permeation of butanol through the membrane.Ethanol cannot undergo a macroscopic phase separation from water because of the hydrophilic nature of water[13,36-38]; thus, the interfacial void between water and ethanol molecules is low, and water can transport through the membrane with ethanol, with ethanol being preferentially transported through the membrane.Furthermore, the enlarged free volume and higher chain mobility of the polymer segment at higher temperatures also favor the transportation of water, which occupied the channels for the permeation of nitrogen;this results in a lower flux and permeability of nitrogen at higher temperatures and a higher flux and permeability of water at higher temperatures.Thus,a higher selectivity can be observed in the ethanol/water/nitrogen mixture than that in the ethanol/nitrogen mixture,and the decreasing trend of the permeability of nitrogen is lower than that of the permeability of ethanol(Fig.5(f));this leads to a decreasing selectivity at higher temperatures in the ethanol/water/nitrogen mixture (Fig.6(c)).
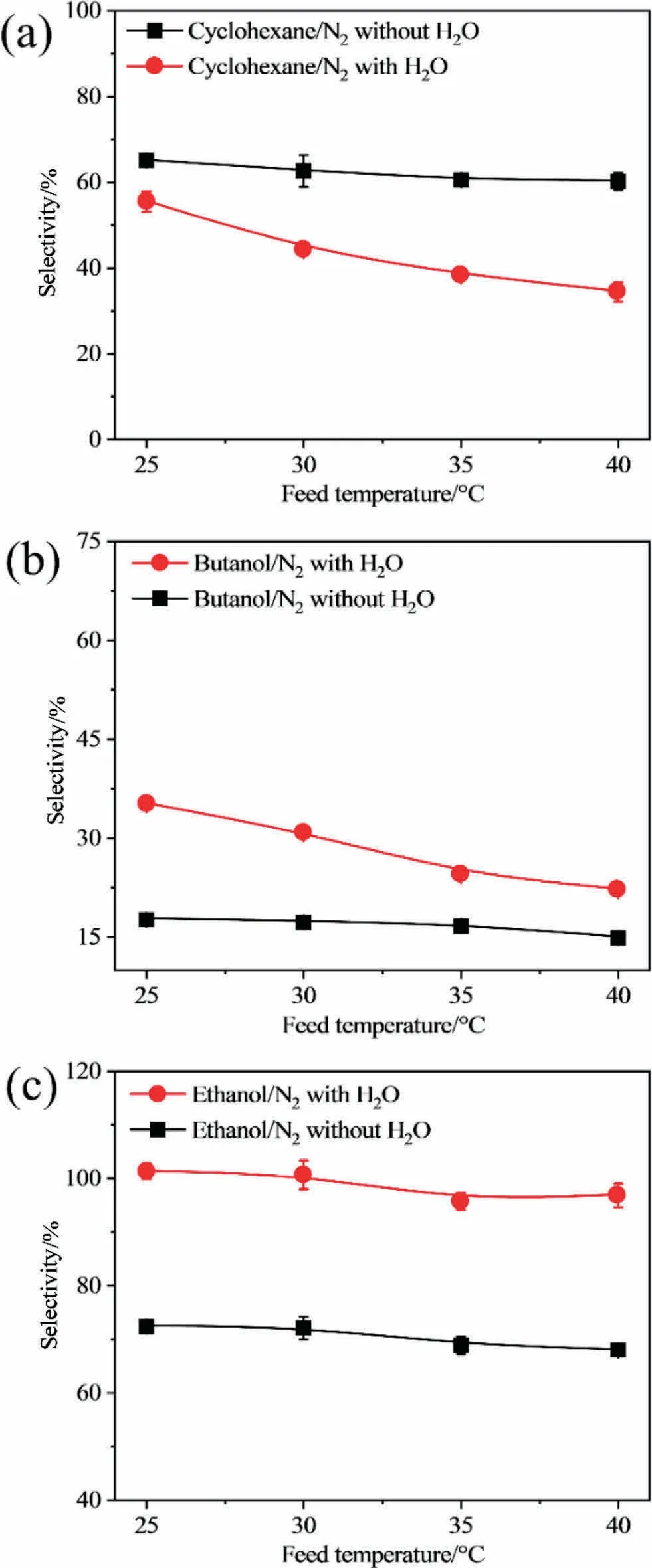
Fig.6.Selectivity of VOC/N2 in different feed temperature: (a) cyclohexane/N2, (b)butanol/N2, (c) ethanol/N2.
3.4.Effect of composition of feed water vapor
It is concluded from the aforementioned experimental results that the existence of water vapor has a notable influence on the performance of the membrane in the separation of binary VOC/nitrogen mixtures.In this section, the effect of the concentration of the feed water was investigated on the performance of the membrane for the separation of a VOC/water/nitrogen mixture.The molar concentration of the feed water was controlled from 0% to 1.6%, and the feed molar concentrations of cyclohexane, ethanol,and butanol were maintained at 2.16%, 1.08%, and 0.18%, respectively.The results are shown in Fig.7.When the concentration of the feed water increases, the flux and permeability of water increase because more water can make contact with the membrane surface and transport through the membrane.More transportation channels are occupied by the water molecules,hindering the transport of cyclohexane molecules through the membrane[13,38-40];thus a lower flux and permeability of cyclohexane can be observed.Furthermore, as discussed above, during the transportation of cyclohexane and water molecules through the membrane, interfacial voids may be formed between the hydrophobic cyclohexane and water molecules, which provide additional channels for the transportation of nitrogen, resulting in a higher flux and permeability of nitrogen (Fig.7(a)).Thus, the lower selectivity of cyclohexane over nitrogen can be observed in Fig.8(a).Furthermore, the increasing trend of the flux of water is higher than that of nitrogen and also leads to the increasing selectivity of water over nitrogen (Fig.8(a)).As the hydrophobicity of the VOCs decrease,the decreasing trend of the flux and permeability of butanol with increasing water concentration is slower than that of the flux and permeability of cyclohexane.The flux and permeability of ethanol increase slightly because the quicker the water molecules speed up,the slower the butanol or ethanol molecules become,when the coupling effect occurs between molecules as discussed above.The low hydrophobicity of butanol and ethanol also suggests that the interfacial void between water and the VOC will be low,and be fewer available channels will exist for the transport of nitrogen.This will lead to a lower flux and permeability of nitrogen with a higher concentration of the feed water.A slight increase can be obtained in the selectivity of butanol or ethanol over nitrogen as shown in Fig.8(b)and(c).Furthermore,the selectivity of water over nitrogen in the ethanol/water/nitrogen mixture is highest, followed by those in the butanol/water/nitrogen mixture, and cyclohexane/water/nitrogen mixture as shown in Fig.8.These results also indicate that the difference in the physicochemical properties between the separated species can markedly affect the performance of the membrane, particularly, when more than two components exist in a mixture.Therefore, researchers should focus on the separation of mixtures containing more than two compounds to study their separation mechanism to determine the fundamental mechanism of their industrial application or to obtain data, because the actual industrial applications involve complex mixtures containing more than three compounds.The interaction between compounds in the separation of ≥three compounds may be more obvious, as in the selectivity of ethanol over water in the binary and ternary mixture,which better explains that the interaction between separated species can notably affect the performance of the membrane separation.

Fig.7.Fluxes (a, c, e) and permeability (b, d, f) of VOCs in separation of various VOC/water/nitrogen mixture versus feed water composition.
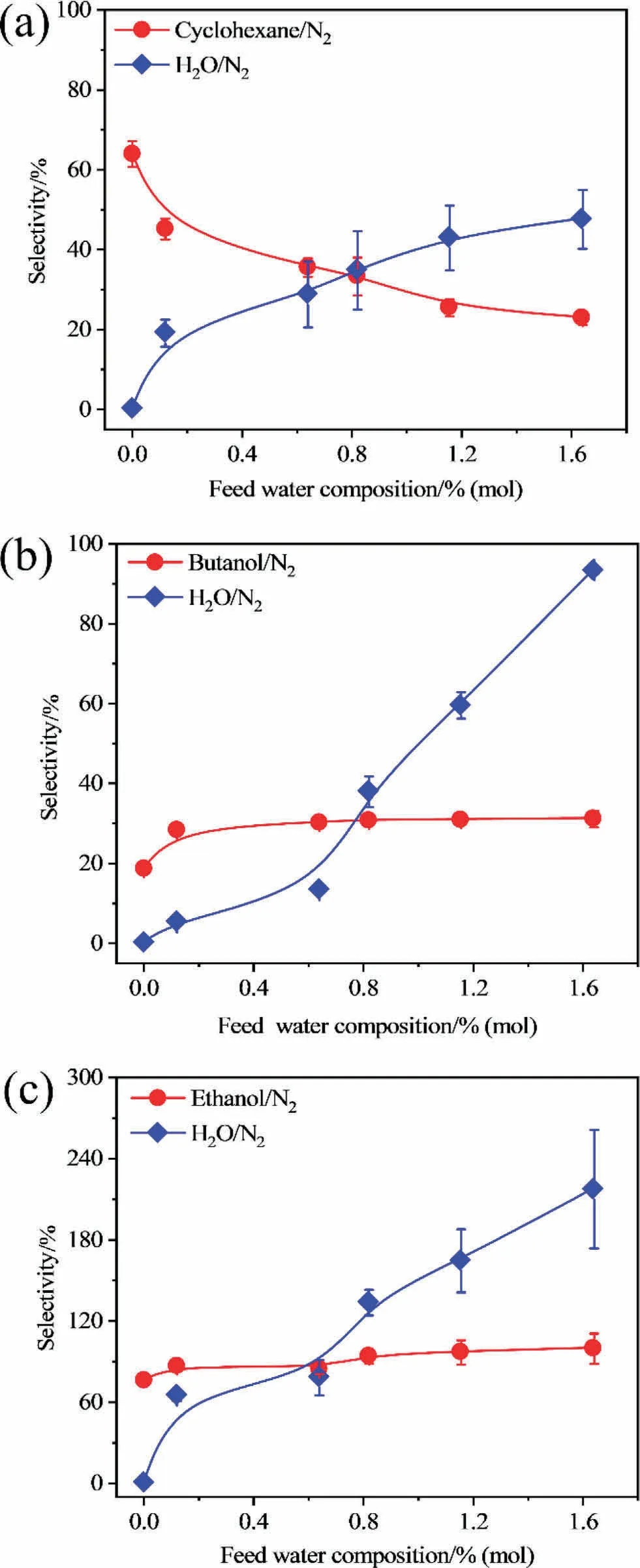
Fig.8.Selectivity of VOC/N2 in different feed water composition: (a) cyclohexane/N2, (b) butanol/N2, (c) ethanol/N2.
4.Conclusions
In this work, the effect of water vapor on the performance of a membrane was investigated in three VOC/water/nitrogen mixtures.Three VOCs including water-immiscible cyclohexane,water-semi-miscible butanol, and water-miscible ethanol were selected to reveal the importance of the coupling effect between separated species in vapor permeation.Different operating parameters were studied, including the concentration of the feed VOC,feed temperature, and the concentration of the feed water.The results showed that the flux and permeability of the VOC showed a similar trend with an increase in the concentration of the feed VOC and feed temperature for the separation of binary and ternary mixtures.Water can inhibit the permeation of a hydrophobic VOC through the membrane,and with a decrease in the hydrophobicity of the VOC,the inhibition will gradually weaken and turn into promotion when the VOC is hydrophilic.However, the effect of water on the permeation of nitrogen was inversely related: the flux or permeability of nitrogen increased in the hydrophobic cyclohexane/water/nitrogen mixture but decreased in the hydrophilic ethanol/water/nitrogen mixture.Therefore, a higher selectivity of a hydrophilic VOC over nitrogen can be observed in the ternary mixture rather than that in the binary mixture.However,in the actual industrial application, it is still necessary to prevent the existence of water vapor in the feed stream or to synthesize a novel membrane that can reject the passage of both nitrogen and water through the membrane if the water vapor cannot be avoided.Therefore, this work can also provide research direction for the future design of membrane materials with a higher performance.
Data Availability
No data was used for the research described in the article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFC2101201,2022YFB3805203), the National Natural Science Foundation of China (22278208).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Intrinsic kinetics of catalytic hydrogenation of 2-nitro-4-acetylamino anisole to 2-amino-4-acetylamino anisole over Raney nickel catalyst
- Experiments and model development of p-nitrochlorobenzene and naphthalene purification in a continuous tower melting crystallizer
- α-Synuclein: A fusion chaperone significantly boosting the enzymatic performance of PET hydrolase
- Mass transfer mechanism and relationship of gas-liquid annular flow in a microfluidic cross-junction device
- Enhanced photocatalytic activity of methylene blue using heterojunction Ag@TiO2 nanocomposite: Mechanistic and optimization study
- Comparative analysis on gas-solid drag models in MFIX-DEM simulations of bubbling fluidized bed
