Clinical efficacy of leprerelin acetate with different dosage forms in central precocious puberty girls
2023-02-23YANGMingmingTANGYongquanGAOQianWANGJieJIANGXueZHOUWendi1
YANG Ming-ming, TANG Yong-quan, GAO Qian, WANG Jie, JIANG Xue, ZHOU Wen-di1,✉
1. Department of Pediatrics, the Huai’an Clinical College of Xuzhou Medical University, Huai’an 223300, China
2. Department of Pediatrics, Binhai People's Hospital, Yancheng 224500, China
3. Department of Pediatrics, the Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University, Huai’an 223300, China
Keywords:
ABSTRACT Objective: To observe the clinical efficacy of different dosage forms of lepraline acetate (LA)in the treatment of girls with central precocious puberty (CPP).Methods: 72 CPP girls treated in the Department of Pediatrics of Huai'an First People's Hospital from February 2021 to August 2022 were included as subjects and divided into two groups: 3-month LA group (n=34)and 1-month LA group (n=38).Both group girls were treated for 6 months.Serum hormone levels, body mass index (BMI), bone age/chronological age (BA/CA) and pelvic color ultrasound were detected at 0 and 6 months after treatment, and the changes of various indexes were compared before and after treatment.Results: 1) There were no significant differences in baseline data between the two groups before treatment (P > 0.05).2) After 6 months of treatment, BA/CA decreased, growth rate slowed down, and predicted adult height increased in both groups (P < 0.05), but there were no significant differences between groups (P > 0.05).3)After 6 months of treatment, there waere no significant differences in luteinizing hormone (LH)inhibition ratio between the 3 month and 1 month dosage groups (P > 0.05).After treatment,the peak value of serum LH and FSH, estradiol level, uterine volume, bilateral ovarian volume,maximum follicle diameter and the number of follicles 4mm were significantly decreased in the two groups, but there were no significant differences between the two groups (P > 0.05).4)There were no significant differences in the levels of thyroid hormone, fasting blood glucose and triglyceride between the two groups before and after treatment (P > 0.05).Total cholesterol levels were increased after treatment (P < 0.05), but there was no significant difference between groups (P > 0.05).5) No serious adverse reactions occurred during the treatment of the two dosage forms of LA, but the 3-month dosage form of LA reduced the treatment cost and improved the treatment compliance.Conclusion: The short-term efficacy of 3-month LA in the treatment of CPP in girls is similar to that of 1-month LA.The 3-month dosage form LA is a safe, effective, and economical method for the treatment of CPP in girls.
1.Introduction
Central precocious puberty (CPP) is an endocrine disorder that is common in girls.It is mainly caused by the premature activation of the hypothalamic-pituitary-gonadal axis (HPGA), which leads to an abnormal increase in the level of sex hormones in children, the premature appearance of secondary sexual characteristics, and an abnormal acceleration of bone growth and development, resulting in premature epiphyseal closure, which affects the lifelong height of the children in adulthood and affects their psychological health.Gonadotropin-releasing hormone agonist (GnRHa) is the standard treatment for this condition.These drugs inhibit gonadotropin secretion through desensitisation and down-regulation of the GnRH receptor, leading to a reduction in gonadal steroid levels to pre-pubertal levels, which in turn reverses or stabilises pubertal development and contributes to growth normalisation.At present,there are 1-month, 3-month, 6-month and 12-month dosage forms in the international arena, and the 6-month and 12-month dosage forms are still in the clinical trial period in foreign countries, and the 1-month dosage form (3.75 mg) of LA and TA are most commonly used in China, while the 3-month form (11.25 mg) of LA is more commonly used in foreign countries, and its therapeutic efficacy is worthy of affirmation[1], Whereas, it is less applied in China and is exempted from entry, and large-scale clinical data have not yet been obtained[2].GnRHa in children: an update from the International Consortium, published in 2019, states that long-acting formulations are the trend[3].As CPP may require several years of treatment, there is an increasing need for the use of long-acting agents to improve treatment adherence and quality of life in children.Therefore, in this study, we used 3-month and 1-month long-acting extended-release dosage forms of LA to treat girls with CPP, respectively, to observe and compare the efficacy of the two dosage forms of LA in girls with CPP in China.This study provides further clinical evidence for the use of the 3-month long-acting form of LA in China.
2.Information and Methods
2.1 Research target
This study is a prospective study, and a total of 72 cases of girls with CPP who were diagnosed and treated in the Department of Paediatrics of Huai’an Clinical College of Xuzhou Medical University from February 2021 to August 2022 were included as the study subjects.The diagnosis of CPP was referred to the Consensus on Diagnosis and Treatment of Central Precocious Puberty[2].Inclusion criteria: ①Meet the diagnostic criteria of CPP; ②Age under 10 years old at the time of diagnosis, weight more than 20kg;③Have not received GnRHa or other drugs that affect the judgement of efficacy before consultation.Exclusion criteria: ①do not meet the diagnostic criteria of CPP; ②secondary precocious puberty caused by central nervous system disease, genital tumour, congenital hypothyroidism, etc.; ③combined with other chronic diseases, such as chronic kidney disease, asthma, epilepsy, haematological disease,etc.; ④poor adherence to the treatment or inability to complete the treatment in accordance with the doctor’s instructions due to other reasons.The study was conducted in accordance with the Declaration of Helsinki and the ethical standards set by the Ethics Committee of Huai’an Clinical College of Xuzhou Medical University (Ethics No.KY-2023-017-01).All children and their parents gave informed consent to the investigation programme.
2.2 Methods of drug administration
The study subjects were divided into two groups according to the dosage form: the 3-month dosage group and the 1-month dosage group.The 3-month dosage group received subcutaneous injections of LA microspheres (Zinatone, 11.25 mg, manufactured by Takeda Pharmaceutical Co., Ltd., Japan) once every 12 weeks,and the 1-month dosage group received subcutaneous injections of LA microspheres (Zinatone, 3.75 mg) once every 4 weeks.The intervention time of both groups was at least 6 months of continuous use, and the children were also instructed on diet, sleep and exercise.
2.3 Observation indicators and methods
All the enrolled children were collected general information by medical professionals, height, weight and sexual characteristics(pubic hair, external genitalia and breasts) were examined at 0, 3 and 6 months of treatment, and growth rate and body mass index(BMI) were calculated; at 0 and 6 months of treatment, GnRHa stimulation test was performed, Dabiga (treprostinil injection,2.5 μg/kg, maximum 100 μg, 0.1 mg aqueous injection, produced by Pfizer ) was injected subcutaneously on an empty stomach at 8:00 am.Blood was collected at 0, 30, 60, and 90 minutes after the injection to measure the serum luteinising hormone (LH) and follicle stimulating hormone (FSH) levels, serum estradiol (E2),insulin-like growth factor-1 (IGF-1), thyroid stimulating hormone(TSH), Free triiodothyronine (FT3), free thyroxine (FT4), fasting blood glucose (FBG), total cholesterol (TC), triglyceride (TG) at 0 minute were measured at the same time; Pelvic ultrasonography was performed to record the uterine diameter, uterine volume, bilateral ovarian volume, the number of follicles 4 mm and the diameter of the largest follicle; bone age (BA) was taken from the left wrist, and bone age was assessed by the Greulich-Pyle method, and the bone age index was calculated to the bone age/actual age(BA/CA), and calculated predicting adult height (PAH) based on Bay-ley-Pinneau method[4].Serum LH, FSH and E2 concentrations were measured by electrochemiluminescence immunoassay.Peak serum LH <4 IU/L after treatment was considered to inhibit the HPGA axis[5].
2.4 Statistical methods
SPSS 26.0 statistical software was used for data analysis.The quantitative data all conformed to the normal distribution, expressed as mean ± standard deviation(±s), and the comparison between the two groups was performed by t-test; the qualitative data were expressed as the number of cases (%), and the comparison was performed by χ2test.P < 0.05 was considered as the difference was statistically significant.
3.Results
3.1 Comparison of pre-treatment baseline data between the two groups
There was no significant difference between the baseline characteristics of the two groups of children before the start of treatment, including age, bone age, actual height, weight, BMI,genetic height, and pubertal stage.See Table 1.
Tab 1 Comparison of baseline data between the LA 3-month depot and 1-month depot groups (±s)
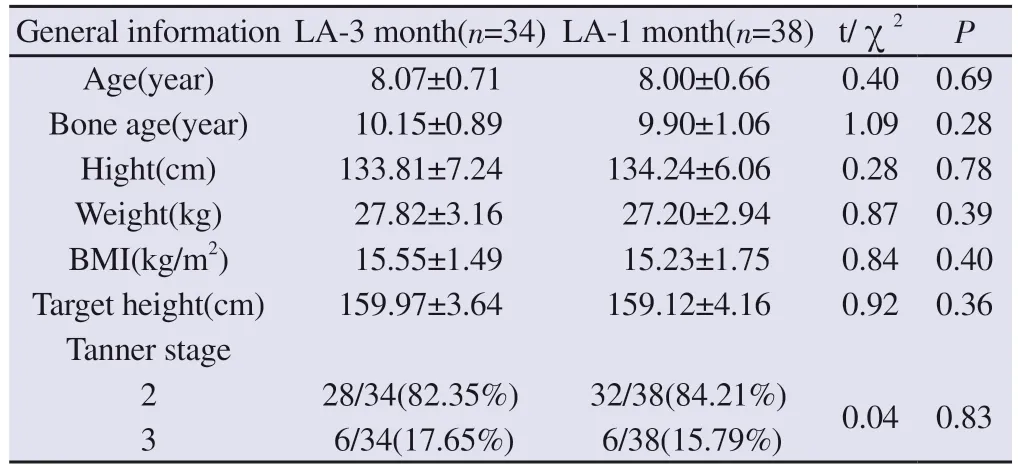
Tab 1 Comparison of baseline data between the LA 3-month depot and 1-month depot groups (±s)
General information LA-3 month(n=34) LA-1 month(n=38) t/χ2 P Age(year) 8.07±0.71 8.00±0.66 0.40 0.69 Bone age(year) 10.15±0.89 9.90±1.06 1.09 0.28 Hight(cm) 133.81±7.24 134.24±6.06 0.28 0.78 Weight(kg) 27.82±3.16 27.20±2.94 0.87 0.39 BMI(kg/m2) 15.55±1.49 15.23±1.75 0.84 0.40 Target height(cm) 159.97±3.64 159.12±4.16 0.92 0.36 Tanner stage 2 28/34(82.35%) 32/38(84.21%) 0.04 0.83 3 6/34(17.65%) 6/38(15.79%)
3.2 Comparison of growth indexes between the two groups of children before and after treatment
There was no significant difference in BA/CA, growth rate, PAH and BMI between the two groups of children before and after treatment.Compared with the pre-treatment, after 6 months of treatment, the BA/CA decreased, growth rate slowed down and PAH increased in both groups, and the difference was statistically significant (P < 0.05).There was a decrease tendency for IGF-1 levels before and after treatment, but the difference was not statistically significant.There was no significant change in BMI after treatment.It is suggested that the effects of the two dosage forms of LA on growth indicators were comparable.See Table 2.
Tab 2 Comparison of growth indexes between the two groups before and after treatment (±s)
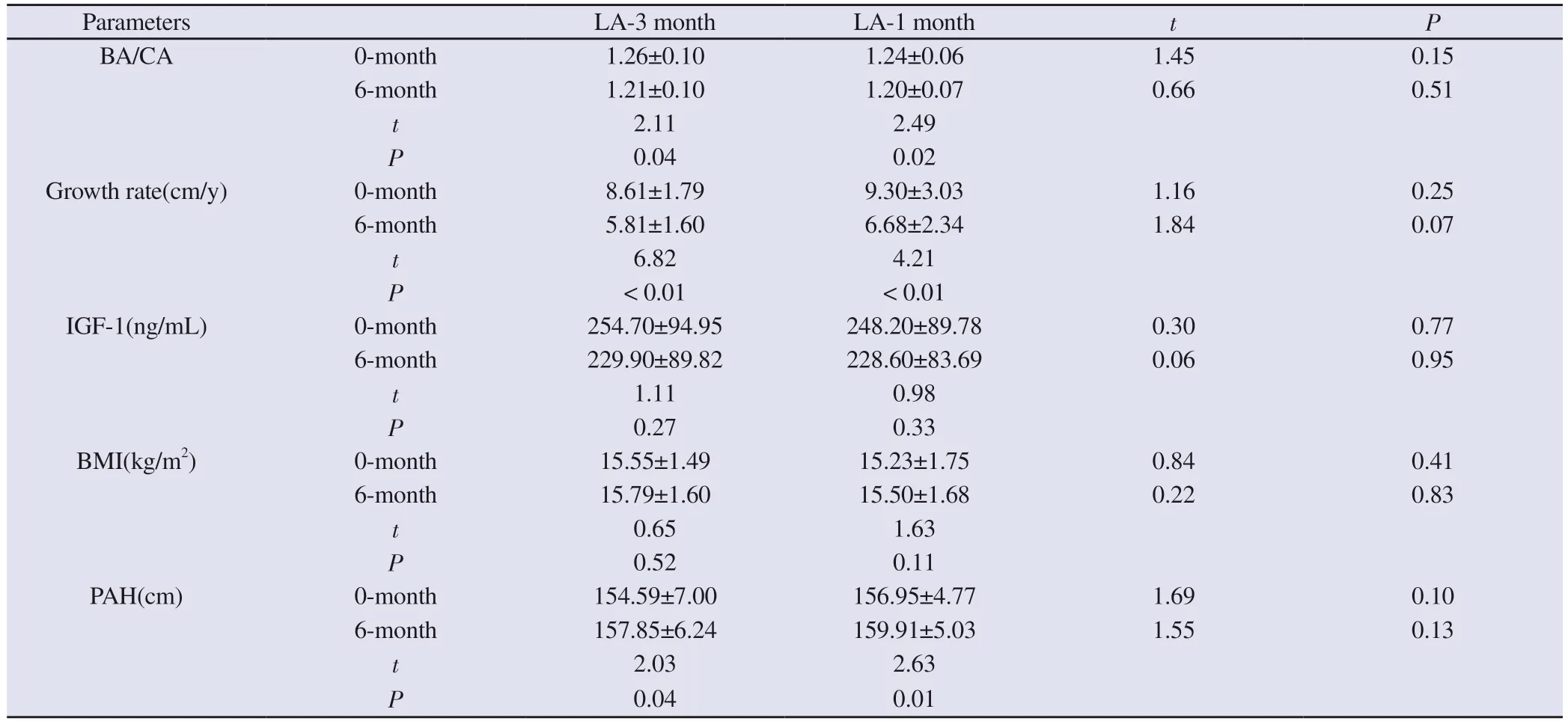
Tab 2 Comparison of growth indexes between the two groups before and after treatment (±s)
Parameters LA-3 month LA-1 month t P BA/CA 0-month 1.26±0.10 1.24±0.06 1.45 0.15 6-month 1.21±0.10 1.20±0.07 0.66 0.51 t 2.11 2.49 P 0.04 0.02 Growth rate(cm/y) 0-month 8.61±1.79 9.30±3.03 1.16 0.25 6-month 5.81±1.60 6.68±2.34 1.84 0.07 t 6.82 4.21 P< 0.01 < 0.01 IGF-1(ng/mL) 0-month 254.70±94.95 248.20±89.78 0.30 0.77 6-month 229.90±89.82 228.60±83.69 0.06 0.95 t 1.11 0.98 P 0.27 0.33 BMI(kg/m2) 0-month 15.55±1.49 15.23±1.75 0.84 0.41 6-month 15.79±1.60 15.50±1.68 0.22 0.83 t 0.65 1.63 P 0.52 0.11 PAH(cm) 0-month 154.59±7.00 156.95±4.77 1.69 0.10 6-month 157.85±6.24 159.91±5.03 1.55 0.13 t 2.03 2.63 P 0.04 0.01
3.3 Comparison of hormone levels and HPGA axis inhibition before and after treatment
There was no significant difference in the peak value of serum LH and FSH, E2 levels between the two groups of children before and after treatment.Compared with the pre-treatment, the peak value of serum LH and FSH, E2 levels of the children in both groups were significantly reduced after 6 months of treatment (P < 0.05).After 6 months of treatment, 32/34 ((94%) children in the LA 3-month dosage group had their LH responses adequately suppressed (peak LH < 4 IU/L), whereas 35/38 (92%) in the 1-month dosage group were suppressed, and there was no significant difference between the two groups (χ2= 0.11, P = 0.74), both of which suggested that the HPGA axis was effectively suppressed.In addition, the Tanner staging before the start of treatment was 2 or 3 in both groups and was evenly distributed.After 6 months of treatment, there was no progression of Tanner stage in both groups.The above results indicate that the two types of LA are equally effective in suppressing the HPGA axis and sex hormone levels in children.See Table 3.
3.4 Comparison of various parameters of pelvic ultrasound before and after treatment in the two groups
Compared with the pre-treatment period, the uterine diameter,uterine volume, bilateral ovarian volume, maximum follicle diameter and the number of follicles 4mm were significantly reduced in the two groups after 6 months of treatment (P < 0.05).And there was no significant difference in the comparison between the two groups,indicating that the gonadal suppression effect of the two dosage forms of LA was comparable.See Table 4.
Tab 3 Comparison of serum peak LH, peak FSH, and E2 levels between the two groups before and after treatment (±s)
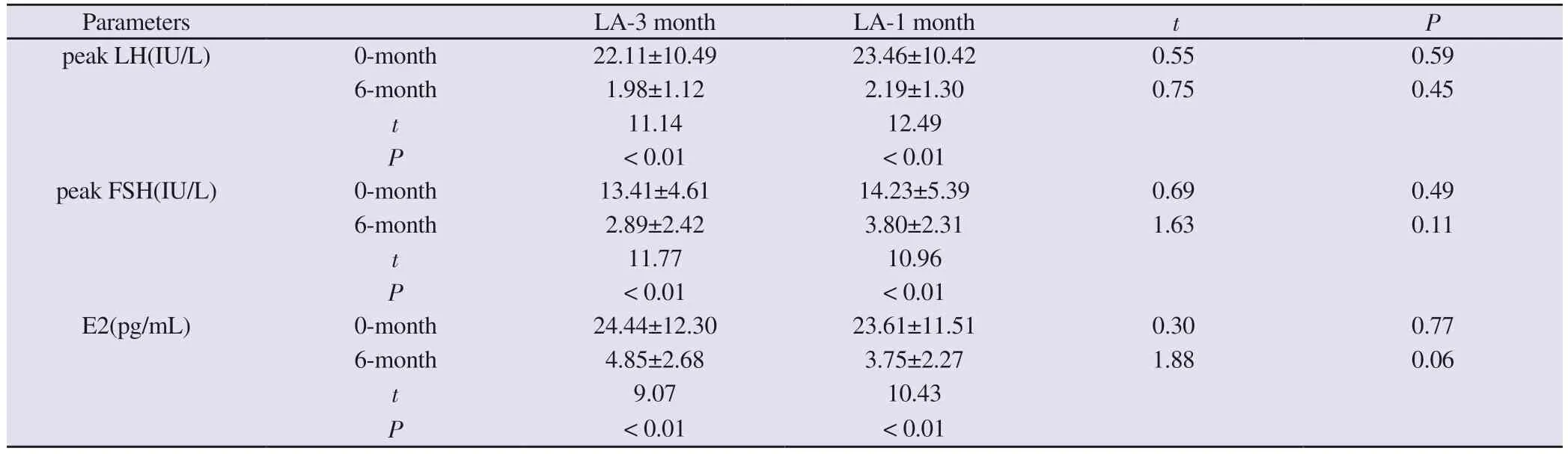
Tab 3 Comparison of serum peak LH, peak FSH, and E2 levels between the two groups before and after treatment (±s)
Parameters LA-3 month LA-1 month t P peak LH(IU/L) 0-month 22.11±10.49 23.46±10.42 0.55 0.59 6-month 1.98±1.12 2.19±1.30 0.75 0.45 t 11.14 12.49 P < 0.01 < 0.01 peak FSH(IU/L) 0-month 13.41±4.61 14.23±5.39 0.69 0.49 6-month 2.89±2.42 3.80±2.31 1.63 0.11 t 11.77 10.96 P < 0.01 < 0.01 E2(pg/mL) 0-month 24.44±12.30 23.61±11.51 0.30 0.77 6-month 4.85±2.68 3.75±2.27 1.88 0.06 t 9.07 10.43 P < 0.01 < 0.01
Tab 4 Comparison of parameters of pelvic ultrasound between the two groups before and after treatment (±s)
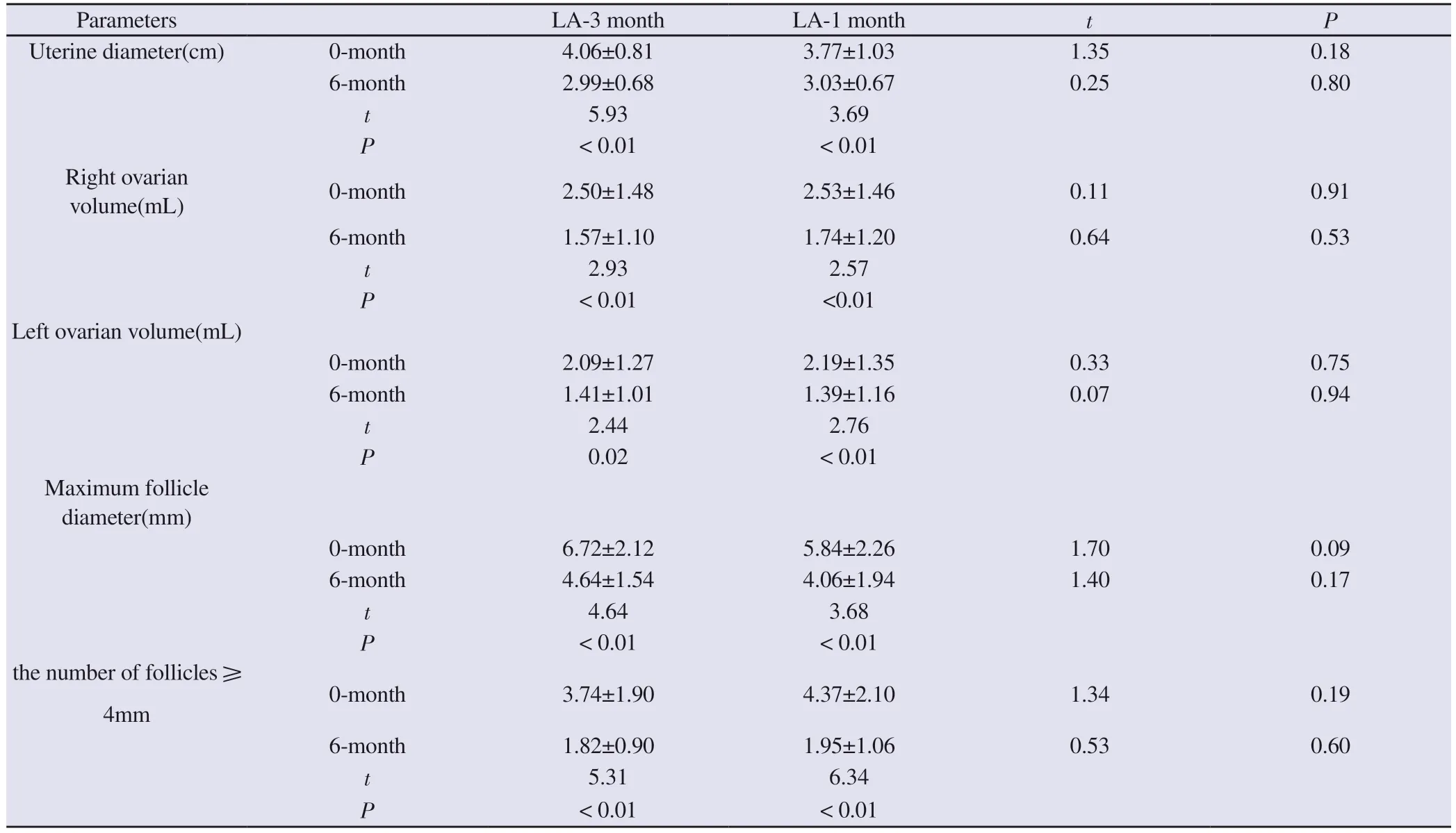
Tab 4 Comparison of parameters of pelvic ultrasound between the two groups before and after treatment (±s)
Parameters LA-3 month LA-1 month t P Uterine diameter(cm) 0-month 4.06±0.81 3.77±1.03 1.35 0.18 6-month 2.99±0.68 3.03±0.67 0.25 0.80 t 5.93 3.69 P < 0.01 < 0.01 Right ovarian volume(mL) 0-month 2.50±1.48 2.53±1.46 0.11 0.91 6-month 1.57±1.10 1.74±1.20 0.64 0.53 t 2.93 2.57 P < 0.01 <0.01 Left ovarian volume(mL)0-month 2.09±1.27 2.19±1.35 0.33 0.75 6-month 1.41±1.01 1.39±1.16 0.07 0.94 t 2.44 2.76 P 0.02 < 0.01 Maximum follicle diameter(mm)0-month 6.72±2.12 5.84±2.26 1.70 0.09 6-month 4.64±1.54 4.06±1.94 1.40 0.17 t 4.64 3.68 P < 0.01 < 0.01 the number of follicles 4mm 0-month 3.74±1.90 4.37±2.10 1.34 0.19 6-month 1.82±0.90 1.95±1.06 0.53 0.60 t 5.31 6.34 P < 0.01 < 0.01
3.5 Changes in metabolic indexes before and after treatment in the two groups
There was no significant difference in TSH, FT3, FT4, FBG and TG between the two groups before and after treatment.After treatment, both the 3-month dosage group (P = 0.04) and the 1-month dosage group (P = 0.04) showed a significant increase in blood TC, but there was no significant difference in the comparison between the two groups.See Table 5.
3.6 Adverse reactions
During the 6-month treatment, one case of transient haematuria in the 3-month dosage group disappeared on its own without special treatment; one case of headache in the 1-month dosage group was relieved on its own without medication, and neither of them affected the course of LA treatment.The remaining children did not see the occurrence of significant adverse reactions, the total incidence of adverse reactions was 2.78% (2/72).
3.7 Comparison of therapeutic economics and adherence
Compared with the 1-month dosage form of LA, the use of the 3-month dosage form of LA can reduce the number of patients’visits to the clinic.Calculated on the basis of half a year of treatment,the number of visits to the clinic has been reduced from one visit permonth to one visit per month in every three months, with a total reduction of four visits, which reduces the patient’s direct medical costs, including the registration fee and the injection fee, etc, and reduces the non-medical costs, including the round-trip travelling fee and the cost of the parents’ lost time in accompanying the patients.Indirect costs are reduced, lowering the cost of treatment.Moreover,the March dose of LA can reduce the number and frequency of injections for children, which improves children’s adherence to treatment.
Tab 5 Comparison of metabolic indexes between the two groups before and after treatment (±s)
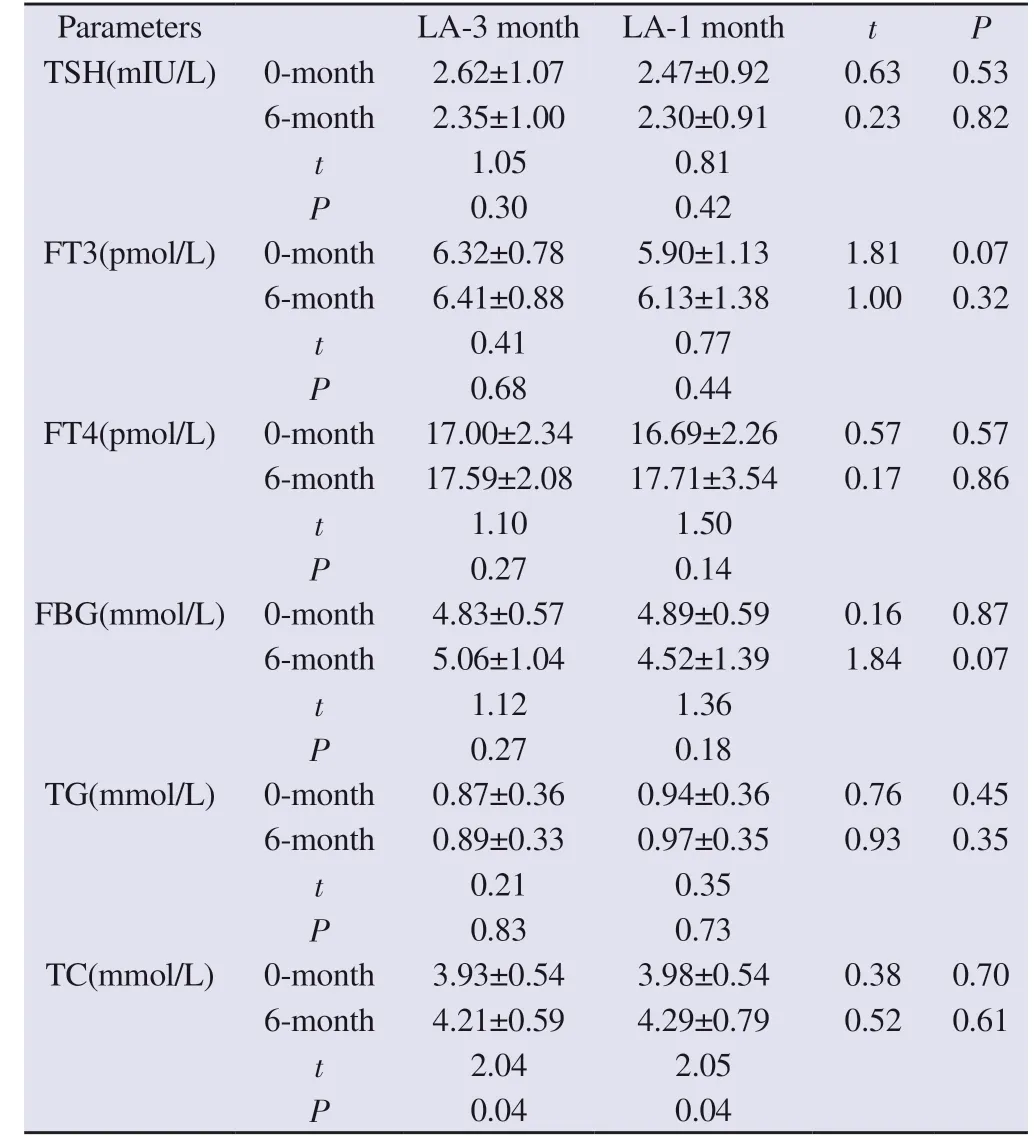
Tab 5 Comparison of metabolic indexes between the two groups before and after treatment (±s)
Parameters LA-3 month LA-1 month t P TSH(mIU/L) 0-month 2.62±1.07 2.47±0.92 0.63 0.53 6-month 2.35±1.00 2.30±0.91 0.23 0.82 t 1.05 0.81 P 0.30 0.42 FT3(pmol/L) 0-month 6.32±0.78 5.90±1.13 1.81 0.07 6-month 6.41±0.88 6.13±1.38 1.00 0.32 t 0.41 0.77 P 0.68 0.44 FT4(pmol/L) 0-month 17.00±2.34 16.69±2.26 0.57 0.57 6-month 17.59±2.08 17.71±3.54 0.17 0.86 t 1.10 1.50 P 0.27 0.14 FBG(mmol/L) 0-month 4.83±0.57 4.89±0.59 0.16 0.87 6-month 5.06±1.04 4.52±1.39 1.84 0.07 t 1.12 1.36 P 0.27 0.18 TG(mmol/L) 0-month 0.87±0.36 0.94±0.36 0.76 0.45 6-month 0.89±0.33 0.97±0.35 0.93 0.35 t 0.21 0.35 P 0.83 0.73 TC(mmol/L) 0-month 3.93±0.54 3.98±0.54 0.38 0.70 6-month 4.21±0.59 4.29±0.79 0.52 0.61 t 2.04 2.05 P 0.04 0.04
4.Discussion
In recent years, with the rapid economic development, improvement of material conditions and changes in dietary habits, the incidence of CPP has been increasing year by year worldwide, and has received widespread attention from the whole society[6, 7].GnRHa has been widely used in the treatment of CPP because of its high safety and efficacy, and so far, the mainstream treatment choice in China is still the 1-month GnRHa preparation[2].However, considering that the treatment course for children with CPP generally needs to last for at least 2 years, the use of a 3-month long-acting GnRHa preparation is expected to improve patient compliance.LA microspheres of 11.25 mg used once every 3 months have been approved by the U.S.Food and Drug Administration for the treatment of CPP in 2011,and was entered China in 2020, which is the first 3-month dosage form approved for the treatment of CPP in China.Its effectiveness and safety have been widely recognised abroad[8], but the ethnicity,diet and lifestyle of our country differ greatly from those of foreign children, and its efficacy needs to be further confirmed.At present,few studies have been reported in China comparing the efficacy of the 3-month dose (11.25 mg) and the 1-month dose (3.75 mg)of GnRHa preparation in the treatment of CPP.In this study, we observed the clinical efficacy of the 3-month dosage form of LA versus the 1-month dosage form of LA in the treatment of girls with CPP in China.The results showed that the efficacy of LA 3-month dosage form and 1-month dosage form was comparable in inhibiting the HPGA axis and bone maturation in girls with CPP, which was consistent with the results of previous studies[9].
The results of this study showed that after 6 months of treatment,peak LH and peak FSH decreased significantly in both groups of children with CPP, and serum E2 levels decreased about 5-6-fold after treatment compared with those at baseline.There is no uniform criterion to determine that LH is suppressed after treatment (peak LH after GnRHa injection), and in our study, we used LH <4 IU/L as a reference[5], and 94% and 92% of the children in the 3-month dosage group of LA and the 1-month dosage group of LA, respectively, were adequately suppressed by the end of the 6-month treatment period.This suggests that the 3-month dosage form of LA is as effective as the 1-month dosage form in suppressing sex hormone levels in children with CPP.The uterine diameter, uterine volume, ovarian volume, maximum follicular diameter and the number of follicles 4 mm were significantly lower in both groups of children compared to the pre-treatment period, further confirming that the two dosage forms of LA had comparable inhibitory effects on the HPGA axis.
In this study, the growth indexes of the children before and after treatment were compared and analysed, and the results showed that compared with the pre-treatment period, both groups of children showed a decrease in BA/CA and growth rate, and a rise in PAH after 6 months of treatment, which gradually converged to the genetic target height.This indicates that the efficacy of the 3-month and 1-month dosage forms of LA is comparable, both of which can inhibit the rapid progression of BA and promote the children’s growth and development to return to the normal developmental process.The baseline age of the children in this study was 8 years old, and although the growth rate slowed down after treatment,there was still a height benefit due to the rise in PAH as the sex hormone action was suppressed and the consequent loss of height was avoided.However, there are inconsistent findings as to whether height benefit can be achieved in children with precocious puberty treated with GnRHa after 8 years of age.Some studies have shown that GnRHa treatment after 8.3 years of age may not result in final height benefit[10], while others have shown that treatment after 8 years of age may improve the final height of girls with CPP[11, 12].
The association between GnRHa treatment and weight gain and obesity has been controversial.Some studies have shown that GnRHa treatment can cause an increase in BMI[13-16].Some other studies have shown that GnRHa treatment does not affect or even reduces BMI[8, 17].In this study, we showed that both the 3-month dose and 1-month dose treatment of LA did not significantly change the BMI of children.In addition, we observed the changes in thyroid function and glucose-lipid metabolism indexes of children before and after LA treatment, and the results showed that FT3 and FT4 tended to increase and TSH tended to decrease in the two groups after treatment, but the differences were not statistically significant,which indicated that both dosage forms of LA treatment did not affect children’s thyroid function, which is in line with the reports of previous studies[18].In addition, there was no significant difference in FBG and TG levels before and after treatment.The blood TC levels of the children in both groups were significantly higher than before treatment, but still within the normal range, which may be related to the disorders of hepatic lipid metabolism caused by the decrease in oestrogen levels after treatment[19].
Common side effects of GnRHa treatment include pain at the injection site, swelling, aseptic abscesses, vaginal bleeding with the first application and polycystic ovary syndrome[2], Polycystic ovary syndrome can affect the reproductive function of patients, and also increase the risk of abnormalities in glucose and lipid metabolism and cardiovascular disease[20].In this study, during 6 months of treatment, one child in the 3-month dosage group developed transient haematuria, and one child in the 1-month dosage group developed headache, both of which resolved on their own without medication and did not affect the course of treatment.No significant adverse reactions were observed in the remaining children.The safety profile of both the 3-month and 1-month dosage forms of LA was confirmed.In addition, the Consensus on Diagnosis and Treatment of Central Precocious Puberty (2015) states that GnRHa treatment aimed at improving height generally lasts more than two years.Compared with the 1-month dosage form LA, the 3-month dosage form LA can effectively reduce the number of patient visits,lower the cost of treatment, and improve children’s adherence to treatment.However, there are several limitations in this study, firstly,the observation time is short, and further extension of follow-up time is needed to observe its long-term efficacy; secondly, boys were not included in the study, and more data on boys can be collected in future studies.
In conclusion, the efficacy of leuprolide 3-month long-acting formulation is comparable to that of 1-month long-acting formulation, both of which can effectively inhibit HPGA axis and sex hormone levels in girls with CPP, inhibit pubertal progression,attenuate BA progression, and increase PAH.no serious adverse effects were observed in the treatment of the two formulations, and their efficacy and safety are in line with the reports of domestic and international studies.In addition, the use of the 3-month long-acting dosage form can effectively reduce the number of visits to the clinic,reduce the cost of treatment, and improve treatment adherence,which has economic advantages[21], and is suitable for promoting its use in China.
Author’s Contribution
Mingming Yang: project design and implementation, data collection, statistical analysis, writing and revising the paper;Wendi Zhou: subject design, supervising and reviewing the article;Yongquan Tang, Qian Gao, and Jie Wang: data collection; Xue Jiang:data collection and analysis
All authors declare no conflict of interest.
杂志排行
Journal of Hainan Medical College的其它文章
- The effect of Huayu Lifei formula on the expression of miR-27a and α-SMA in lung tissue of bleomycin-induced rat lung fibrosis model
- Prevalence and influencing factors of cognitive frailty in elderly with diabetes mellitus in China: A meta-analysis
- Correlation between metabolic dysfunction-associated fatty liver disease and liver fibrosis based on Fibrotouch
- The effect of miR-129-5p in pancreatic cancer cells on apoptosis through targeted of HMGB1
- Pharmacodynamic study of cannabidiol on bleomycin-induced pulmonary fibrosis in rats
- Exploring the mechanism of moist exposed burn ointment for the treatment of diabetic ulcer based on network pharmacology and molecular docking
