Synergistic effect of CuO coupled with MoS2 for enhanced photodegradation of organic dyes under visible light
2023-02-28UmsalamaAbuelgasimAbubakrYasinZhixinJiaZiwenQinTianyuGuoRuihuaZhaoJianpingDu
Umsalama Abuelgasim Abubakr Yasin, Zhixin Jia, Ziwen Qin, Tianyu Guo, Ruihua Zhao,,4,Jianping Du,,5,
1 College of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan 030024, China
2 College of Chemistry, Taiyuan University of Technology, Taiyuan 030024, China
3 College of Environment Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China
4 Shanxi Kunming Tobacco Co.Ltd., Taiyuan 030032, China
5 Shanxi Key Laboratory of Gas Energy Efficient and Clean Utilization, Taiyuan 030024, China
Keywords:CuO/MoS2 Heterostructure Organic pollutants Photocatalytic degradation
ABSTRACT A series of MoS2-modified CuO (CuO/MoS2) heterostructures were successfully fabricated.The photodegradation properties of organic dyes were explored in detail under visible light.The photocatalytic results demonstrate that the CuO/MoS2-3 heterostructure delivers superior degradation rates towards methyl violet dye (MV) and rhodamine B (RhB), reaching 99.8% and 95.3% within 30 min, respectively.The decent photodegradation activity is due to improved visible light adsorption and faster transfer of electron-hole pairs.The radical trapping experiments show that superoxide radicals (·O-2) and holes(h+)are the main active species in the removal of MV.Furthermore,the CuO/MoS2-3 composite possesses the prominent stability and recyclability.This work offers a highly sustainable technique for designing a high-efficiency photocatalyst to remove environmental pollutants.
1.Introduction
With the growth of the world’s population and the development of industrialization [1], millions of tons of wastewater are produced every day from various industries [2] and directly discharged into nearby water sources without any primary or secondary treatment, which consist of different types of carcinogenic, persistent and bio-accumulative inorganic (e.g.,heavy metals and earth rare metals) and organic pollutants (e.g.,dyes,pharmaceuticals,personal care products,phenols,herbicides and pesticides) [3,4], causing severe negative consequences for humans, animals and the environment [5].Hence, wastewater treatment has been an increasing concern for environmentalists.Among the various techniques,semiconductor photocatalysis technology has been considered as a viable way to solve the environmental concern due to environmental friendliness, high activity[6] and low cost [7].Among the inspired transition metal oxides,copper oxide (CuO) as a p-type semiconductor has attracted more attention in the photocatalytic field due to stability, excellent catalytic activity,availability and non-toxicity[8].Unfortunately,the fast electron-hole recombination limits its actual application [9].To conquer the shortcoming, modulation of unique morphology,an extension of visible light absorption range and regulation of band structure have been devoted to enhancing the photocatalytic activity [10].Encouragingly, fabricating heterojunction has been proven to be one of the best alternatives to boost the charge transfer ability [11].Currently,molybdenum disulfide (MoS2) has more concern due to its tunable optical response and unique layered structure [12].For instance, Shelly et al.[13] synthesized binary MoS2/WO3composite and the result showed that the formation of strong interfacial contact between MoS2and WO3separated the charges for a long time, which enhanced the photocatalytic performance for degradation of rhodamine B dye.Yu et al.[14]fabricated novel Z-scheme MoS2/Bi2WO6composite, and the experimental results showed that MoS2/Bi2WO6demonstrated significantly higher photodegradation activity than pure MoS2and Bi2WO6, which may be attributed to the stable Z-scheme heterostructure, the fast separation of photoinduced electrons and holes.
According to the aforementioned research, it has been confirmed that constructing a heterojunction with MoS2can effectively improve the photocatalytic performance.Therefore, it is significant to synthesize CuO/MoS2heterostructure with outstanding activity for efficient photocatalytic degradation of organic pollutants from wastewater.
In light of the above analysis,a series of CuO/MoS2heterostructures were fabricated by a facile method at room temperature.The photocatalytic performance was further investigated by using different pollutants under visible light.In the meantime, the photocatalytic mechanism of the CuO/MoS2hybrid was also studied according to the radical-trapping experiments and band structures.
2.Experimental
2.1.Materials
Ammonium molybdate tetrahydrate (H24MO7N6O24·4H2O) and isopropyl alcohol (IPA) were purchased from Aladdin Chemistry Co.Ltd (China).Ethylene diamine tetraacetic acid (EDTA), methyl violet and ascorbic acid(AA)were obtained from Shanghai Macklin Biochemical Co.Ltd (China).Copper sulfate pentahydrate(CuSO4·5H2O), thiourea (CS(NH2)2, sodium hydroxide (NaOH),methyl orange, cetyltrimethylammonium bromide (CTAB) and absolute ethanol were purchased from Tianjin Chemical Reagent Co.Ltd(China).Rhodamine B and methyl blue were obtained from National Chemical Reagent Co.Ltd (China).All analytical grade chemicals were used without further treatment.
2.2.Synthesis of CuO material
CuO nanorods were synthesized by a hydrothermal route with improved method[15].Typically,25 mmol CuSO4·5H2O was ultrasonically dissolved in 30 ml of distilled water for 30 min and 0.82 mmol CTAB was added to the above solution under magnetic stirring.Subsequently, 10 ml of NaOH solution (1 mol·L-1) was added dropwise with continuously stirred for 1 h till the precipitate formed.Then,the solution was transferred into a 100 ml autoclave and heated at 160 °C for 11 h.After that, it was allowed to cool at ambient temperature.Finally, the sample was respectively washed with distilled water and absolute ethanol before being dried at 60 °C overnight and further calcined for 4 h at 400 °C.
2.3.Preparation of MoS2 material
MoS2was prepared by a hydrothermal method with improved method[16].In a typical process,18.7 mmol of ammonium molybdate tetrahydrate and 45.9 mmol of thiourea were initially dissolved in 70 ml of distilled water.Then, the solution was stirred for 60 min to obtain a homogeneity.Thereafter,the above solution was transferred to a 100 ml Teflon-lined autoclave and hydrothermally treated at 180°C for 20 h.The obtained product was washed three times by a centrifuge using deionized water and absolute ethanol, respectively.Finally, the sample was dried at 60 °C overnight.
2.4.Fabrication of CuO/MoS2 composite
Typically, 12.5 mmol of as-obtained CuO and a certain amount of as-prepared MoS2(0.62, 1.24, 1.86 and 2.48 mmol) were dissolved in 30 ml of deionized water, respectively.The above mixture was stirred for 1 h at room temperature and then washed with distilled water and absolute ethanol, respectively.The above samples were dried at 60°C overnight.The obtained products were named as CuO/MoS2-1, CuO/MoS2-2, CuO/MoS2-3 and CuO/MoS2-4, corresponding to 0.62, 1.24, 1.86 and 2.48 mmol of MoS2,respectively.
2.5.Characterization
The phase composition and crystal structure of the sample were investigated by an XRD diffractometer(German Bluker D8),operating with Cu Karadiation as an X-ray source.The morphology was detected by scanning electron microscopy equipped with EDX(SEM,Hitachi S-4800,Japan)and transmission electron microscopy with an accelerating voltage of 200 kV(TEM,Hitachi H-800,Japan).The composition and surface chemical valence were observed by X-ray photoelectron spectroscopy (XPS, ESCALAB 250 Xi, USA).The BET surface area and pore size were studied by N2adsorption-desorption isotherms at 77 K (Micromeritics, Tristars Ⅱ3020).UV-Vis diffuse reflectance spectroscopy (UV-1800) was used to investigate the optical property.The separation ability of electron-hole pairs was studied by photoluminescence(PL)spectra with a fluorescence spectrometer (Hitachi F-4700, Japan) with an excitation wavelength of 300 nm.Photocurrent spectroscopy and electrochemical impedance spectra (EIS) were carried out on an electrochemical workstation (VersaSTAT-3, Ametek Princeton, U.S.A)with a three-electrode chemical system in 0.5 mol·L-1Na2SO4solution, including a saturated calomel electrode (SCE), platinum wire and glassy carbon electrode(GCE)loaded with 2 mg of photocatalyst,which are served as the reference electrode,counter electrode and working electrode, respectively.A 300 W xenon lamp was used as a light source.
2.6.Photocatalytic measurements
The photocatalytic performance of the as-synthesized sample was determined by degradation of multi-organic dyes under visible-light irradiation by a 300 W Xe lamp with a filter plate(λ >420 nm).Typically,100 mg of the photocatalyst was dissolved in a 100 ml dye solution with a concentration of 15 mg·L-1.Before the reaction, the solution was stirred in the dark for 30 min to attain the adsorption-desorption equilibrium and then irradiated with visible light to initiate the photocatalytic reaction under continuous stirring.Then, 4 ml of suspension from the above mixture was taken out,and the catalyst was separated from the solution by using a 0.22 μm microporous membrane filter at 15 min intervals.The dye concentration was investigated by a UV-Vis spectrophotometer (U-T6PC).Furthermore, the stability of the photocatalyst was tested under the same conditions, and the catalyst was centrifuged, dried and reused three times.The degradation rate was calculated according to the following Eq.(1) [17]
where C0is the dye concentration at time 0 and Ctis the concentration at time t.
3.Results and Discussion
3.1.Characterization analysis
The morphologies of the as-prepared samples are analyzed by SEM technique.As depicted in Fig.1(a), the pure CuO possesses nanorods-like morphology with a length of ca.1.5 μm.The MoS2exhibits the microflower-like structures that were self-assembled from ultrathin nanosheets (Fig.1(b)).Moreover, the CuO/MoS2-3 sample is comprised of irregular and smooth nanoparticles with the size of less than 50 nm (Fig.1(c)).These facts indicate that the introduction of MoS2destroys the morphology and structure of CuO.The EDS analysis was performed to identify the elemental components of the CuO/MoS2-3 photocatalyst.The EDS mappings suggest that Cu, O, Mo and S elements are uniformly dispersed in the CuO/MoS2-3 sample (Fig.1(d)), proving that the CuO/MoS2is successfully prepared.
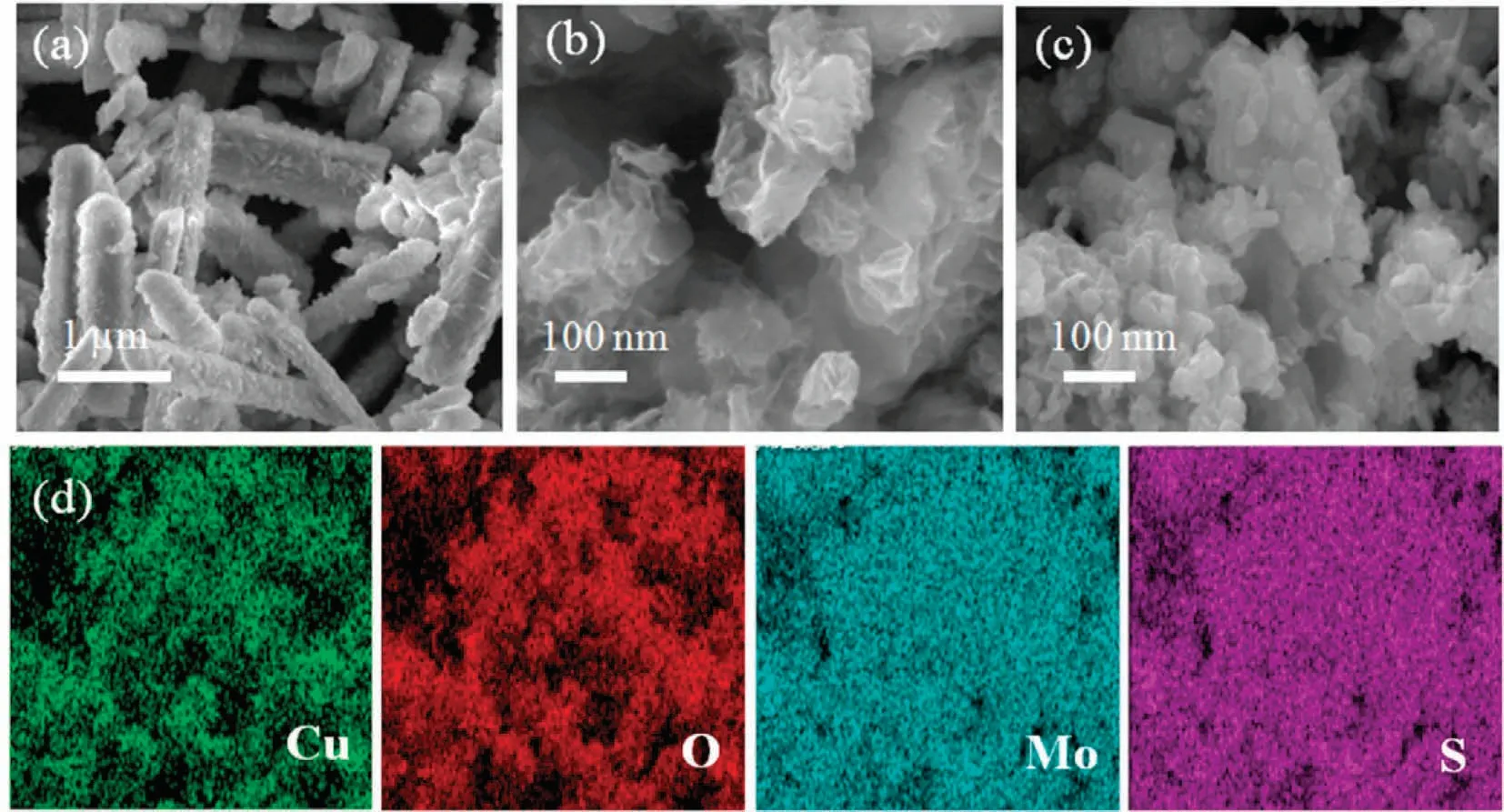
Fig.1.SEM images of (a) CuO, (b) MoS2, (c) CuO/MoS2-3 and (d) elemental mappings of CuO/MoS2-3.
The composition and the crystallinity of the as-prepared samples were investigated by XRD technique(Fig.2).It is observed that the diffraction peaks at 33.7°, 35.3°, 38.6°, 46.2°, 49.4° and 52.7°correspond to the characteristic reflections of (1 1 0), (0 0 2), (2 0 0),(-1 1 2),(-2 0 2)and(0 2 0)planes of monoclinic CuO structure(JCPDS No.80-0076).Additionally, the weak peaks appeared at 14.2°, 32.4° and 57.9° are attributed to the (0 0 2), (1 0 0) and (1 1 0) planes of the hexagonal phase of MoS2(JCPDS No.77-1716)(Fig.S1 in Supplementary Material)[13].The existence of no other peaks suggests that the CuO/MoS2samples were successfully fabricated without impurity.In addition,the peak intensities increase as changing the amount of MoS2in all the samples,confirming that addition of MoS2influences the crystalline and growth of CuO.
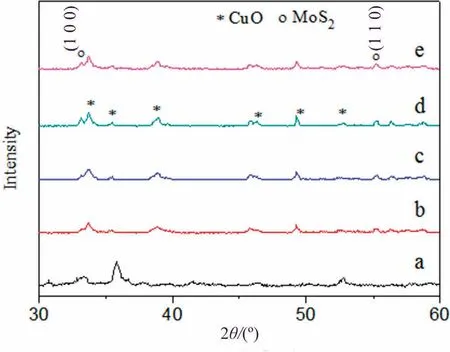
Fig.2.XRD patterns of (a) CuO, (b) CuO/MoS2-1, (c) CuO/MoS2-2, (d) CuO/MoS2-3 and (e) CuO/MoS2-4.
The microstructures and crystal planes of the CuO/MoS2-3 are analyzed by TEM and high-resolution TEM (HRTEM).As viewed in Fig.3(a), the CuO/MoS2-3 sample is composed of the nanorods intersected nanosheet structure.Magnified image suggests that the length of nanorods is about 50 nm and CuO nanorod is located between nanosheets of MoS2(Fig.3(b), 3(c)).The orange region(Fig.3(c)) is further enlarged and shown in Fig.3(d), and the heterojunction interface and lattice fringes of CuO and MoS2can be clearly observed.The spacings of 0.25 nm and 0.62 nm correspond to the (0 0 2) monoclinic phase of CuO [18] and the (0 0 2) plane of MoS2[14], respectively (Fig.3(d)).

Fig.3.(a), (b), (c) TEM and (d) HRTEM images of the CuO/MoS2-3 material.
The nitrogen adsorption-desorption curves were performed to analyze the Brunauer-Emmett-Teller (BET) surface area and pore size distribution.Obviously, the typical IV-type isotherms and a H3 hysteresis ring in the P/P0range of 0.9-1.0 can be observed for the three samples (Fig.4(a)).The specific surface areas of the CuO,MoS2and CuO/MoS2-3 are 1.93,5.22 and 6.06 m2·g-1,respectively.That is to say, the CuO/MoS2-3 exhibits a slight increase in the BET surface area compared with CuO and MoS2.Furthermore,the pore size distributions estimated using BJH method show that the pore sizes of CuO,MoS2and CuO/MoS2-3 are ca.7.88,17.13 and 19.98 nm, respectively (Fig.4(b)).Increased specific surface area may provide more active sites, hence, enhancing photocatalytic performance of the photocatalyst [19].
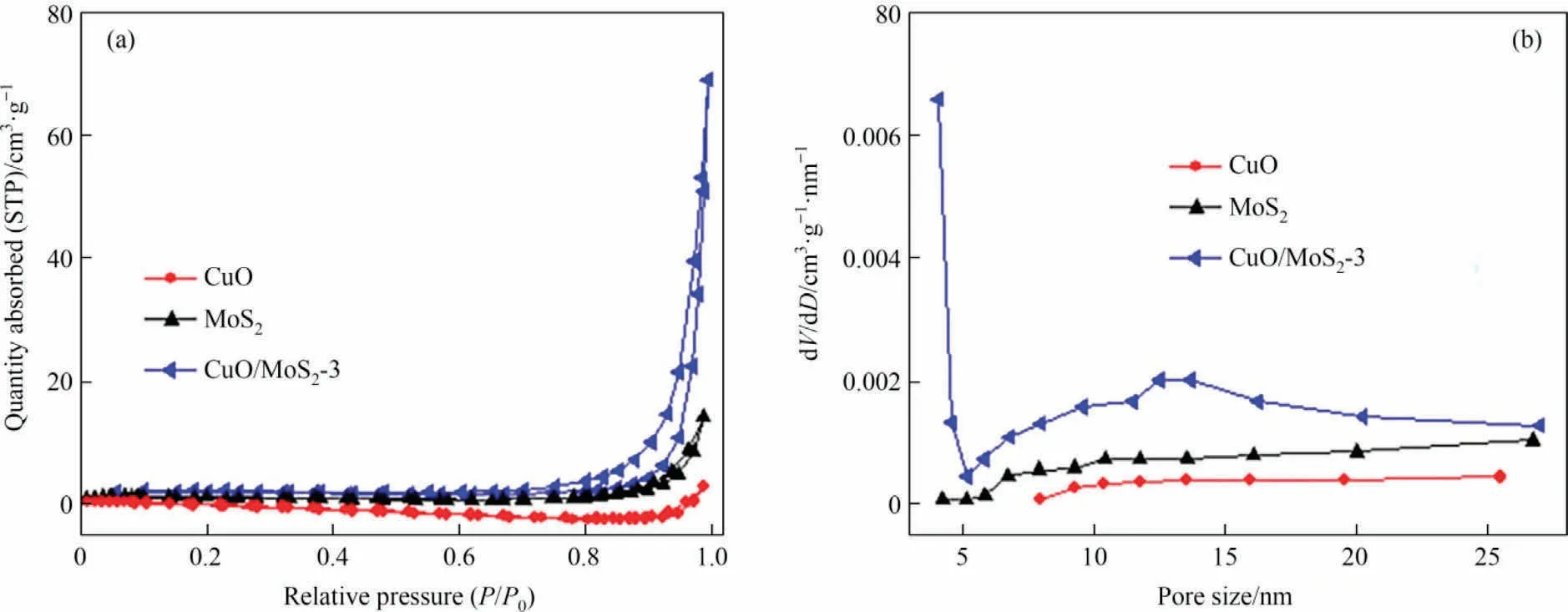
Fig.4.(a) N2 adsorption-desorption isotherms and (b) pore size distributions of the CuO, MoS2 and CuO/MoS2-3.
The element compositions and chemical states of the CuO/MoS2-3 composite were analyzed by the XPS technique.Fig.S2 displays the survey spectrum peaks of Mo, S, Cu, O and C, verifying that the CuO/MoS2composite was successfully prepared.The C 1s peak, used as a calibration reference, has binding energy at 284.6 eV [20].The high-resolution XPS spectrum shows that the two bands at 232.3 and 235.5 eV belong to Mo 3d5/2and Mo 3d3/2, respectively (Fig.5(a)) [21].For S 2p (Fig.5(b)), the peaks at 168.4 and 169.7 eV are assigned to S 2p3/2and S 2p1/2, respectively [22].The binding energies located at 933.4 and 953.8 eV can be indexed to Cu 2p3/2and Cu 2p1/2,respectively,and the satellite peak energies of 941.4, 943.9 and 962.6 eV confirm the presence of Cu2+inCuO/MoS2-3 (Fig.5(c)) [23].The binding energy around 531.2 eV indicates the presence of oxygen (Fig.5(d)) [24].The above results confirm the successful preparation of the CuO/MoS2composite.
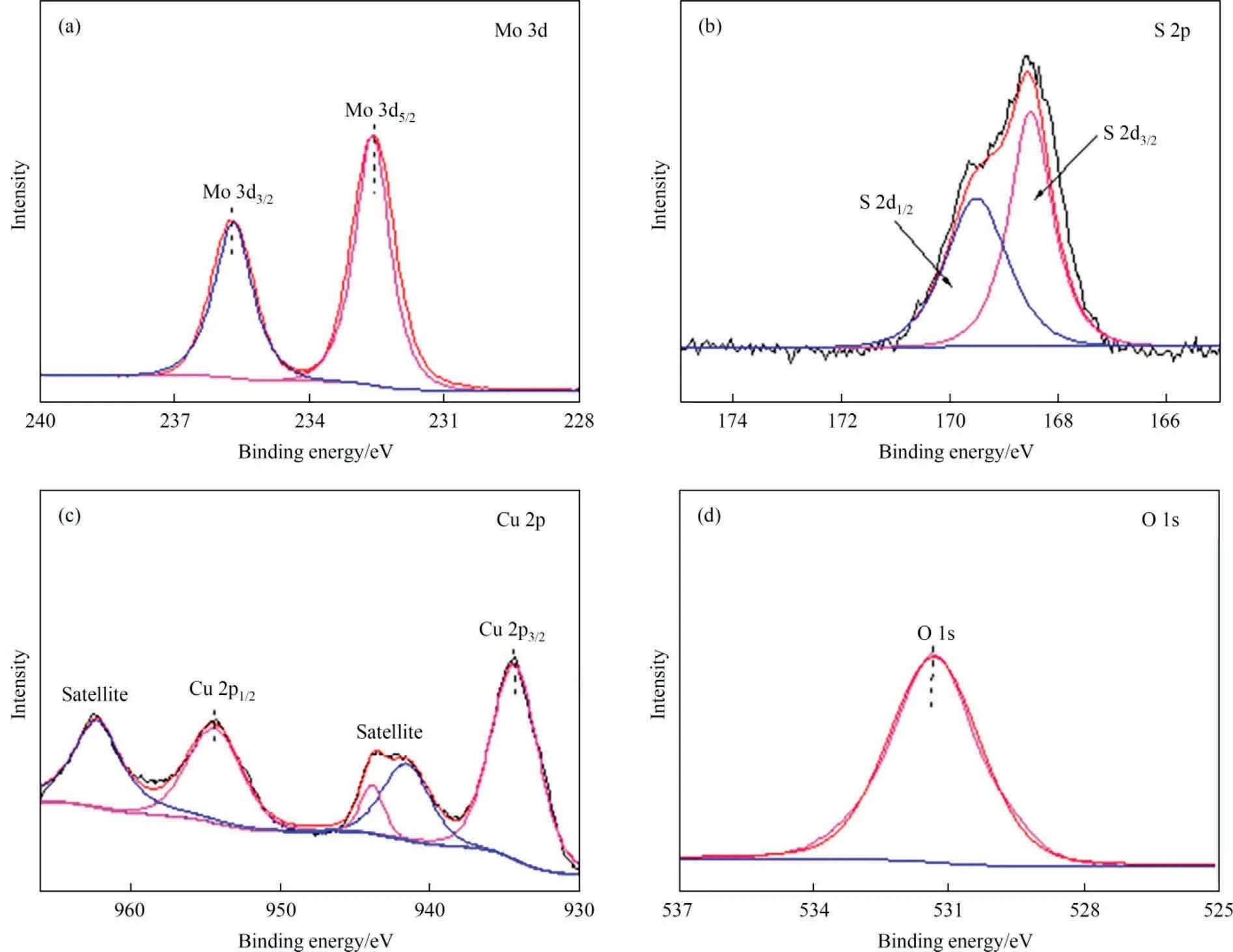
Fig.5.XPS spectra of (a) Mo 3d, (b)S 2p, (c) Cu 2p,(d) O 1s for the CuO/MoS2-3 sample.
The optical absorption of the as-prepared samples was determined by UV-Vis spectra and the results are shown in Fig.6(a).It can be found that the adsorption band edge of the CuO/MoS2-3 was red shifted after adding MoS2, indicating visible light adsorption improved.In addition, the bandgap energy of semiconductors can be calculated according to the Eq.(2) [1]:
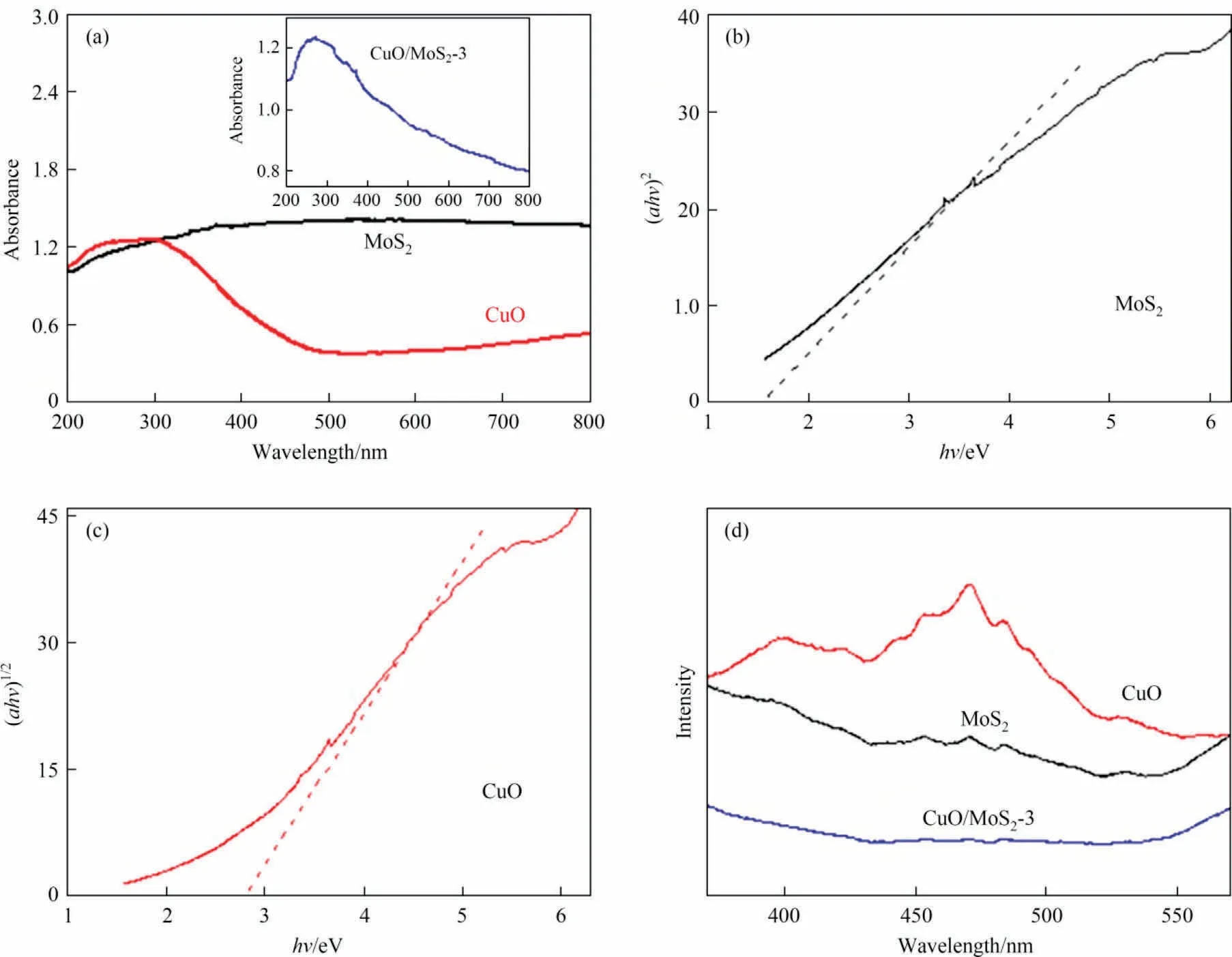
Fig.6.(a) UV-Vis DRS spectra of CuO and MoS2 (inset: CuO/MoS2-3), (b), (c) the bandgaps energy of MoS2 and CuO and (d) photoluminescence spectra.
where α, h,ν, A, and Egrepresent the absorption coefficient, Planck constant, light frequency, proportionality constant and bandgap energy, respectively.The letter n is type of electronic transition,and n is equal to 4 for direct bandgap semiconductors and 1 for indirect bandgap semiconductors.Hence, the value of 1 is for CuO [9]and 4 for MoS2[14].The bandgaps are calculated to be 1.57 and 2.97 for the MoS2and CuO, respectively (Fig.6(b), (c)).Obviously,the introduction of MoS2can reduce the band gap, enhance the optical absorption ability and result in high photocatalytic activity as a result.
The separation efficiency of electron-hole pairs is analyzed by photoluminescence spectra (PL).As shown in Fig.6(d), the PL intensity of the CuO/MoS2-3 shows a noticeable decrease compared with pure CuO and MoS2, revealing that adding MoS2with a suitable amount can efficiently separate electron-hole pairs and frustrate photocarrier recombination,resulting in excellent photocatalytic activity.
To have better insight into the separation capacity of the lightgenerated electron-hole pairs, EIS Nyquist plots (Fig.7(a)) and the transient photocurrent response (Fig.7(b)) are further studied.Moreover, the CuO/MoS2-3 has the smaller arc radius compared to pure CuO,proving that it exhibits outstanding charge separation efficiency.Encouragingly, the CuO/MoS2-3 possesses slight larger photocurrent density than the pure CuO.As a result, the CuO/MoS2-3 has high charge separation efficiency due to the formation of heterojunction and the synergetic effect between CuO and MoS2,and more photogenerated carriers will involve in the photocatalytic degradation.

Fig.7.(a) Nyquist plots of the electrochemical impedance spectra (EIS) and (b) transient photocurrent response.
3.2.Photocatalytic activity
Photocatalytic activity of the as-prepared samples is investigated by degradation of methyl violet(MV)under visible light irradiation, as exhibited in Fig.8.The adsorption-desorption equilibrium was achieved for 30 min in the dark condition before irradiation.During the photocatalytic degradation process, selfdegradation of MV is difficult without introducing the photocatalyst.Moreover, the pure CuO shows a low degradation rate for MV with a degradation efficiency of only 28% and MoS2exhibits 69%removal efficiency within 30 min.More importantly,the doping of MoS2markedly enhances the photocatalytic activity and the degradation rates within 30 min decrease in the sequence: CuO/MoS2-3 (99.8%) >CuO/MoS2-4 (93.0%) >CuO/MoS2-2 (85.8%) >Cu O/MoS2-1(82.5%)(Fig.8(a),(b)).Notably,the CuO/MoS2-3 delivers the highest degradation rate, which is attributed to the enhanced visible light adsorption and rapid separation efficiency of photoexcited electron-holes.The result reveals that the suitable doped MoS2amount plays a crucial role in boosting photoactivity.As expected, the photodegradation property of the CuO/MoS2-3 is superior to that of the reported CuO and CuO-based materials(Table S1).Furthermore, the kinetics was studied using a quasifirst order kinetic model (3) [25]:
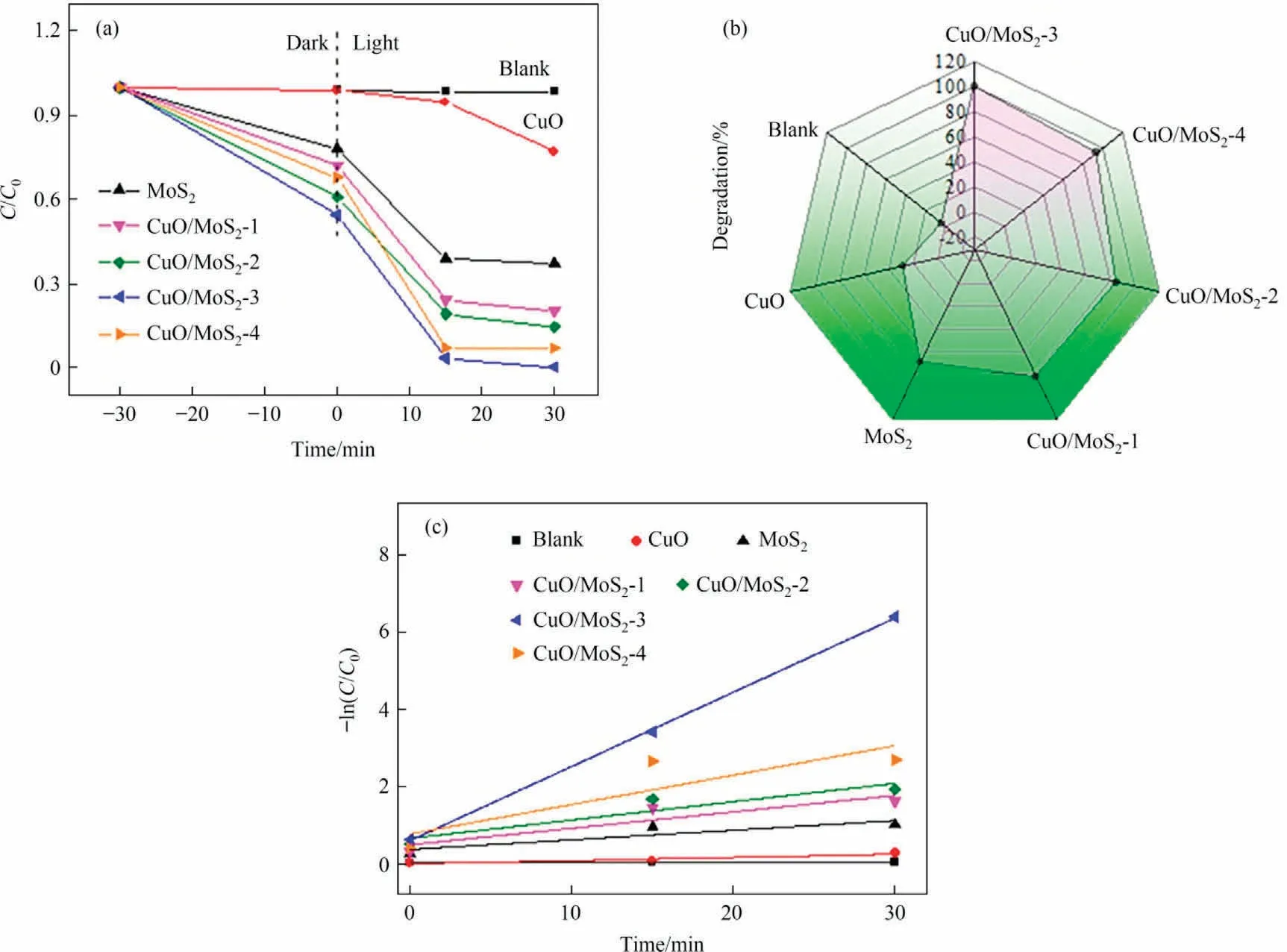
Fig.8.(a)Photocatalytic degradation of MV over the as-prepared photocatalysts under visible light,(b)photodegradation rate of MV after 30 min and(c)the rate constant k values for the as-prepared samples.
where C and C0represent the concentration of MV at time t and 0,respectively.k and t are the reaction rate constant and reaction time, respectively.The obtained rate constant k of CuO/MoS2-3 is 0.139 min-1, which is higher than that of the CuO/MoS2-4(0.047 min-1), CuO/MoS2-2 (0.031 min-1), CuO/MoS2-1(0.031 min-1), MoS2(0.019 min-1) and CuO (0.008 min-1), respectively(Fig.8(c)).This result confirms that the CuO/MoS2-3 composite can efficiently degrade MV organic dye.Seen from the solution photos (Fig.S3) after the degradation, the solution color gradually becomes colorless, suggesting that a suitable MoS2amount has a significant effect on enhancing the photodegradation performance of CuO.
To enlarge the application of the photocatalyst, degradation efficiencies of various organic pollutants (RhB, MB and MO) are also explored for the optimum CuO/MoS2-3 composite (Fig.9(a),(b).It can be found that degradation rates are 95.3%within 30 min for RhB,22.8%for MB and 31%for MO within 45 min,and reaction rate constants are 0.880 min-1, 0.0056 min-1and 0.00046 min-1for RhB,MB and MO,respectively(Fig.9(c)).These results confirm that the CuO/MoS2-3 material can be used as an effective photocatalyst for the photocatalytic degradation of various organic dyes,especially MV and RhB.
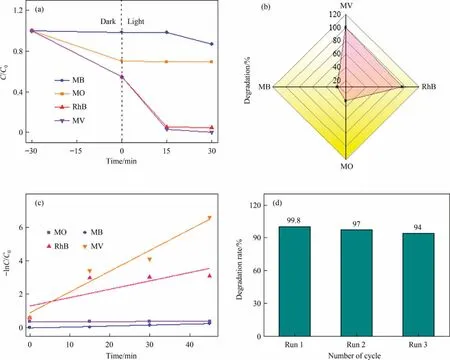
Fig.9.(a)Photocatalytic degradation of various dyes(15 mg·L-1)over the CuO/MoS2-3,(b)photodegradation rate after 30 min for RhB and MV and after 45 min for MO and MB under visible light and (c) the rate constant k values for degradation of different pollutants and (d) reusability of the CuO/MoS-3 for degradation of MV.
Given that reusability is an essential factor for practical application, the reusability of the CuO/MoS2-3 composite is evaluated for three cycles.After each reaction, the catalyst was washed, dried and then reused.As presented in Fig.9(d), the degradation efficiency shows no significant decrease, reaching 94% within 30 min after three successive cycles, indicating the excellent reusability of the CuO/MoS2-3 composite in photocatalytic reaction.Moreover, the XRD pattern of the CuO/MoS2-3 sample after three cycles was characterized (Fig.S4), and the used CuO/MoS2-3 still keeps the structure of pure CuO/MoS2-3, indicating the steady structure.The SEM image reveals that the CuO/MoS2-3 still maintains the same irregular nanoparticles morphology (Fig.S5).Therefore, the above results demonstrate the excellent reusability and high stability of CuO/MoS2-3 heterostructure.
3.3.Photocatalytic mechanism
Active species were investigated by using different scavengers including isopropyl alcohol (IPA), ascorbic acid (AA) and ethylene diamine tetraacetic acid (EDTA) for capturing ·OH, ·O-2and h+,respectively.As viewed in Fig.10, the degradation rate decreases from 99.8% to 63.0% and 58.0% after adding AA and EDTA, while there was no change in the photodegradation activity after adding IPA (Fig.10(a) and (b)).This result confirms that h+and ·O-2have essential roles for boosting the photocatalytic activity.However,·OH does not affect the photocatalytic property.To further understand the photocatalytic mechanism, XPS valence band (VB) spectrum was tested to investigate the valence band of the CuO/MoS2-3.From Fig.10(c), the VB positions (EVB) are determined to be 1.15 eV and -0.34 eV for CuO and MoS2, respectively.Moreover,the CB positions(ECB)of CuO and MoS2can be calculated by the following equation (4) [26]:
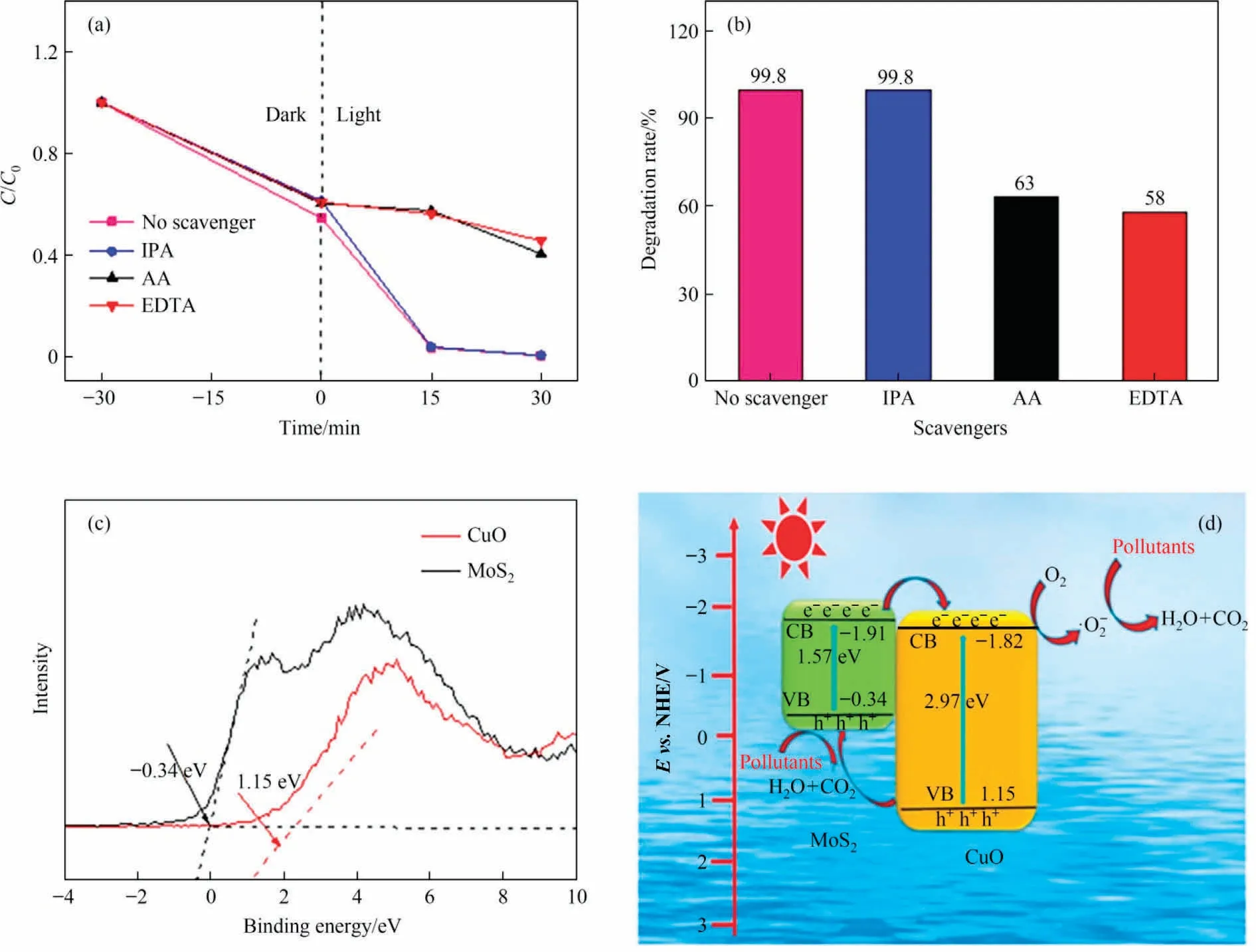
Fig.10.(a),(b)Effect of various scavengers on the degradation of MV,(c)XPS-VB spectra of as-obtained samples,(d)possible mechanism of photocatalytic degradation of MV over the CuO/MoS2-3 heterojunction.
where ECBand EVBare the conduction and valence band edge,Egrepresents the bandgap energy.According to the UV-Vis DRS results,the Egvalues of the CuO and MoS2are 2.97 eV and 1.57 eV,respectively.The obtained ECBof CuO is -1.82 eV, while ECBof MoS2is -1.91 eV.Given the above discussions,the photocatalytic degradation mechanism is depicted in Fig.10(d).Under the visible light irradiation,photogenerated electrons were transferred from CB of MoS2to CB of CuO.In contrast,holes were transferred from VB of CuO to VB of MoS2[21].The formation of the CuO/MoS2heterostructure noticeably boosts the charge separation efficacy, which enhances the photocatalytic performance.Moreover, CB of CuO is -1.82 eV is lower than the reduction potential of (O2/·O) (-0.33 eV/NHE) [1].As a result, the accumulated electrons in the CB of CuO can be scavenged by dissolved O2and converted into active ·O, while the EVBof MoS2is -0.34 eV is smaller than the standard reduction potential of H2O/·OH(2.38 eV/NHE) [5], which is not enough to generate ·OH, thus, h+can directly oxidize MV.The transfer process of photogenerated electron-hole pairs and photodegradation MV processes are summarized as following steps:
4.Conclusions
The CuO/MoS2heterostructure was successfully constructed and used for photodegradation of various organic dyes.The result suggests that the CuO/MoS2-3 can efficiently boost the photocatalytic property with the degradation rate of 99.8% for MV within 30 min under visible light and also exhibits high photoactivity for degradation of RhB dye (95.3% within 30 min).The improved photocatalytic activity is ascribed to the synergistic effect of MoS2and CuO in the CuO/MoS2composite resulting in improved visible light adsorption and rapid separation of light-induced electron-hole pairs.The proposed mechanism reveals that h+and·O2-become the main active species in the photocatalytic degradation of methyl violet.This work provides a hopeful strategy for designing high-performance photocatalysts for wastewater purification field.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the National Natural Science Foundation of China (51572185), Natural Science Foundation of Shanxi Province(202203021211158 and 20210302123173), and the Key Research and Developmen program of Shanxi Province (International Cooperation, 201903D421079) for the financial support.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.06.017.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Intrinsic kinetics of catalytic hydrogenation of 2-nitro-4-acetylamino anisole to 2-amino-4-acetylamino anisole over Raney nickel catalyst
- Experiments and model development of p-nitrochlorobenzene and naphthalene purification in a continuous tower melting crystallizer
- α-Synuclein: A fusion chaperone significantly boosting the enzymatic performance of PET hydrolase
- Influence of water vapor on the separation of volatile organic compound/nitrogen mixture by polydimethylsiloxane membrane
- Mass transfer mechanism and relationship of gas-liquid annular flow in a microfluidic cross-junction device
- Enhanced photocatalytic activity of methylene blue using heterojunction Ag@TiO2 nanocomposite: Mechanistic and optimization study
