NiCrAl-NiC封严涂层在盐雾环境下的腐蚀行为研究
2023-02-07刘天孙杰宋佳杨国昊孙海静王保杰
刘天,孙杰,宋佳,杨国昊,孙海静,王保杰
NiCrAl-NiC封严涂层在盐雾环境下的腐蚀行为研究
刘天1,孙杰1,宋佳2,杨国昊1,孙海静1,王保杰1
(1.沈阳理工大学,沈阳 110159;2.中国航发沈阳黎明航空发动机有限责任公司,沈阳 110043)
通过盐雾试验,研究NiCrAl-NiC封严涂层在高湿度、高盐分的环境中工作的腐蚀行为及机理。使用热喷涂的方法,在GH907基体上,以NiAl作为粘结层,喷涂制备NiCrAl-NiC封严涂层,并针对该涂层进行连续盐雾试验。对不同盐雾试验时间得到的试样进行电化学(极化曲线以及电化学阻抗谱)测试、XPS测试、SEM观察及EDS测试,研究涂层的腐蚀行为。在500倍电子显微镜下观察得涂层表面腐蚀在表面孔隙附近发生,且腐蚀产物随腐蚀时间的增加而不断增加。EDS结果显示,腐蚀前期O元素含量增加,在连续盐雾时间达到72 h后,观察到Cl、Fe元素,且涂层表面的腐蚀产物出现裂隙。这个结果说明电解质溶液已侵入涂层内部,使基体发生腐蚀。涂层在盐雾试验前期,极化曲线所得腐蚀电流密度有所下降,说明涂层耐蚀性增强。盐雾腐蚀后期,腐蚀电流密度增加,涂层耐蚀性下降。试验前期,腐蚀产物的积累使得涂层的耐蚀性提高,由于腐蚀速率随时间逐渐增加,试验后期涂层内部已经发生腐蚀,腐蚀产物为金属氧化物和氢氧化物。
NiCrAl-NiC封严涂层;盐雾试验;电化学测试;腐蚀
随航空发动机技术的发展,气路密封技术对航空发动机机械效率的不断提高起到了重要的作用。据文献,发动机叶片与机匣的间隙每增加1%,其效率就会减小1.5%,相应地耗油率就增加3%[1]。针对气路密封技术的研究,航空发动机用密封技术有蜂窝封严技术[2]、刷式封严技术[3]、热喷涂封严涂层技术[4]等,由于热喷涂封严涂层维修方便,制造工艺简单,耐磨性与抗冲蚀性配合最佳,热稳定性好等而被广泛应用[5-11]。
当封严涂层在高湿度、高盐分的环境中工作时,会因为涂层与环境之间相互作用而导致比较严重的腐蚀,因此,封严涂层在环境中的腐蚀破坏行为就成为一个比较严峻的挑战[12-16]。Ni等[17]针对CuAl-NiC耐磨封严涂层的腐蚀失效机制,以5%(质量分数)NaCl溶液为腐蚀介质长时间浸泡,试验发现,涂层的孔隙率是腐蚀发生的关键,其提供了腐蚀介质进入不同层之间界面的路径,进而诱导各层间电偶腐蚀的发生,甚至会出现一个大阴极–小阳极电池,大大加快腐蚀恶化速度。Lei等[18]以Al-BN为研究对象,进行浸泡试验,在Al-BN涂层系统的腐蚀过程中,面层的Al-BN层、粘结层和基体之间发生了电偶相互作用。Al-BN层作为阳极,NiAl层和基体作为阴极。涂层体系中Al-BN层的铝基体由于电偶相互作用而发生自发点蚀。在后续研究中,Al-BN涂层的电偶腐蚀速率由低到高再到最后稳定可分为3个阶段,沉淀腐蚀产物Al(OH)3的空间位阻效应可能是最重要的腐蚀速率控制机制,包括Al(OH)3在孔隙的沉淀,限制了O2向阴极的传输,以及堵塞孔内的局部酸化[19]。李垚等[20]发现,NiCrAl涂层在盐雾环境腐蚀156 h后,表面发生严重腐蚀,腐蚀产物在涂层表面堆积,且存在裂隙,为腐蚀介质深入涂层内部致使粘结层发生腐蚀提供了前提,电化学结果显示,涂层的耐蚀性有所下降。
NiCrAl-NiC封严涂层中,NiCrAl合金可保障涂层在高温下的抗氧化性,Ni基合金具有优秀的耐冲蚀性,采用NiC可为涂层提供良好的自润滑性能。目前,国内外对于NiCrAl-NiC封严涂层在海洋环境中的腐蚀行为,特别是对于腐蚀机制的分析研究还有待于加强。本文针对NiCrAl-NiC封严涂层在高湿度、高盐分的环境,进行了盐雾试验,并分析了其腐蚀行为及机理。
1 试验
试验用NiCrAl-NiC封严涂层由大气等离子喷涂方法制备,主要喷涂工艺参数见表1。NiCrAl-NiC封严涂层面层厚1.5 mm,粘结层NiAl层约厚0.15 mm,喷涂在厚度为2 mm的 GH907基体上。试验前,将涂层系统切割为暴露面积为1 cm2的小样品,其他5面用硅胶密封,导线焊接在基体未被喷涂一侧,确保只有涂层一面暴露在盐雾中。试验按照GB/T 1012—2012进行,溶液采用5%的NaCl溶液,试验设备型号为DCTC1200P盐雾箱,试样腐蚀时间为96 h,采用24 h连续喷雾,每24 h取出1组试样,其他试验参数见表2。

表1 喷涂主要工艺参数

表2 盐雾腐蚀试验参数
使用CS350电化学工作站测得极化曲线及电化学阻抗谱图,试验介质为3.5%的NaCl溶液,试验采用三电极体系,参比电极使用饱和甘汞电极,辅助电极为铂电极,工作电极为被测试样。盐雾试验前对试样进行处理,被测试面积为1 cm2,扫描速率为2 mV/s。电化学阻抗谱图采用ZSimpWin软件进行拟合。
腐蚀前后样品利用TESCAN-MIAR4型扫描电子显微镜(SEM)500倍下观察微观形貌,以及其自带能谱(EDS)分析腐蚀产物元素组成。最后利用X射线光电子能谱(XPS)分析腐蚀96 h后试片的腐蚀产物各元素的化学组成和状态。
2 结果与讨论
2.1 微观形貌及成分分析
图1为500倍下测得涂层的SEM形貌。从图1a可以看出,未经腐蚀的原始NiCrAl-NiC涂层较为疏松,深色区域为孔隙,多孔结构可以有效改善涂层的可磨耗性能,在部分区域可以看到球形颗粒,这是等离子喷涂过程未充分熔融的NiCrAl-NiC粉料,主要呈现堆叠形貌。图1b是经过24 h盐雾试验后的图像,涂层孔隙明显减少,这是因为发生了腐蚀,且腐蚀产物多在孔隙周围生成,覆盖了表面部分孔隙。随着腐蚀时间的延长,涂层表面被破坏,腐蚀产物堆积表面逐渐趋于平整,图像中成片白色区域为疏松的腐蚀产物。腐蚀后期,在涂层表面出现裂隙,这为腐蚀介质深入涂层提供了通道。图1 f为涂层腐蚀96 h后裂隙处放大形貌,可以看出,腐蚀产物为絮状分布在裂隙周围。

图1 不同中性盐雾腐蚀时间后NiCrAl-NiC涂层的SEM形貌
图1中各区域的能谱结果见表3。在未进行腐蚀的情况下,在涂层表面发现微量O元素(Point1),这是因为在热喷涂过程中,涂层粉料被熔融时氧化生成的Al2O3[21]。经过24 h盐雾腐蚀后,O元素有增加的趋势,表面出现绿色和白色腐蚀产物。从EDS结果元素组成可认为是Al和Ni被腐蚀后产生化合物[22],且当盐雾时间达到72 h之后,Cl、Fe元素相继出现,这是腐蚀产物在表面积累的结果。由于面层与粘结层中都无Fe元素,故可以证明Fe元素来自基体GH907,腐蚀介质已经进入涂层到达基体,并发生反应。

表3 图1中区域EDS结果
2.2 电化学分析
不同时间点NiCrAl-NiC涂层盐雾腐蚀后在3.5% NaCl溶液中所测得的极化曲线如图2所示。利用Tafel曲线外推法得到腐蚀过程的动力学参数,结果见表4。由于涂层孔隙存在实际测试面积会大于1 cm2,测试结果仅反映此NiCrAl/NiC涂层系统耐蚀性能随盐雾时间的变化。未经盐雾腐蚀涂层的腐蚀电流密度(corr)为5.44×10–4A/cm2,经历48 h腐蚀涂层的corr降低至2.29×10–4A/cm2。涂层发生腐蚀,使得涂层的耐蚀性能提高,随着盐雾时间延长,腐蚀产物的增加,48 h时腐蚀产物已可以在多孔结构的涂层中阻挡腐蚀介质的进一步侵入,起到一定的保护作用。在之后的试验中,涂层表面液膜持续更新,涂层的腐蚀会不断进行。盐雾96 h时,corr逐渐增大到4.40×10–4A/cm2,耐蚀性逐渐减退。在盐雾试验结束时,涂层的耐蚀性比未腐蚀的试样好,这是其表面产生腐蚀产物的结果。腐蚀24~94 h,Tafel阳极斜率(a)逐渐增大,因为电极的极化过程被孔隙及涂层表面中生成的腐蚀产物电阻所阻止,这也证明了腐蚀产物在孔隙中积累[23]。
涂层腐蚀科学领域中,电化学阻抗谱(EIS)是一种强有力且无损的电化学表征测试[24]。NiCrAl-NiC涂层经过不同时间盐雾腐蚀后,在3.5% NaCl溶液中所测的Nyquist图与Bode图如图3、4所示。由Nyquist图可以看出,经过盐雾腐蚀后,NiCrAl-NiC涂层的图像有2个明显的容抗弧。相应地,图4b由相位角对lg作图的曲线上同样可以看出有2个峰值,高频率所对应的峰由双电层电容(即涂层电容)的容抗弧引起。Nyquist图中,经过腐蚀后的电容回路的直径较未腐蚀有明显增加,耐蚀性有所提高,且直径随着盐雾时间的延长而迅速减小,说明腐蚀速率增大[25],而低频率所对应的峰是相对应于双层的电荷转移电容引起的。随试验时间的延长,腐蚀加剧扩展正在原本孔隙的基础上可能扩大的孔隙通道,增加Cl–深入涂层的几率,导致基体腐蚀。图4b相位角曲线的下降正解释了这一点,且最大相位角(即最高峰值)有向较低频率方向移动的趋势,这与基体和腐蚀介质接触的面积增加有关。图4a由||(阻抗模值)对lg作图的曲线上,高频区所对的平滑部分对应的是溶液电阻。进行盐雾腐蚀后的||值较未被腐蚀的||值高,耐蚀性有所提高。NiCrAl-NiC涂层是多孔结构,其表面积更大,且有更大吸附和集中腐蚀介质到孔隙中的趋势,而腐蚀后的产物更有抵抗腐蚀的能力,并且填充了部分空隙,使得涂层的耐蚀性比腐蚀前提高,这也解释了腐蚀96 h后的corr值比未发生腐蚀情况低的原因。
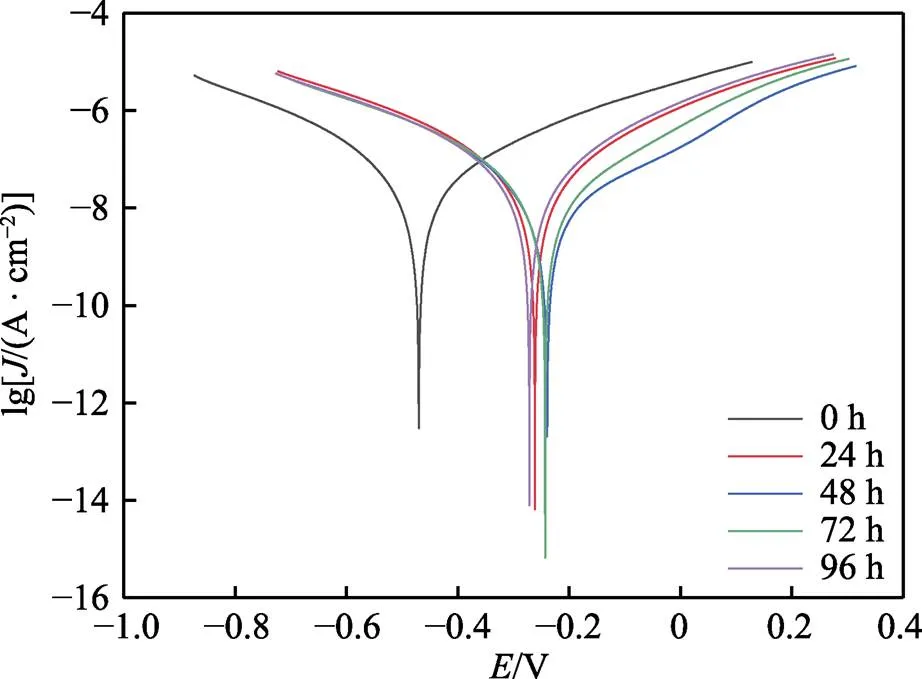
图2 NiCrAl-NiC封严涂层盐雾极化曲线
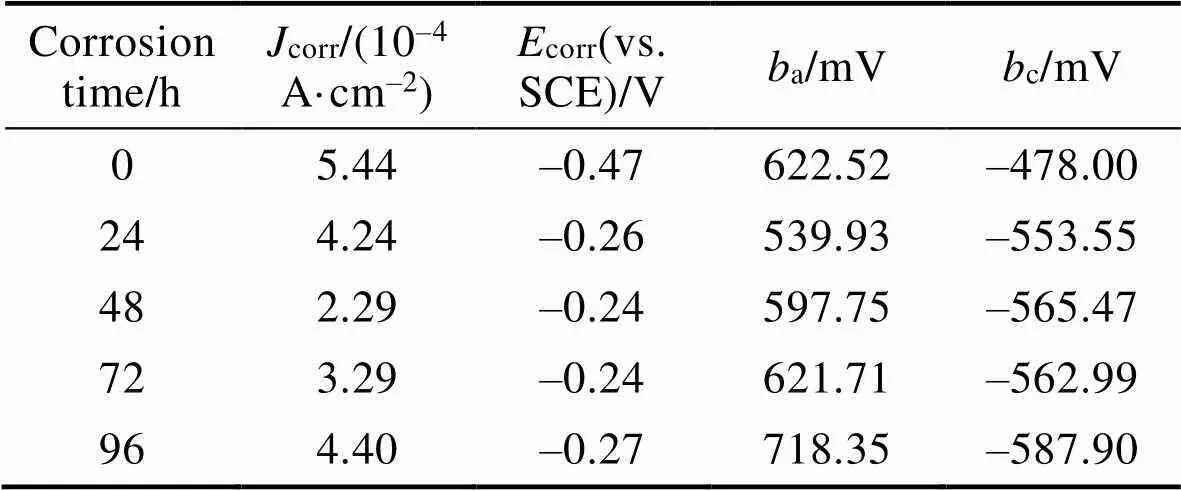
表4 NiCrAl-NiC封严涂层盐雾电化学参数

图3 NiCrAl-NiC涂层不同盐雾腐蚀时间的Nyquist图
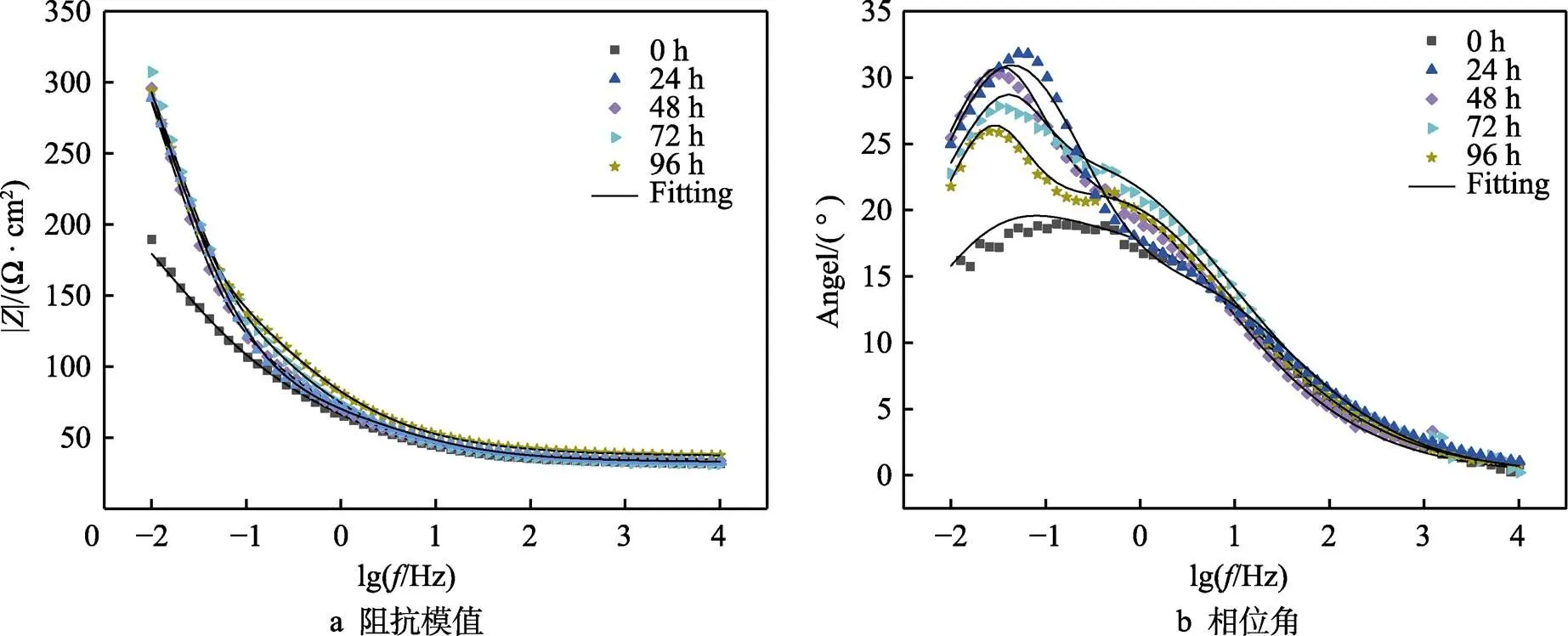
图4 NiCrAl-NiC涂层不同盐雾腐蚀时间Bode图
以图5所示的等效电路图模型拟合电化学阻抗谱,0~48 h的等效电路如图5a所示,72、96 h等效电路如图5b所示。电路中的元件定义如下:s为溶液电阻,1为涂层电容相对电阻元件,1为孔隙电阻,二者与涂层的介电性能有关,双电层电容2相对的2为电荷转移电阻,电路元件同样为双电层电容。等效电路中以常相位角元件代替线性电化学元件,即理想电容器,其原因是实际过程与理想电容行为的偏差,由被测电极表面的不均匀性、表面粗糙度、电极几何形状导致的电流或电位分布不均匀性、表面层的孔隙度和不均匀导电性造成的。的阻抗如下所示:
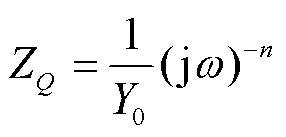
式中:0和为可调参数,可通过非线性拟合过程获得。介于0~1,在粗糙表面的情况下,暴露于电解质溶液的真实表面大于几何定义的表面,因此不统一[26]。从图4b可以看到,随着腐蚀时间的延长,相位角曲线呈下降趋势,说明涂层电容值增大,这与盐雾腐蚀介质侵入涂层有关。随着腐蚀产物的逐渐产生,腐蚀产物在涂层空隙中由疏松变得致密,在72、96 h时,双电层电荷转移电容接近理想电容。在NiCrAl- NiC涂层系统上获得的EIS参数的拟合结果见表5。
图5 盐雾腐蚀后NiCrAl-NiC涂层等效电路
Fig.5 Equivalent circuit diagram of NiCrAl-NiC coating after salt spray corrosion
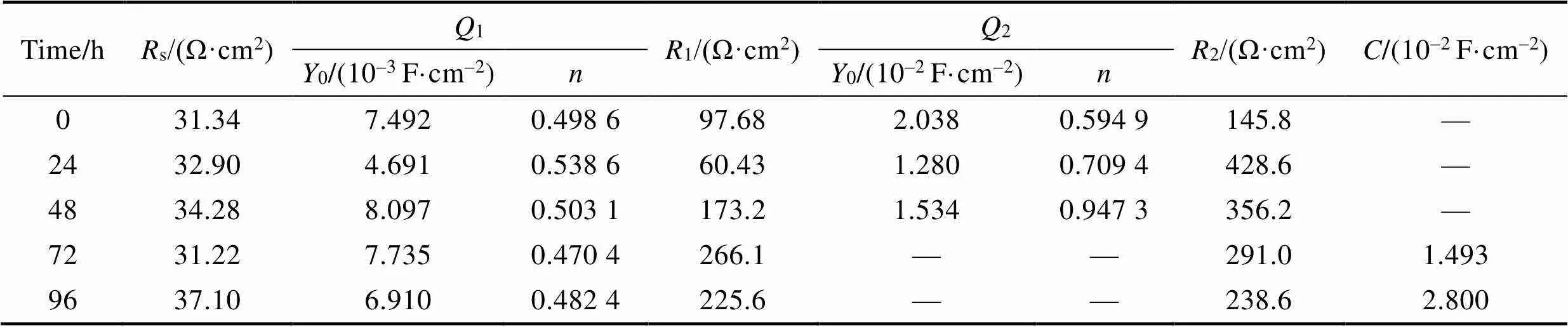
表5 在NiCrAl/NiC涂层系统上获得的EIS参数的拟合结果
2.3 XPS分析
通过XPS深度分析经盐雾腐蚀96 h后NiCrAl- NiC涂层的腐蚀产物,结果如图6所示。首先在总谱中可以确定涂层表面包含Fe、Al、Cr、Ni、O、Cl元素(见图6a),样品在0~1 350 eV内进行扫描,并在284.8 eV的结合能下用标准C1s峰值进行荷电校正。从图6b中可以看出,Al2p3/2拟合后,可以得到2个拟合峰,峰位置结合能分别是74.06、74.73 eV,与Al2O3和AlCl3的谱图吻合[27],这与Al被腐蚀有关。虽然上述分析中原始涂层含有Al2O3,但也不排除腐蚀后产生的可能。图6c中Fe2p3/2轨道峰拟合后的结果中,结合能在712.49 eV的峰对应FeOOH,708.45 eV位置的峰证明了Fe2O3的存在[28-29]。图6d中,Ni2p1/2轨道峰拟合后,在873.71、874.82 eV出现的峰归属于Ni(OH)2和Ni,这与上文分析相呼应。图6e中对O1s轨道峰进行拟合后,531.5、532.5 eV出现的峰分别对应Ni(OH)2、Al2O3的谱图,533.4 eV处的峰分配给Fe-O结构,与文献中描述Fe2O3峰位置相近[30-32]。
2.4 腐蚀机制分析
NiCrAl-NiC涂层系统盐雾腐蚀实际上是一个电化学腐蚀过程,SEM图像可以表明多孔的结构更有吸附腐蚀介质的趋势。在高湿度高电解质的盐雾作用下,液膜附着在涂层表面,且暴露在空气中氧气也会被吸附。由于液膜很薄,其含量很容易达到饱和,极易发生氧化还原反应。
盐雾腐蚀初期较为活泼的Al、Ni,在湿热环境下作为阳极失去电子,见式(2)、(3)。EDS结果中,O元素增加表明阴极反应为吸氧腐蚀,见式(4)。
Al→Al3++3e (2)
Ni→Ni2++2e (3)
O2+2H2O+4e→4OH–(4)
Al3+与水接触会发生式(5)的反应[29],OH–与Ni2+生成具有保护性腐蚀产物,见式(6),这也解释了初期涂层的耐蚀性有所提高的现象。被腐蚀部位pH值也会降低,由于电解液较薄,但同时不断刷新,故腐蚀会进一步进行。
2Al3++3H2O→Al2O3+6H+(5)
Ni2++2OH–→Ni(OH)2(6)
无论是反应生成Al2O3,还是涂层制备过程中产生Al2O3,其从热力学角度上讲,在溶液薄膜中都易发生水合反应,见式(7),而电解液薄膜中大量的Cl–会同Al2O3中的OH–争夺Al3+进而形成可溶的氯化物,见式(8)—(10)。
Al2O3+3H2O→2Al(OH)3(7)
Al(OH)3+Cl–→Al(OH)2Cl+OH–(8)
Al(OH)2Cl+Cl–→Al(OH)Cl2+OH–(9)
Al(OH)Cl+Cl–→AlCl3+OH–(10)
多孔的涂层结构,加之涂层部分可以被溶解,为腐蚀介质进入涂层系统内部提供了通道。腐蚀持续进行,当电解质与基体接触时,与基体中Fe发生反应,见式(11)。被溶解的Fe2+随电解质薄膜扩散至涂层表面,与OH–结合生成Fe(OH)2,之后在O2的作用下氧化成Fe(OH)3,见式(12)、(13)。
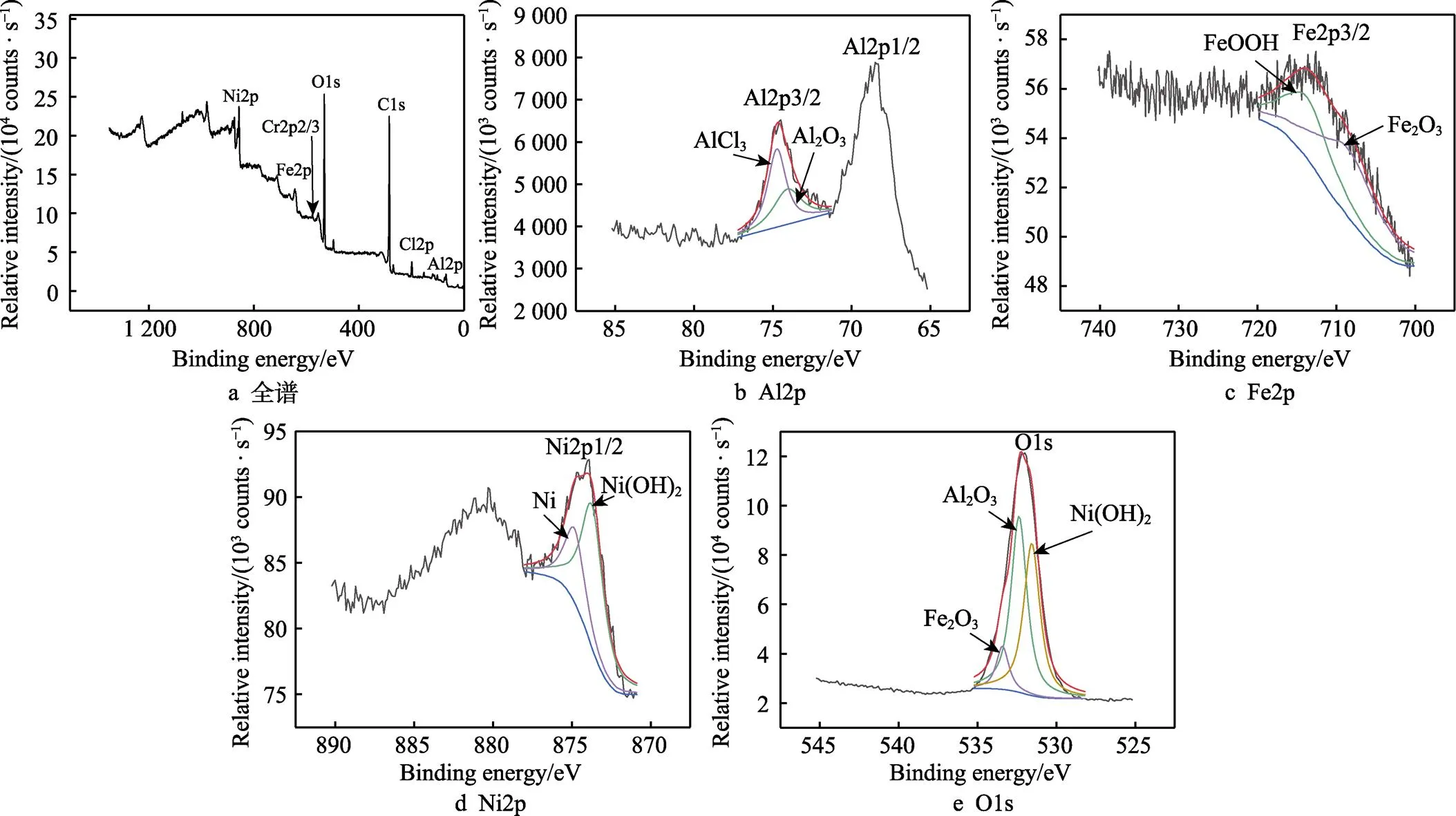
图6 盐雾腐蚀96 h后的NiCrAl-NiC涂层XPS谱图
Fe→Fe2++2e (11)
Fe2++2OH—→Fe(OH)2(12)
4Fe(OH)2+O2+4H2O→4Fe(OH)3(13)
同时,在湿热的环境中,Fe2+存在的情况下,有电解质薄膜中存在的Fe(OH)3可以发生如下转化反应,见式(14)、(15)。
Fe(OH)3→FeOOH+H2O (14)
Fe(OH)3→Fe2O3·3H2O→Fe2O3+3H2O(15)
随着腐蚀持续发生,若随腐蚀产物积累,涂层内部或基体形成闭塞电池腐蚀,呈现自催化反应,那么对涂层内部破坏更为严重。
3 结论
1)NiCrAl-NiC涂层经过连续盐雾腐蚀期间,前48 h腐蚀电流密度减小,后48 h腐蚀电流密度增加,相应涂层耐蚀性先增大、后减小,这是涂层腐蚀与腐蚀产物积累共同作用的结果。
2)盐雾试验中,NiCrAl-NiC涂层的腐蚀速率随时间增大,腐蚀后的涂层出现很多裂隙,涂层表面分布并不均匀,且涂层包含多种元素,使得不同元素之间形成电势差。涂层表面积累的腐蚀产物主要为金属的氧化物和氢氧化物,溶液侵入到基体的情况下,可能造成各层之间发生电偶腐蚀,严重情况下会导致涂层脱落,影响航空发动机使用寿命。
[1] 张俊红, 鲁鑫, 何振鹏, 等. 航空发动机可磨耗封严涂层技术研究及性能评价[J]. 材料工程, 2016, 44(4): 94-109.
ZHANG Jun-hong, LU Xin, HE Zhen-peng, et al. Technique Application and Performance Evaluation for Abradable Coating in Aeroengine[J]. Journal of Materials Engineering, 2016, 44(4): 94-109.
[2] LU Bin, MA Xiao-jian, WU Cai-guang, et al. The Wear of Seal Fins during High-Speed Rub between Labyrinth Seal Fins and Honeycomb Stators at Different Incursion Rates[J]. Materials (Basel, Switzerland), 2021, 14(4): 979.
[3] 张甜甜, 黄传兵, 兰昊, 等. 镍基耐高温自润滑刷式封严涂层研究[J]. 航空制造技术, 2017, 60(8): 24-29.
ZHANG Tian-tian, HUANG Chuan-bing, LAN Hao, et al. Investigation of Ni-Based Brush Seal Coatings with Self-Lubricating Property at Elevated Temperature[J]. Aeronautical Manufacturing Technology, 2017, 60(8): 24-29.
[4] LI He-ping, KE Zhi-qiang, LI Jing, et al. An Effective Low-Temperature Strategy for Sealing Plasma Sprayed Al2O3-Based Coatings[J]. Journal of the European Ceramic Society, 2018, 38(4): 1871-1877.
[5] ZHANG Feng, LAN Hao, HUANG Chuan-bing, et al. Corrosion Resistance of Ti3Al/BN Abradable Seal Coating[J]. Acta Metallurgica Sinica (English Letters), 2014, 27(6): 1114-1121.
[6] 黄伟. 高温可磨耗封严复合涂层的制备及性能研究[D]. 武汉: 武汉理工大学, 2011.
HUANG Wei. Study of Preparation and Properties of Abradable Composite Seal Coating Used in High Temperature[D]. Wuhan: Wuhan University of Technology, 2011.
[7] WANG Zhe, DU Ling-zhong, LAN Hao, et al. Preparation and Characterization of YSZ Abradable Sealing Coating through Mixed Solution Precursor Plasma Spraying[J]. Ceramics International, 2019, 45(9): 11802-11811.
[8] CAO Y X, LIU W, DU L Z, et al. Preparation and Property of Al/hBN Plasma Sprayed Abradable Sealing Coating[J]. Rare Metal Materials and Engineering, 2012, 41(S2): 813-816.
[9] WANG Zhe, DU Ling-zhong. Stabilization of a Novel Mixed Solution Precursor Used for Preparing YSZ Abradable Sealing Coatings[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 562: 354-360.
[10] ZHANG Xi-qian, YANG Qiong-lian, CHEN Lin, et al. Fabrication and Characterization of 8YSZ Ceramic Based Abradable Seal Coatings by Atmospheric Plasma Spraying [J]. Ceramics International, 2020, 46(17): 26530-26538.
[11] XIA Zhi-xin, WANG Chuan-yang, ZHAO Dong, et al. Substructure Formation Mechanism and High Temperature Performance in CoNiCrAlY Seal Coating by Laser Melting Deposition with Inside-Laser Coaxial Powder Feeding[J]. Surface and Coatings Technology, 2019, 367: 108-117.
[12] ZHANG Jia-ping, XUE Wei-hai, DUAN De-li, et al. Effect of the Corrosion of Seal Coatings Used in Aero Engines on Their High-Speed Wear Behaviour[J]. Journal of Thermal Spray Technology, 2020, 29(8): 1958-1967.
[13] 赵丹, 孙杰, 赵忠兴. 钛合金与NiAl封严涂层的电偶腐蚀行为研究[J]. 稀有金属, 2012, 36(2): 224-228.
ZHAO Dan, SUN Jie, ZHAO Zhong-xing. Galvanic Corrosion Behavior of Titanium Alloy and NiAl Seal Coating [J]. Chinese Journal of Rare Metals, 2012, 36(2): 224-228.
[14] USMAN B J, SCENINI F, CURIONI M. Corrosion Testing of Anodized Aerospace Alloys: Comparison between Immersion and Salt Spray Testing Using Electrochemical Impedance Spectroscopy[J]. Journal of the Electrochemical Society, 2020, 167(4): 041505.
[15] ZHANG Feng, XU Cun-guan, LAN Hao, et al. Corrosion Behavior of an Abradable Seal Coating System[J]. Journal of Thermal Spray Technology, 2014, 23(6): 1019-1028.
[16] 孙杰, 石超, 赵丹. NiAl/AlBN封严涂层的电偶腐蚀行为[J]. 材料工程, 2015, 43(11): 44-49.
SUN Jie, SHI Chao, ZHAO Dan. Galvanic Corrosion Behavior of NiAl/AlBN Seal Coating[J]. Journal of Materials Engineering, 2015, 43(11): 44-49.
[17] NI Yu-meng, ZHANG Fan, NJOKU D I, et al. Corrosion Mechanism of CuAl-NiC Abradable Seal Coating System—The Influence of Porosity, Multiphase, and Multilayer Structure on the Corrosion Failure[J]. Journal of Materials Science & Technology, 2021, 88: 258-269.
[18] LEI Bing, LI Man, ZHAO Zhong-xing, et al. Corrosion Mechanism of an Al-BN Abradable Seal Coating System in Chloride Solution[J]. Corrosion Science, 2014, 79: 198-205.
[19] LEI Bing, PENG Ming-xiao, LIU Ling, et al. Galvanic Corrosion Performance of an Al–BN Abradable Seal Coating System in Chloride Solution[J]. Coatings, 2020, 11(1): 9.
[20] 李垚, 马彬, 赵九蓬, 等. 盐雾条件下NiCrAl涂层的耐腐蚀性研究[J]. 稀有金属材料与工程, 2010, 39(12): 2181-2184.
LI Yao, MA Bin, ZHAO Jiu-peng, et al. Corrosion Resistance of NiCrAl Coatings in the Salt Spray Tests[J]. Rare Metal Materials and Engineering, 2010, 39(12): 2181-2184.
[21] 项民, 骆军华, 张琦. 盐雾腐蚀对热障涂层高温循环氧化性能的影响[J]. 航空学报, 2006, 27(1): 138-141.
XIANG Min, LUO Jun-hua, ZHANG Qi. Influence of Salt Spray Corrosion on High-Temperature Cyclic Oxidation Behavior of Thermal Barrier Coatings[J]. Acta Aeronautica et Astronautica Sinica, 2006, 27(1): 138-141.
[22] XU Cun-guan, DU Ling-zhong, YANG Bin, et al. Study on Salt Spray Corrosion of Ni-Graphite Abradable Coating with 80Ni20Al and 96NiCr-4Al as Bonding Layers[J]. Surface and Coatings Technology, 2011, 205(17/18): 4154-4161.
[23] DUAN Hong-ping, DU Ke-qin, YAN Chuan-wei, et al. Electrochemical Corrosion Behavior of Composite Coatings of Sealed MAO Film on Magnesium Alloy AZ91D [J]. Electrochimica Acta, 2006, 51(14): 2898-2908.
[24] YUE Yuan-yuan, LIU Zhong-xia, WAN Ting-ting, et al. Effect of Phosphate-Silane Pretreatment on the Corrosion Resistance and Adhesive-Bonded Performance of the AZ31 Magnesium Alloys[J]. Progress in Organic Coatings, 2013, 76(5): 835-843.
[25] SHAO Fang, YANG Kai, ZHAO Hua-yu, et al. Effects of Inorganic Sealant and Brief Heat Treatments on Corrosion Behavior of Plasma Sprayed Cr2O3-Al2O3Composite Ceramic Coatings[J]. Surface and Coatings Technology, 2015, 276: 8-15.
[26] 曹楚南. 腐蚀电化学原理[M]. 3版. 北京: 化学工业出版社, 2008: 175-176.
CAO Chu-nan. Principles of electrochemistry of corrosion[M]. 3rd Edition. Beijing: Chemical Industry Press, 2008: 175-176.
[27] DING Jian, LIU Xin, WANG Yu-jiang, et al. Effect of Sn Addition on Microstructure and Corrosion Behavior of As-Extruded Mg-5Zn-4Al Alloy[J]. Materials (Basel, Switzerland), 2019, 12(13): 2069.
[28] KOCIJAN A, DONIK Č, JENKO M. Electrochemical and XPS Studies of the Passive Film Formed on Stainless Steels in Borate Buffer and Chloride Solutions[J]. Corrosion Science, 2007, 49(5): 2083-2098.
[29] GUO Yang, ALI R, ZHANG Xing-zhong, et al. Raman and XPS Depth Profiling Technique to Investigate the Corrosion Behavior of FeSiAl Alloy in Salt Spray Environment[J]. Journal of Alloys and Compounds, 2020, 834: 155075.
[30] XU Wen-hua, HAN En-hou, WANG Zhen-yu. Effect of Tannic Acid on Corrosion Behavior of Carbon Steel in NaCl Solution[J]. Journal of Materials Science & Technology, 2019, 35(1): 64-75.
[31] OLIVARES O, LIKHANOVA N V, GÓMEZ B, et al. Electrochemical and XPS Studies of Decylamides of Α-Amino Acids Adsorption on Carbon Steel in Acidic Environment[J]. Applied Surface Science, 2006, 252(8): 2894-2909.
[32] WAN Kai, LI Chang-cheng, XING Cui-juan, et al. Enhanced Anticorrosion Properties of Epoxy Coatings from Al and Zn Based Pigments[J]. Chemical Research in Chinese Universities, 2015, 31(4): 573-580.
Corrosion Behavior of NiCrAl-NiC Seal Coating in Salt Spray Conditions
1,1,2,1,1,1
(1. Shenyang Ligong University, Shenyang 110159, China; 2. AECC Shenyang Liming Aero-Engine Co., Ltd., Shenyang 110043, China)
With the development of aero-engine technology, gas path sealing technology plays an important role in the continuous improvement of aero-engine mechanical efficiency. It is widely used due to the advantages of convenient maintenance, simple manufacturing process and good thermal stability of thermal spraying seal coating. When the seal coating is applied in the environment of high humidity and high salt, its composite metal coating will be seriously damaged. Therefore, the work aims to study the corrosion behavior of NiCrAl-NiC seal coating in high humidity and high salt environment by the salt spray test.
The NiCrAl-NiC seal coating was prepared by atmospheric plasma spraying. The surface layer of NiCrAl-NiC seal coating was 1.5 mm thick and the bonding layer of NiAl was about 0.15 mm thick. It was sprayed on the GH907 substrate with a thickness of 2 mm by thermal spraying, and the salt spray test was continuously carried out to the coating. The other five sides of the sample were sealed with silica gel to ensure that only one side of the coating was exposed to salt spray. The test was carried out in accordance with GB/T 1012—2012. The solution was analyzed with 5wt.% pure NaCl solution. The test equipment was DCTC1200P salt spray box. The corrosion time of the samples was 96 h. The samples were continuously sprayed for 24 h, and a set of samples was removed every 24 h. The samples obtained after different time of salt spray test were stored in CS350 electrochemical workstation. The three-electrode cell was used (the reference electrode was saturated calomel electrode, the auxiliary electrode was platinum electrode, and the working electrode was the tested sample) to carry out polarization curve test, electrochemical impedance spectrum test, and scanning electron microscope (TESCAN-MIAR4) observation. Then, the energy dispersive spectrometer spectrum of each selected point collected by the energy spectrometer was used to analyze the elements. X-ray photoelectron spectroscopy test was carried out to the samples after 96 h of salt spray corrosion, and the composition of corrosion products was deeply analyzed. It was observed that the coating surface was loose and had many pores under 500 times electron microscopes, and the corrosion occurred near the surface pores. The results of the energy dispersive spectrometer showed that the content of O element increased in the early stage of corrosion. After continuous salt spray time reached 72 h, Cl and Fe elements were observed, and cracks appeared on the corrosion products on the coating surface. This result showed that the electrolyte solution intruded into the interior of the coating, resulting in corrosion of the substrate. The XPS results after 96 h of corrosion showed that the corrosion products were Al2O3, Ni(OH)2, FeOOH, etc. In the early stage of salt spray test, the corrosion current density obtained by polarization curve decreased, indicating that the corrosion resistance of the coating was enhanced. In the later stage of salt spray corrosion, the corrosion current density increased and the corrosion resistance of the coating decreased. The accumulation of corrosion products in the early stage of the test improves the corrosion resistance of the coating. Due to the gradual increase of corrosion rate with time, corrosion has occurred in the coating in the later stage of the test, and the corrosion products are metal oxides and metal hydroxides.
NiCrAl-NiC seal coating; salt spray test; electrochemical test; corrosion
TG172
A
1001-3660(2023)01-0206-08
10.16490/j.cnki.issn.1001-3660.2023.01.021
2021–12–01;
2022–04–06
2021-12-01;
2022-04-06
沈阳理工大学高水平成果建设项目(SYLUXM202105);沈阳理工大学科研创新团队支持项目(SYLUTD202004)
High Level Achievement Construction Project of Shenyang LiGong University (SYLUXM202105); Research Innovation Team Support Project of Shenyang LiGong University (SYLUTD202004)
刘天(1998—),男,硕士研究生,主要研究方向为材料的腐蚀与防护。
LIU Tian (1998—), Male, Postgraduate, Research focus: corrosion and protection of materials.
孙杰(1971—),男,博士,教授,主要研究方向为材料腐蚀电化学行为及防护方法。
SUN Jie (1971—), Male, Doctor, Professor, Research focus: material corrosion electrochemical behavior and protection methods.
刘天, 孙杰, 宋佳, 等. NiCrAl-NiC封严涂层在盐雾环境下的腐蚀行为研究[J]. 表面技术, 2023, 52(1): 206-213.
LIU Tian, SUN Jie, SONG Jia, et al. Corrosion Behavior of NiCrAl-NiC Seal Coating in Salt Spray Conditions[J]. Surface Technology, 2023, 52(1): 206-213.
责任编辑:刘世忠
