Breakthroughs on tailoring membrane materials for ethanol recovery by pervaporation
2023-01-30XiaZhanXueyingZhaoZhongyongGaoRuiGeJuanLuLuyingWangJidingLi
Xia Zhan, Xueying Zhao, Zhongyong Gao, Rui Ge, Juan Lu, Luying Wang, Jiding Li
1 Key Laboratory of Cleaner Production and Integrated Resource Utilization of China National Light Industry, Beijing Technology and Business University, Beijing 100048, China
2 The Institute of Medicinal Plant Development, The Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC), Beijing 100193, China
3 Beijing Key Laboratory of Lignocellulosic Chemistry, Beijing Forestry University, Beijing 100083, China
4 The State Key Laboratory of Chemical Engineering, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
Keywords:Pervaporation Ethanol recovery Microstructure Polymeric membrane Mixed matrix membrane
ABSTRACT Bioethanol,as a clean and renewable fuel,has gained increasing attention due to its major environmental benefits.Pervaporation (PV) is a promising and competitive technique for the recovery of ethanol from bioethanol fermentation systems due to the advantages of environmental friendliness, low energy consumption and easy coupling with fermentation process.The main challenge for the industrial application of ethanol perm-selective membranes is to break the trade-off effect between permeability and selectivity.As membrane is the heart of the pervaporation separation process, this article attempts to provide a comprehensive survey on the breakthroughs of ethanol perm-selective PV membranes from the perspectives of tailoring membrane materials to enhance PV separation performance.The research and development of polymeric and organic/inorganic hybrid membranes are reviewed to explore the fundamental structure–property-performance relationships.It is found that mixed matrix membranes with welldesigned membrane structures offer the hope of better control overphysi-/chemical microenvironment and cavity/pore size as well as size distribution,which may provide both high permeability and membrane selectivity to break the trade-off effect.The tentative perspective on the possible future directions of ethanol perm-selective membranes is also briefly discussed, which may provide some insights in developing a new generation of high-performance PV membranes for ethanol recovery.
1.Introduction
Due to the emerging global energy and environmental crisis,people have been forced to seek for alternative energy sources to fossil fuels [1].Ethanol biofuel, as one of the most promising candidates,has been received great attention in recent years due to its pollution-free and renewable characteristics [2].According to the statistical data released by the Renewable Fuels Association(EthanolRFA), the total global production of fuel ethanol in 2020 is 26059 million gallons.The United States, Brazil, European Union,and China account for 53%,30%,5%and 3%of the total ethanol biofuel global production in the same period, respectively.
From a technical viewpoint, one approach to make bioethanol competitive economically in fuels market would be the conversion of the traditional batch or sequential-batch fermentation process currently employed in the industry to one based upon a truly continuous fermentation [3–5].This can be achieved by coupling an ethanol recovery operation with the fermentation process,thereby keeping the ethanol concentration in the fermentation broth at a level which is minimally inhibitory to the fermentation organism[4].Compared with traditional separation technologies, such as vacuum distillation, solvent extraction and gas stripping distillation,pervaporation(PV)was a more competitive process for recovering ethanol from biomass fermentation broths due to the advantages of environmental friendliness, low energy consumption and easy coupling with fermentation process [6].The breakthrough of pervaporation in industrial applications was achieved in 1982 by GFT (Gesellschaft f″ur Trenntechnik, Hamburg, Germany) with the first PV apparatus established in Brazil for dehydration of ethanol/water azeotropic mixtures.After that,pervaporation for dehydration of alcohols/water mixtures has developed rapidly in large scale applications.However, the industrial applications of pervaporation in ethanol recovery from aqueous mixtures and fermentation broth has been proved much more challenging [7,8].Ordinarily, ethanol perm-selective membranes with the intrinsic feature of larger ethanol molecules permeating preferentially and smaller water molecules being intercepted,confronts more severe challenges than water perm-selective membranes, since the kinetic diameter of ethanol molecules (0.45 nm)is larger than that of water molecules (0.26 nm) [9].Theoretically,the similarity between ethanol and water molecules as well as the strong interactions among liquid molecules and membrane materials increases the difficulty in achieving high separation efficiency[7–12].Moreover, both experimental and molecular simulation results showed that the coupling effect formed by the strong hydrogen bonding between ethanol and water molecules in membranes led to decreased diffusion rate of both ethanol and water,which depressed permeation flux and membrane selectivity of ethanol perm-selective membranes [12,13].Therefore, the pervaporation performance of ethanol perm-selective membranes,especially membrane selectivity,is still at a low level which hinders its industrial application.Bruggenetal.[8]proposed that the application of pervaporation in the production of bioethanol should not be paralyzed by the search of membranes with a selectivity that may prove unrealistic, since it has been shown that increasing the permeability has a better potential to reduce the process cost.
As for a specific separation system, the key factor determining permeability and selectivity is membrane.Most ethanol permselective membranes reported are prepared from hydrophobic polymers, inorganic and hybrid materials [14–17].Polymers are the most widely used ethanol perm-selective materials owing to their low cost, easiness for processing, and tunable microstructure.However, polymeric membranes are limited by their poor swelling resistance, low chemical and thermal stability,and especially the intrinsic trade-off effect between permeability and selectivity [18].In comparison, inorganic membranes have some unique superiorities, such as higher membrane selectivity,higher chemical and thermal stability, and more favorable solvent swelling resistance properties [19,20].Nevertheless, inorganic membranes have obvious inherent limitations such as poor film-forming properties, high brittleness, high cost, and thus are more difficult to be fabricated into large defect-free membranes[21].Hybrid membranes can inherit superior attributes from both the polymeric and inorganic membranes by integrating polymer with inorganic materials, thus widely explored in recent years[22,23].
This review compiles and analyzes the latest works over the last 5 years in polymeric and hybrid pervaporation membranes for ethanol recoveryviapervaporation.To retrieve the related articles, searches were performed in the Web of Science database.The following keywords were included ‘‘pervaporation”, and‘‘ethanol/water mixtures” OR ‘‘bioethanol” OR ‘‘biofuel” OR ‘‘ethanol perm-selective” OR ‘‘PDMS” OR ‘‘silicon rubber” OR ‘‘mixed matrix membrane”.The following criteria were considered for the inclusion of articles in the study: (1) ethanol perm-selective membranesviapervaporation, (2) published online between January 1, 2016 until November 20, 2021, (3) English language, (4)original research studies and review articles, books, book chapters, thesis, letter to the editor and conference were excluded from the study.The framework of this review is established along with the following topics, namely, the rational design of membrane materials–the precise regulation of membrane macro-/mic ro-structure–the optimization of membrane mass transport mechanisms, as shown in Fig.1.The relationship between membrane macro-/micro-structure and pervaporation performance is summarized and discussed by taking inspiration from the previous works, which will provide some new ideas and strategies for developing a new generation of ethanol perm-selective membranes with high-performance.Furthermore, the tentative perspective on the future directions of ethanol perm-selective membrane is briefly proposed.

Fig.1. The framework of this review.
2.Fundamentals of Pervaporation
2.1.Transport mechanisms of PV
The membrane structure-performance relationship is always one of the key scientific issues for the design and preparation of membrane materials and membranes with high separation performance, and a deep insight into mass transport mechanisms is essential to elucidate membrane structure-performance relationship [23,24].There are several transport mechanisms proposed for describing the mass transfer in pervaporation membranes,including solution-diffusion mechanism [25,26], facilitated transport mechanism [27], and pore-flow mechanism [28],etc.Due to the complex interaction between membrane materials and permeates, the mass transfer in the membrane is quite complicated and there is no such a universal model to characterize every detail of mass transfer in membranes [24].
2.1.1.Solution-diffusionmechanism
The solution-diffusion mechanism is currently the most widely applied transport mechanism in pervaporation.There are three distinct and consecutive steps in pervaporation process as shown in Fig.2: (I) sorption of permeates onto the membrane surface;(II) diffusion of permeates across the membrane driven by chemical potential difference and(III)finally desorption at the permeate side of the membrane.Sorption and diffusion steps are rategoverned steps since the desorption step is usually extremely fast.Sorption process is governed by thermodynamics while diffusion process is determined by mass transport kinetics.It is proposed that an appropriate chemical microenvironment to ensure high solubility and a well-designed microstructure to ensure high diffusivity are indispensable for the ideal membranes [7].

Fig.2. Schematic of the solution-diffusion mechanism.
Hydrophobic microenvironment and strong interaction between membrane materials and ethanol molecules are in favor to obtain high ethanol solubility.The highly hydrophobic groups are in favor of lowering the chemical potential of ethanol molecules at equilibrium state,thus increasing the driving force of sorption process.Moreover, the employed hydrophobic groups are of water-repellency,thus intensifying the selective capture of ethanol molecules from ethanol/water mixtures.Besides, the strong interactions between membrane materials and ethanol molecules are beneficial to weakening the coupling effect between ethanol and water molecules, thus inhibiting the diffusion of water along with ethanol molecules.According to the solubility parameter theory[29],the closer the solubility parameters of ethanol and membrane materials,the higher ethanol perm-selectivity would be.The most extensively adopted materials for ethanol perm-selective PV membranes are polymers with hydrophobic chains or low-surfaceenergy groups [30–37].
The diffusivity of small molecules across membranes is primarily associated with physi-/chemical properties of penetrants (i.e.,kinetic diameter, shape and polarity) and membrane materials (i.e., free volume, flexibility and intermolecular forces), as well as the intermolecular interactions among penetrants and membranes.The polarity of ethanol(relative polarity:0.654)is different from that of water molecule (relative polarity: 1) [9].As kinetic diameter of ethanol molecules (0.45 nm) is larger than that of water molecules(0.26 nm),the intrinsic feature of larger molecules permeating preferentially, and smaller molecules being intercepted increases the difficulty in achieving high ethanol diffusion selectivity.Ordinarily, the competitive diffusion of ethanol and water molecules in the membrane generally results in much lower ethanol diffusion selectivity than ethanol sorption selectivity [38–40].The selective diffusion of ethanol molecules becomes the ratecontrolling step,while selective sorption is the dominant contribution step in most pervaporation process for ethanol recovery.Therefore, improving diffusion selectivity is crucial to enhance membrane selectivity for ethanol recoveryviapervaporation.It is found that incorporation of hydrophobic porous fillers into polymer matrix is an efficient method to improve ethanol diffusion selectivity due to the enlargement of cavity size and precise control over cavity size distribution in polymer as well as construction of suppressed water transport pathways [40–42].
2.1.2.Facilitatedtransportmechanism
The facilitated transport mechanism is usually used to explain the preferential permeation of gas or liquid molecule through a mixed matrix membrane.Fillers in the membrane act as facilitated transport carriers to accelerate the transfer of the targeted species in addition to the normal Fickian diffusion [43].According to the mobility of facilitated transport carriers,facilitated transport membranes can be categorized into three types, including mobile carrier, semi-mobile carrier and fixed-site carrier [27,44].As for ethanol perm-selective membranes, most of the membranes are fixed-site carrier membranes.In a fixed-site carrier membrane such as mixed matrix membranes as shown in Fig.3, reversible chemical reactions (E + C⇌EC) take place between the carriers(C) and the permeating species (ethanol) in the feed, whereas C and EC are constrained within the membrane [7], which lead to a preferential diffusion of ethanol through the membrane and membrane selectivity for ethanol.
2.1.3.Pore-flowmechanism
The pore-flow model proposed by Okada and Matsuura [45] is an alternative model to clarify the mass transport phenomena during pervaporation, especially for the pervaporation membrane with micropores.The model assumes that there are straight cylindrical pores across the membrane and the pervaporation is taking place in the pores.Pervaporation consists of three consecutive steps in series as shown in Fig.4: (i) the permeants transport through the liquid-filled portion of the pore; (ii) a liquid-tovapor-phase change takes place inside the pore;(iii)the permeants transport through the vapor-filled portion of the pore[23].In contrast to the solution-diffusion model,the pore-flow model concentrates on clarifying the permeation phenomenon of a liquid mixture through a porous asymmetric membrane.Many works[28,46] published on poly(vinylidene fluoride) (PVDF) membranes for ethanol recovery from aqueous solutions are typical examples.Sukitpaneenitetal.[47]developed a modified pore-flow model by factoring in the contribution of Knudsen flow to vapor transport,which was ignored by the pore-flow model.Based on the plenty of works,it is concluded that the solution-diffusion model is more applicable for those membranes without pores, while pore-flow model is a better alternative for those with pores.
2.2.Evaluation of membrane performance
The comprehensive PV performance of membranes is usually evaluated by permeation flux(J,kg∙m-2∙h-1)and separation factor(α).In the case of a binary ethanol/water mixture, the permeation flux and separation factor can be experimentally obtained according to the following equations:
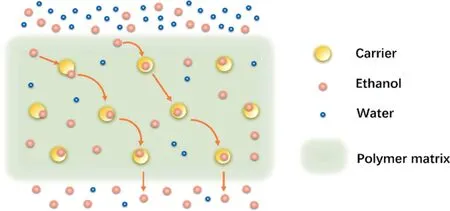
Fig.3. Schematic of the facilitated transport mechanism.
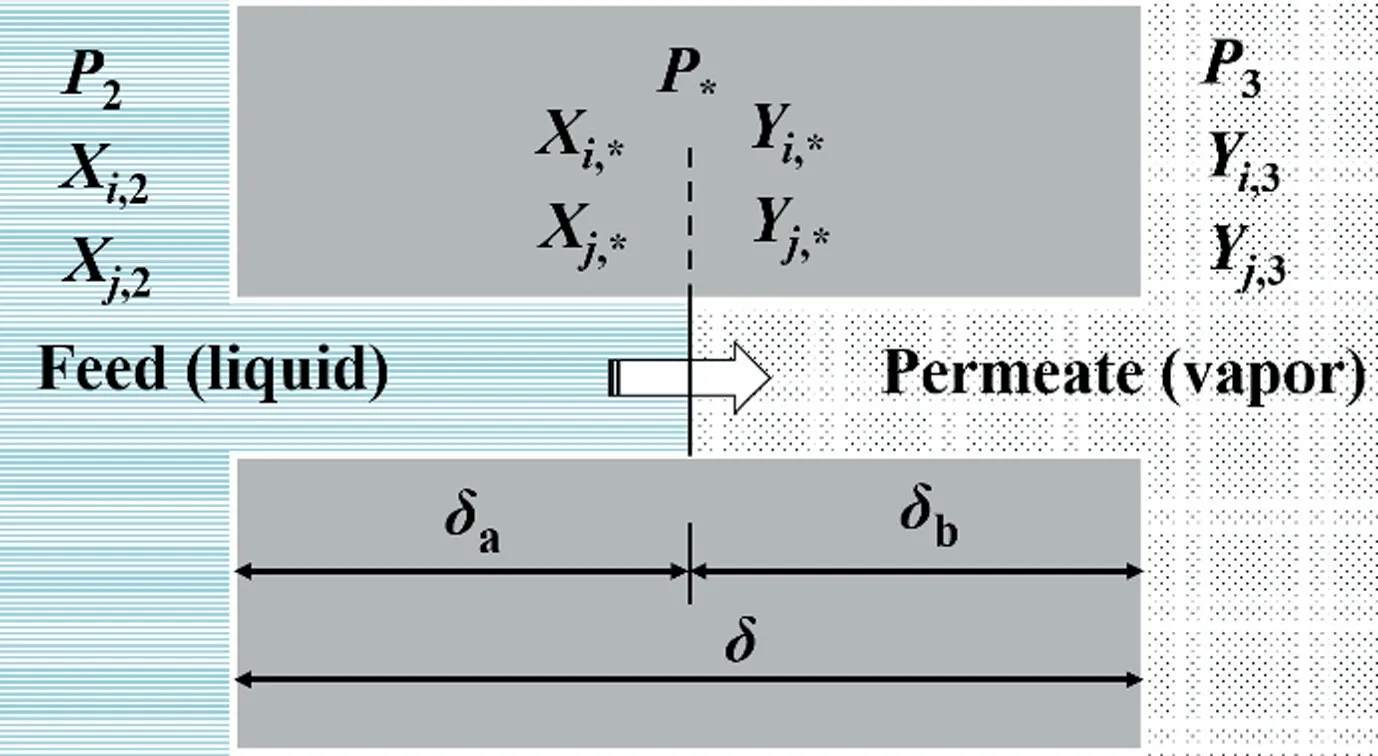
Fig.4. Schematic of the pore-flow model [47].

whereQrepresents the total quantity(in gram or mole) of the collected liquid through the effective membrane areaAin timet.Where α stands for the enrichment factor,yE(yW) andxE(xW) are the weight percent of ethanol(water)in the permeate and feed side,respectively.
To evaluate the overall performance of a membrane,pervaporation separation index (PSI, kg∙m-2∙h-1) is defined as a product of permeation flux and separation factor,

Moreover, Tothetal.[48,49] created a simply and valuable method called membrane flash index (MFLI) to evaluate PV membranes’ capabilities in liquid separation compared to traditional distillation, which is mainly based on the comparison of available theoretical maximum distillate compositions.

The values of permeation flux and separation factor strongly depend on both the intrinsic properties of the membranes and the operation conditions(such as feed concentration,feed temperature,and permeate pressureetc.).As operation conditions change,the values of permeation flux and separation factor change as well,which makes it difficult to evaluate the intrinsic pervaporation performance of membranes.Furthermore, these values may lead to confusion in understanding the real trend of membrane intrinsic properties as operation conditions change.Therefore,three driving force normalized terms are proposed to evaluate the intrinsic pervaporation performance of membranes, including membrane permeabilities (Pi), permeances (Pi/l) and selectivities (ßij) [50–52]
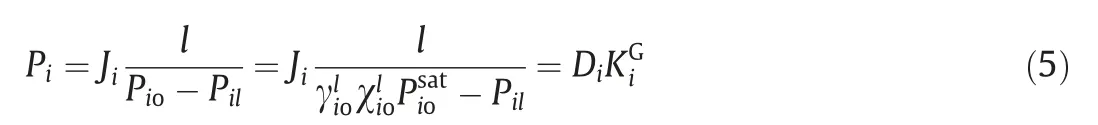
Membrane permeability, a component flux normalized for membrane thickness and driving force.Diis the membrane diffusion coefficient(cm-2∙s-1)of componenti,and(cm3(STP)∙cm-3-∙cmHg-1, 1 cmHg=1333.22 Pa) is the sorption coefficient of componenti.WhereJistands for the permeation flux of componenti;lis membrane thickness;PioandPilrepresented the permeate and feed side pressures of componenti, respectively;is the activity coefficient of componentiin feed solution;is the mole fraction of componentiin feed solution;represented the saturated vapor pressure of componentiin feed solution.The activity coefficient γ can be calculated with the UNIFAC model through Aspen Plus simulation software or predicted using the Wilson equation [51].
There are several IUPAC-type units for membrane permeability(Pi)[50],including Barrers(1 Barrer=1×10–10cm3(STP)∙cm∙cm-2-∙s-1∙cmHg-1), m3(STP)∙m∙m-2∙s-1∙kPa-1(1 m3(STP)∙m∙m-2∙s-1-∙kPa-1=1.33 × 1014Barrer), g∙m∙m-2∙h-1∙kPa-1[42] and mol∙m∙m-2∙h-1∙kPa-1[39].

Membrane permeance (Pi/l) is most commonly reported as gas permeation units (GPU) (1 GPU=1 × 10-6cm3(STP)∙cm-2∙s-1-∙cmHg-1, 1 m3(STP)∙m-2∙s-1∙kPa-1=1.33 × 108GPU).
Selectivity is determined by the relative permeability of two components.Permeability, permeances and selectivity represent the intrinsic transport properties of a material to a penetrant,which is considered to be a preferred way to evaluate the contribution of intrinsic properties of membrane to pervaporation performance [50].
3.Membranes for Ethanol Recovery via Pervaporation
Macro-structures and micro-structures of membranes are key factors determining the PV performance.According to membranes’macro-structures, pervaporation membranes can be divided into three groups, including free-standing homogeneous membranes,supported composite membranes, and asymmetric porous membranes.Compared with the first two dense membranes,asymmetric porous membranes exhibited higher permeability and lower selectivity due to their porous properties [28].Free-standing homogeneous membranes usually can’t meet the industrial application due to low permeability and poor mechanical properties,which are often used for the selection of membrane materials in the initial stage of laboratory research.Supported composite membranes are generally composed of porous supports and a thin dense selective layer,which provide both low trans-membrane resistance and comparable selectivity [53,54].The selection of macrostructure of membrane plays an important role in pervaporation performance due to the different permeation characteristics of small molecules across membranes.Chang’s group and our group[55,56] found that multi-layer composite membranes with multilayer configuration showed much better pervaporation performance than common bi-layer composite membranes due to the different concentration profile and mobility of the penetrants in membranes.As for micro-structure of membranes, free volume and cavity size distribution in polymers,pores in porous materials,and interfacial structures between organic and inorganic phases as well as micro-environment in membranes have significant effect on the sorption and diffusion properties of small molecules in membranes, which consequently determine the pervaporation performance [41,42,57].As both macro-structures and microstructures are strongly dependent on membrane materials, the structure-property-performance relationships are discussed from the perspectives of tailoring membrane materials for the enhancement of PV performance in this review.
3.1.Polymeric membranes
According to the solubility parameter theory [58], Flory–Huggins theory [59] and solution-diffusion model [24–26], the polymeric materials with strong hydrophobicity, weak polarity and similar solubility to ethanol are generally selected to enhance the sorption selectivity of alcohols in membranes, and the polymeric materials with larger free volume are selected to weaken or eliminate the adverse effects to the ethanol diffusion selectivity due to larger size of ethanol molecules.Therefore, hydrophobic polymers are commonly developed as the most versatile and prospective membrane materials for ethanol recovery via pervaporation,including silicon-containing polymers and other polymers.The PV performance of polymeric membranes are listed in Table S1(Supplemetary Material).
3.1.1.Silicon-containingpolymers
There are mainly two types silicon-containing polymers, silicone rubber and glassy polysilypropyne as shown Fig.5.Silicone rubber, featured with hydrophobic backbone comprised entirely of Si–O bonds, such as polydimethylsiloxane (PDMS), exhibits an ethanol sorption selectivity [60].Molecular diffusion across PDMS membranes is strongly dependent on free volumes in membranes,which provide diffusion channels with low transport resistance.Based on the positron annihilation lifetime spectroscopy characterization,it was generally found that there are two kinds of free volume cavities in cross-linked PDMS membranes, including small free volume cavity (network pores reflected by lifetime τ3) and large free volume cavity (aggregate pores reflected by lifetime τ4)[41,42].The network pores refer to the interstitial small cavities in the cross-linked site while the aggregate pores refer to the free volume between the clusters of networks.Based on the PALS results reported in literatures [41,42], the radii (r3) of small free volume cavity of PDMS membranes was ranging from 0.19 to 0.33 nm,and radii(r4)of large free volume cavity was ranging from 0.38 to 0.43 nm.Benefiting from PDMS’ large free volume cavity and low glass transformation temperature, PDMS possesses good permeability for small molecules which is in favor of ethanol diffusion in membranes.The ethanol/water separation factors reported for pristine silicone rubber membranes are in the range of 4.4–10.8,with permeation fluxes generally far below 1.000 kg∙m-2∙h-1[61].O’Brien’ group [62] made an economic analysis of ethanol production by a PV process coupled with a fermentor and concluded that a membrane with a permeation flux of over 0.150 kg∙m-2∙h-1and a separation factor of no less than 10.3 would be cost-competitive with distillation alone on a commercial scale.Vaneetal.[6]proposed that extractants with ethanol–water separation factors closer to 20 might be required to be competitive with distillation on an energy basis.Therefore, the low separation factor and permeation flux of PDMS has become the main bottleneck restricting its industrial application.

Fig.5. The chemical structures of silicon-containing polymers.
The significant variation in pervaporation performance of PDMS membrane is mainly related to the different preparation conditions(functional side groups in silicone rubber [63], molecular weight[64,65], support layer [54,66], crosslinking conditions [67,68],membrane thickness[69–71])and operation conditions(operation pressure,feed temperature,feed concentration,and feed flow rate,etc.) [72].Typical silicone rubber includes PDMS, poly(octhylmethyl siloxane) [72,73], poly(phenyl methyl siloxane) (PPMS)[74],poly(ethoxy methyl siloxane)(PEOMS)[63],and poly(trifluropropyl methyl siloxane) (PTFMS) [63]etc.Based on the swelling experiments and solubility parameters theory, it was found that the affinities to ethanol of these membranes followed the order,PPMS > PEOMS > PDMS > PTFMS, which was consistent with the order of separation factors.The introduction of phenyl groups,ethoxy groups,and trifluropropyl led to low chain mobility of silicone rubber and resulted in low diffusion of permeating molecules[63].
The porous substrate used for preparation of PDMS composite membranes are commonly PVDF[66,75],polysulfone[53,76],polyimide[54],and cellulose acetate[74,77],ceramic supports[78],etc.The intrusion of PDMS casting solution into porous support can’t be ignored, which might bring unfavorable effects on permeation flux due to the increased transport resistance.Some works[54,77] proposed pre-wetting method for the support layer with water or other solvents to alleviate the intrusion of PDMS casting solution into porous support.The permeance of silicone membrane could be enhanced by decreasing the thickness of silicone selective layer [69,70,79].However, PDMS composite membranes with ultra-thin PDMS selective layer sometimes lead to non-selective defects in active layer and consequently the lower separation factor.Jadav’s group [79] found that ultra-thin PDMS membranes exhibited relatively higher amount of the chain aggregates and a relatively loose structure responsible for the low selectivity but high flux in the separation of ethanol from water by pervaporation.Zongetal.[69] fabricated sub-micron-thick (700 nm) PDMS composite membranes without defects using a facile scale-up coating method by decreasing viscosity of the membrane solution, which showed a flux as high as 2.4 kg∙m–2∙h-1and separation factor of 8.6 at 40 °C for 5% (mass) ethanol aqueous solution.Results indicated that the coating with an optimum PDMS solution viscosity was an efficient approach for the large-scale fabrication of submicron-thick membranes.Jiaetal.[70] proposed the use of lowsurface-energy monomer into casting solution to enhance its spreading ability for ultrathin silicone membrane fabrication.An ultrathin poly(dimethyldiethoxylsilane) (PDMDES) active layer with thickness of submicrometer size was obtained, and the PDMDES composite membrane exhibited a superior flux of 7.566 kg∙m–2∙h-1and maintained a comparable separation factor when compared to traditional PDMS membranes.
Crosslinking is proved to be an important and inevitable step to improve PDMS film-forming properties and structure stability.The selection of crosslinker is ordinarily dependent on the PDMS types,such as hydroxyl-terminated PDMS and two-component vinylterminated PDMS as shown in Fig.5.Although crosslinking reagents only accounts for a small proportion in crosslinked PDMS membranes, ranging from 1% to 10%, the effect of crosslinking reagent on the separation performance is remarkable.Several groups [67,80] found that PDMS membranes fabricated using phenyl-containing tri-functional crosslinkers exhibited a higher permeate flux and separation factor than the membrane prepared using the tetra-functional crosslinker because their less crosslinked structures increased the free volume and enhanced hydrophobicity.Qin’s group [35,81] proposed an ultraviolet (UV)-induced crosslinking strategy and realized the ultrafast and continuous fabrication of the PDMS membrane,in which UV crosslinking of PDMS could be completed within 30 s as shown in Fig.6.Liuetal.[35]prepared 3-methacryloxypropylmethyldimethoxysilane (PTFPMS)UV-crosslinked PDMS-PTFPMS block copolymer membranes for ethanol pervaporation and found that the strong C–F bond contributed to enhancement of membrane hydrophobicity and an outstanding separation performance.Zhengetal.[33] and Zhuetal.[34]also fabricated fluorinated PDMS membrane using fluoroalkylsilane (FAS) as cross-linking agent, which exhibited much higher total flux and separation factor than the pristine PDMS membrane due to the anti-biofouling and hydrophobic properties.The results illustrated that improving PDMS membrane’s hydrophobicity was an effective way to improve the membrane selectivity for ethanol/water separation.The variation of pervaporation performance of PDMS crosslinked with different reagents might be attributed to the changes in hydrophobicity, fraction of free volume and the cavity size and distribution raised from different crosslinked networks.

Fig.6. Fabrication processes of PDMS membrane via (a) conventional thermal-crosslinking approach and (b) UV-polymerization approach [81].
To achieve superior membrane performance of PDMS membrane, various physical and chemical modification methods are often employed either at the preparation stage or by a posttreatment step.Physical modification of PDMS membranes can be achieved by surface coating with hydrophobic polymers [37],blending with polymer or hydrophobic molecules [82,83], interpenetrating with polymers [60],etal.Heetal.[37] prepared polydivinylbenzene (PDVB)-coated PDMS composite membranes with super-hydrophobic surface, which showed a higher ethanol recovery performance with the separation factor and total flux increased by 13% and 30%, respectively.Puspasarietal.[82] fabricated cellulose/PDMS blending membranes for ethanol recovery,which displayed an excellent pervaporation performance with a 100% increase of the separation factor when compared with pure PDMS due to the good miscibility of the blending polymers.Cabezasetal.[83] prepared PDMS-coated [P6,6,6,14][Tf2N] ionic liquid(IL) gel membranes, which showed ultrahigh separation factor of 483 for ethanol and low permeation flux of 5.824 × 10–3kg∙m-2∙h-1due to the hydrophobic character of the ionic liquid.Leónetal.[84] modified PDMS with hydrophobic 1-dodecanol and found that the separation factor increased with the concentrations of 1-dodecanol in the polymeric layer while total flux decreased.Ahmedetal.[85] prepared PDMS/PS interpenetrating polymer network (IPN) membranes by sequential IPN technique,which exhibited a higher selectivity but a somewhat lower permeability compared with pristine PDMS membranes.This was ascribed to that the crosslinking of rigid PS polymer and physical entanglement between molecules led to higher degree of network chain segment concentration and crosslinking density, which reinforced swelling resistant and suppressed the membrane permeability.
Chemical modification of PDMS membranes are usually achieved via grafting,block copolymerization andin-situpolymerization,which can endow the membrane with intrinsic advantages of both or more polymers and enhance the PV performance of pristine membrane [86].Yunetal.[87] prepared PDMS-graft copolyimide membrane exhibiting an ideal property for membrane material, such as high organic perm-selectivity, process ability and durability to several organic solvents.The high permeability of the membrane would be resulted from the continuous phase of flexible PDMS domain, and the mechanical strength would be derived from the hard shell consisted of polyimide backbone.Jiaetal.[88] employed a copolymerization modification method to fabricate poly(vinyltriethoxysilane)/hydroxy silicone oil (PVTESHSO) membrane, which showed the best performance with the separation factor of 6.6 and flux up to 8.16 kg∙m-2∙h-1when separating 9% (mass) ethanol aqueous solution at 35 °C.Sietal.[89]reported that fluoromonomer was introduced into methacrylate-PDMS membrane solution and was initiatedin-situUVpolymerization to fabricate fluoro-modified PDMS membranes.Compared with pristine PDMS membrane, the fluoro-modified PDMS membranes showed a simultaneously enhanced permeation flux and separation factor for butanol/water separation.
Another typical silicon-containing polymeric membrane material for ethanol recovery is poly [1-(trimethylsilyl)-1-propyne](PTMSP),which is a super glassy polymer with better process ability and scalability as well as much higher free volume than silicone rubber membranes [90,91].Consequently, PTMSP exhibits much higher separation factor and permeation flux than PDMS,with separation factor ranging from 8 to 26, and permeation flux ranging from 0.052–7.600 kg∙m-2∙h-1[24].However, the glassy polymer PTMSP suffer from rapid physical aging as polymer chains undergo physical relaxation with time and are prone to converge towards a thermodynamic equilibrium,resulting in the reduction in free volume and decay of both permeability and selectivity over time.Several works found similar decline phenomenon in PV performance of PTMSP membranes with various thickness, and the decline becomes more serious for thinner membranes or at higher operation temperature [92].
Some methods [93,94] have been developed to stabilize the PTMSP membranes and to further improve the ethanol-selective separation performance for brightening the application prospects of this material, such as grafting with PDMS, semi-IPN method,copolymerization with other polymers,etc.Nagase’s group [95]found that all PTMSP membranes grafted with PDMS in any proportion delivered higher selectivity to ethanol than pristine PTMSP and PDMS alone due to the introduction of a short PDMS side chain into a PTMSP backbone.But the commercial application of PTMSP membranes is still limited at this time due to the decay of pervaporation performance.Further research should be concentrated on developing novel preparation and modification methods of PTMSP-based membranes to resist the physical aging.
3.1.2.Otherpolymers
Benefiting from the good hydrophobicity, fluoro-containing polymers are employed for the ethanol recovery via pervaporation as shown in Fig.7.Sukitpaneenitetal.[28] prepared PVDF asymmetric hollow fiber membranes for ethanol–water separation which demonstrated remarkable high fluxes of 3.500–8.80 kg∙m-2-∙h-1and reasonable ethanol–water separation factors of 5–8.Aroujalianetal.[96] used a porous polytetrafluoroethylene (PTFE)membrane (0.2 mm pore diameter) for ethanol/water separation which produced an ethanol flux of 0.465 kg∙m-2∙h-1with selectivity near 2.25 at 60 ℃.Nakaoetal.[97] applied a commercially available microporous PTFE membrane (0.2 μm pore diameter)into a continuous ethanolic fermentation system.It displayed an attractive permeation flux with a value of 5.7 kg∙m-2∙h-1, while the ethanol concentration in the permeate was 6–8 times higher than that in feed.Compared with dense membranes, the hydrophobic porous membranes exhibited higher permeability but lower selectivity due to their porosity, which was strongly restricted by membrane morphology, pore size, and pore distribution.Zhengetal.[98]proposed a two-step reaction method to tailor the porosity and hydrophobicity of polyacrylonitrile (PAN)ultrafiltration membrane with perfluoro1-octanesulfonyl fluoride.The fluoroalkyl grafted membranes exhibited a high flux ofca.10.5 kg∙m-2∙h-1and a separation factor ofca.12 for ethanol recovery from aqueous solution via vapor permeation, which outstripped most polymeric membranes.The research provided a new insight into the membrane modification and process design for the application of fluoro-containing porous membranes in ethanol recovery.

Fig.7. The chemical structures of other ethanol perm-selective polymers.
Apart from the investigations in silicone-containing polymers and fluoro-containing polymers for ethanol recovery via pervaporation, several researches have been devoted to the development of new ethanol perm-selective materials, such as poly(etherblock-amide) (Pebax) [52], polymer of intrinsic microporosity(PIM-1) [99], polyphosphazene heteropolymers (PPP) [100],polysiloxaneimide (PSI) [101],polystyrene-bpolydimethylsiloxane-b-polystyrene (SDS) [102] and styrenebutadienestyrene (SBS) [103]etc., as shown in Fig.7.Liuetal.[104]reported that PEBA 2533 membranes exhibited poor separation factor and permeation flux especially with thinner selective layer due to the weak affinity between ethanol and Pebax membrane.Shietal.[105] fabricated membranes composed of octamethyl cyclotetrasiloxane, vinyl heptamethyl cyclotetrasiloxane,styrene, and divinyl benzene via the concentrated emulsion polymerization method, which displayed a permeation flux of 0.070 kg∙m-2∙h-1and a separation factor of 7.4 for 30% (mass)ethanol aqueous solution.Huangetal.[100] prepared a series of polyphosphazene heteropolymers with different hydrophobic pendant groups for ethanol/water separation, and the polyphosphazene membrane with –OCH2CF3substituting groups showed the highest separation performance both in permeation flux and separation factor, which were 0.26 kg∙m-2∙h-1and 6.1, respectively.Songetal.[101] reported a novel composite membranes using polysiloxaneimide (PSI) as a selective layer formed by nonsolvent induced phase inversion, which showed the highest separation factor of ~3 with corresponding flux of 0.5 kg∙m-2∙h-1.Shinetal.[102] demonstrated the efficacy of polystyrene-bpolydimethylsiloxane-b-polystyrene (SDS) triblock copolymer membranes forinsituproduct removal of biofuels by pervaporation during ABE fermentations,which exhibited higher permeabilities than a commercially available cross-linked PDMS membrane.Santoroetal.[103]prepared styrene-butadienestyrene(SBS)dense symmetric flat membranes and composite membranes made of SBS coated on a highly porous support of Fluoroplast F-42.It was found that an intermediate layer of PDMS or poly urethane (PU)was an effective way to improve the ethanol/water selectivity of the composite flat membranes in comparison to pure flat SBS membrane.
Thongsukmaketal.[106] used tri-n-octylamine (TOA) liquid membrane with coated porous hollow fibers for ethanol recovery.The selectivity of ethanol increased considerably to as much as 113 at 54 °C and the permeation flux of ethanol for ~10% (mass) ethanol in feed obtained was 0.016 kg∙m-2∙h-1.Rdzaneketal.[107]prepared two kinds of supported ionic liquid membranes (SILMs)immobilized with the help of PEBAX forn-butanol recovery.To prevent the ionic liquids from leaching out, an additional amount of PEBAX was applied directly on the IL-PEBA layer.It was found that incorporation of IL increased membrane selectivity, while decreased permeation flux due to the depressed water flux by IL.The PV performance of SILMs forn-butanol recovery suggested that hydrophobic IL might be a promising ethanol perm-selective material.
As reviewed above, the pristine ethanol perm-selective polymers still can’t meet the requirements of industrial application,including both high permeability and ethanol selectivity.Moreover, the membrane selectivity and permeability of pristine polymer membrane present typical trade-off effect, and the comprehensive separation performance, especially the separation factor should be effectively enhanced furthermore for industrial application.
3.2.Organic/inorganic hybrid membranes
Organic/inorganic hybrid membranes can inherit superior attributes from both the polymeric and inorganic membranes and overcome the limitation of both polymeric and inorganic membranes[7].According to combination ways of polymeric and inorganic materials, there are two major classes of hybrid structures,concrete-like structure and sandwich-like structure.In sandwichlike structure, multilayers are constructed by combining inorganic layers and organic layers together.In concrete-like structure, the dispersed phase of multifunctional fillers is embedded in polymer continuous phase, which result in mixed matrix membranes(MMMs)[108].Based on the literature reports,most hybrid membranes are mixed matrix membranes due to the easiness of producibility and excellent pervaporation performance.The rapid development of MMMs also provides a new thought for the design and preparation of PV membranes.
3.2.1.Sandwich-likehybridmembranes
The hybrid membranes fabricated by the layer by layer (LbL)self-assembly method often exhibit ‘‘sandwich-like structure”.But in this review,sandwich-like hybrid membrane refers to membranes with multi selective layers composed of two or more polymeric/organic layersviadifferent interactions.Ikegami’s group[21] reported silicalite-1 membrane with silicone rubber coating for concentration of ethanol and fermented ethanol by pervaporation,with the highest separation factor of 125 and permeation flux of 0.14 kg∙m-2∙h-1.Most hybrid membranes with sandwich-like structure used for ethanol recovery are inorganic membranes covered with organic layer so as to alleviate the defects or pores of inorganic membranes by penetration or modification with organic materials, which has been proved to be an effective method to enhance the selectivity of organic membranes.
Panetal.[36] prepared superhydrophobic ZIF-8 /PDMS/PVDF hybrid membrane with a nano-level bud-like ZIF-8 layer grown on PDMS membraneviaZIF-8 particle dip-casting, secondary seeded growth, and hydrophobic modification bynoctadecylphosphonic acid for ethanol/water separation.The resultant optimal sandwich-like hybrid membrane displayed a separation factor of 17.4 and total flux of 0.64 kg∙m-2∙h-1with 5%(mass) ethanol aqueous solution at 30 °C.The strategy of constructing superhydrophobic inorganic layer on PDMS membrane might provide another promising method of preparing sandwichlike hybrid membranes.
3.2.2.Mixedmatrixmembranes(MMMs)
MMMs usually consist of a continuous polymer matrix phase and a dispersed inorganic filler phase, hoping to marry the high intrinsic permeability and separation properties of advanced fillers with the robust processing and mechanical properties of polymers[18].Based on the literature reported, the most common used ethanol perm-selective MMMs are still based on the PDMS matrix.The rich diversity of inorganic fillers provides plenty of ways to increase membrane hydrophobicity, optimize the macro- and micro-structure of MMMs and construct preferred transport pathways for ethanol molecules.The PV performance of various MMMs for ethanol recovery from aqueous solution are listed in Table S2.To obtain desirable MMMs with high pervaporation performance,the advancement of MMMs still faces several major challenges as shown in Fig.8: (a) homogeneous particle dispersion; (b) integration of particles and matrix; (c) ultra-thin selective layer without defects; (d) continuous mass transfer pathways; (e) better control over cavity size and distribution.Plenty of research focused on the effect of various preparation conditions of MMMs on pervaporation performance, including filler type, particle size, particle loading,polymer matrix, pre-crosslinking of polymer matrix, filler dispersion method, the interface compatibility and interactionetc.
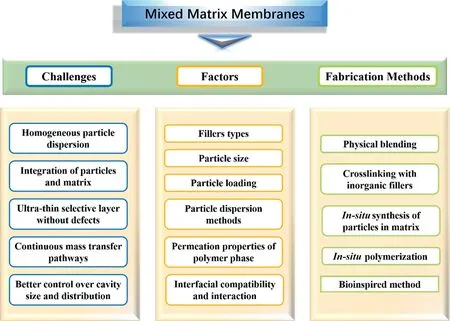
Fig.8. The challenges, factors, and fabrication methods of mixed matrix membranes.
Fillerstypes
According to the differences of dimensions, fillers are commonly divided into three categories, one-dimensional (1D), twodimensional (2D), and three-dimensional (3D) fillers as shown in Fig.9.Typical 1D and 2D fillers used in ethanol recovery include carbon nanotubes (CNTs) [109,110] and graphene oxide (GO)[111,112].3D materials are mainly MFI-type zeolite (silicalite-1,ZSM-5) [113–115], silica [116,117], metal organic frameworks(MOFs) [118–122], and polyhedral oligomeric silsesquioxanes(POSS) [39,42]etc.
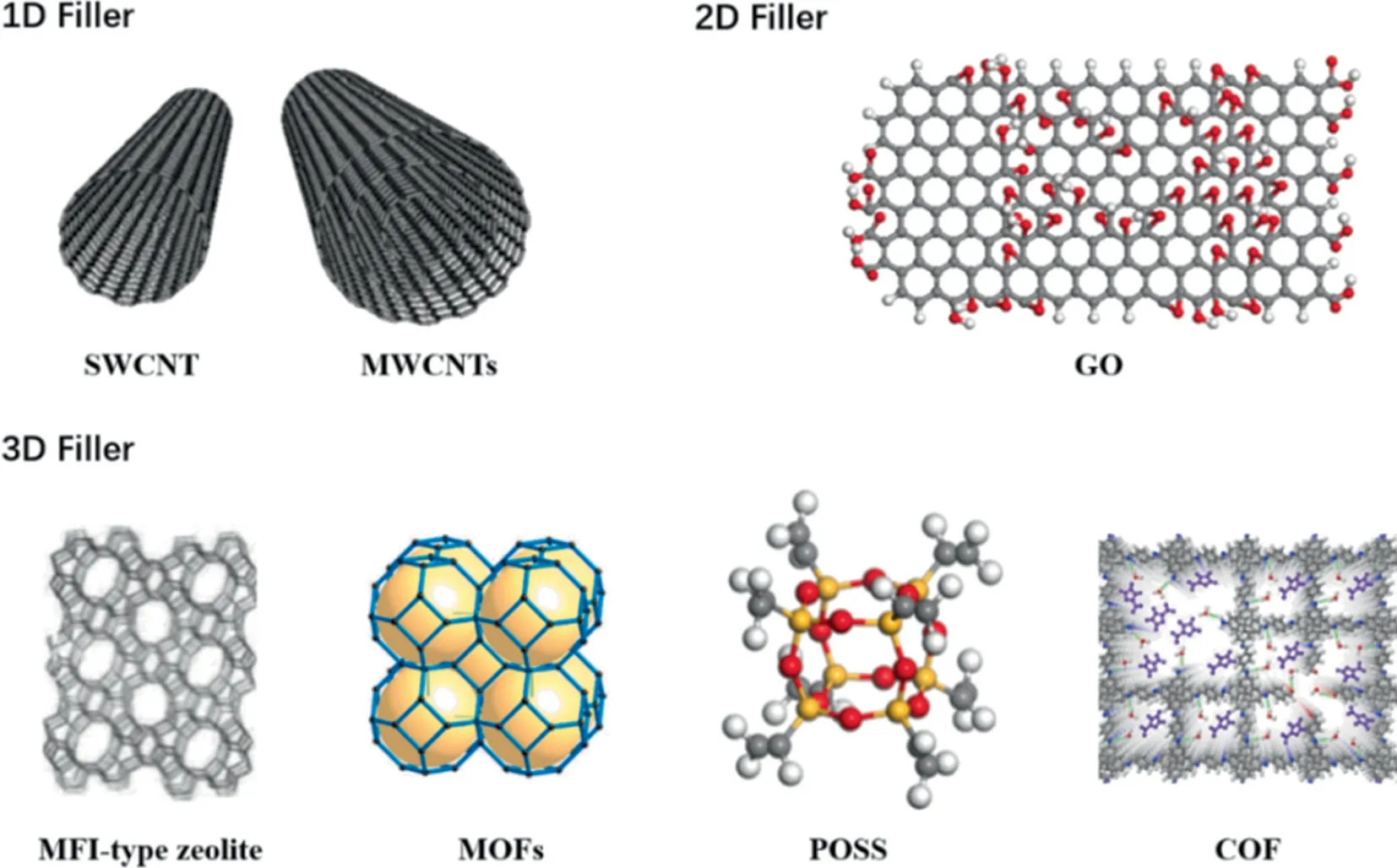
Fig.9. 1D, 2D, and 3D fillers commonly used in MMMs for ethanol recovery.
Nowadays,the mixed matrix membranes composed of CNTs/GO and polymers,are more widely used for pervaporation dehydration rather than for ethanol recovery from aqueous solution.This is mainly ascribed to the hydrophilicity and super-fast water transport channels provided by CNTs and GO [123].However, based on the comparison of the channel diameter of CNTs (0.7 to 2.0 nm)and interlayer distance of GO(0.6–1.2 nm)with the kinetic diameter of ethanol and water molecules (0.43 and 0.26 nm,respectively),it is possible for ethanol to transfer through the CNTs 1D channel or interlayer capillary pores between GO layers.Moreover,it was also confirmed experimentally that the almost frictionless interface at the carbon-nanotube wall or the non-oxidation zone of GO interlayer space resulted in very rapid diffusion of ethanol molecules [123,124].However, there are only several investigations on CNTs/GO filled MMMs for ethanol recovery via pervaporation.Xueetal.[109], Marjanietal.[110] and Albertoetal.[112] prepared CNTs/PDMS, CNT/polyamide (PA), and GO/PIM-1 MMMs,respectively.The MMMs displayed better PV performance than pristine polymeric membranes, but the improvement was still very limited.
To enhance the PV performance of MMMs,surface modification of GO was deployed to improve the surface hydrophobicity.Zhuetal.[111]reported theinsitugrowth of ZIF-8 particles on GO surface to fabricate ZIF-8@GO/PDMS MMMs as shown in Fig.10.The separation factor and permeation flux of ZIF-8@GO/PDMS MMMs for ethanol/water separation were 22.2 and 0.444 kg∙m-2∙h-1,respectively.The enhanced pervaporation performance was attributed to the synergistic effect of GO nanosheet as a strong barrier and hydrophobic ZIF-8 nanoparticles with the continuous inner channels.Tangetal.[125] and Lietal.[126] prepared ionic liquid(IL) modified GO/Pebax MMMs and ZIF-8 modified GO/Pebax MMMs for butanol recovery from aqueous solution, which exhibited better butanol recovery performance than pristine Pebax membranes due to the good affinity and hydrophobicity of IL/ZIF-8 for butanol.The results mentioned above suggest that chemical micro-environment of GO may play an important role in the solution-diffusion process of ethanol/water molecules.Surface modification of GO nanosheets with hydrophobic materials might be a promising method for MMMs to enhance alcohol selectivity by improving GO surface hydrophobicity and benefiting from the synergistic effect of GO and other ethanol perm-selective fillers.Amreietal.[127] prepared CNT-GO/PDMS MMMs, which showed the highest ethanol selectivity of 22.5 with 0.75% (mass) of CNT/GO loading due to the synergistic effect of CNT and GO.
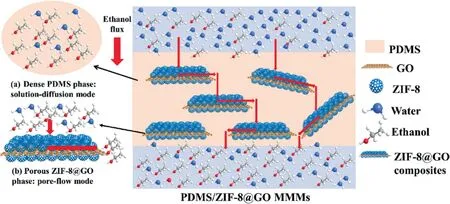
Fig.10. Schematic of ethanol recovery by ZIF-8@GO/PDMS MMMs [111].
MMMs prepared with 3D fillers, such as MFI-type zeolite(Silicalite-1, ZSM-5) [113–115], MOFs [118], silica [116,117], and POSS [39,42] are extensively investigated for ethanol recovery and show great potential performance.According to the literature reports, the most widely studied fillers and polymer in the past three decades were MFI-type zeolite and PDMS due to the medium pore size(nearly 0.55 nm)of zeolite and their hydrophobic properties.The MFI-type zeolite includes silicalite-1 composed of pure silica and ZSM-5 in which some Si atoms are partially substituted by Al.In general,silicalite-1/PDMS MMMs showed better selectivity for ethanol recovery than ZSM-5/PDMS MMMs due to the stronger hydrophobicity of silicalite-1.Jiaetal.[128]prepared silicalite-1/PDMS MMMs with filler loading up to 77%(mass)which showed the highest separation factor value of 59 reported for ethanol recovery so far and low permeation flux of 0.071 kg∙m-2∙h-1at 22°C.In order to obtain high selectivity,the zeolite loading is generally as high as 40%–77%(mass),while the permeation flux is still at a low level or even lower than that of pristine PDMS membranes.Moreover,the poor structure adjustable variability of zeolite is not conducive to the regulation of the internal pore structure, which restrains the further development of zeolite/PDMS MMMs.
Metal organic frameworks (MOFs) are composed of metal ions linked by organic ligands which offer an exceptionally high surface area, unique spatial structure adjustable variability and appealing compatibility with polymers.Zeolitic imidazole frameworks(ZIFs),a subclass of MOFs,maintains the similar pore structures with MFItype zeolite and exhibit great potential in ethanol recovery.Khanetal.[129], Panetal.[32] and Yinetal.[130] prepared ZIF-67/PDMS, ZIF-91/PDMS, and ZIF-71/PDMS MMMs, respectively, for the separation of ethanol/water mixtures via pervaporation.Compared to unfilled PDMS membranes, MMMs with 20%–40% ZIFs loading showed much better PV performance than pristine PDMS membranes.The incorporation of MOFs into PDMS has been proved to be an effective way to improve both separation factor and permeation flux with lower filler loading than that of zeolite,while the MOFs stability in organic solvents is still a great challenge to its industrial application in ethanol recovery from fermentation broths.
Polyhedral oligomeric silsesquioxanes (POSS) is a nano-sized cage-like hybrid inorganic/organic composite with hydrophobic Si–O–Si cage framework and external organic polar or nonpolar functional groups.The external organic functional groups can reduce the non-selective voids generated at the polymer-filler interface, making them compatible or miscible with most polymers.Leetal.[52] and our group [131] prepared POSS/Pebaxand POSS/PDMS MMMs, respectively, which showed much better PV performance than pristine polymer membranes.It was found that the best PV performance of POSS/Pebax and POSS/PDMS MMMs could be achieved with only 2% and 1.59% POSS loading, respectively.The POSS/PDMS MMMs showed the optimal flux of 2.2 kg∙m-2∙h-1(243% higher than that of pure PDMS) and corresponding separation factor of 9.3 (31% higher than that of pure PDMS) at 60 °C.Liuetal.[41] also found that methyl-POSS could finely control the conformation and topology of PDMS polymer chains with molecular-interaction-driven tunable free volumes.As applied to the bio-butanol recovery from aqueous solutions,the prepared POSS/PDMS MMMs exhibited a simultaneous increase in permeability and selectivity.The results certificated that POSS is a very promising candidate to prepare MMMs for ethanol recovery due to its hydrophobic properties, multi-functional groups and well-defined cage-like structure.Except for POSS,silica was proved to be a good choice for silicone based composite membranes due to the similar Si–O structure[132,133].Claesetal.[90]fabricated silica filled PTMSP MMMs, which exhibited a flux as high as 9.5 kg∙m-2∙h-1with an ethanol/water separation factor of 18.3.Sunetal.[134]prepared organophilic nano-silica filled PDMS MMMs with an excellent separation factor of 30.1 and permeation flux of 0.114 kg∙m-2∙h-1at 60 °C, which was ascribed to the enhanced solution and diffusion selectivity as well as accessible free volumes in the matrix.
With the rapid development of inorganic particles, many novel fillers were prepared and employed to modify polymer membranes, such as clay [135], hollow silicalite sphere [136], microporous silicalite-1 [137], MCM-41@ZIF-8 [138] and lotus leaf powder [37],etc.Samantaetal.[135] prepared nano-sized clay filled copolymer of butyl acrylate and styrene by emulsion polymerization in water.The MMMs with 2% clay loading yielded the best result with permeation flux of 0.34 kg∙m-2∙h-1and an ethanol selectivity of 26.4 at 30°C for 5%(mass)ethanol in water.Kamelianetal.[137] prepared a novel microporous silicalite-1/PDMS mixed matrix membrane by spraying on a flat ceramic membrane.The best performances of permeation flux of 3.64 kg∙m-2∙h-1and separation factor of 17.2 were obtained for separating 5%(mass)ethanol aqueous solution at 60 °C.
Except for inorganic fillers, many advanced microporous organic polymers (MOPs), such as covalent organic frameworks(COFs), polymers of intrinsic microporosity (PIMs), porous aromatic frameworks (PAFs), conjugated microporous polymers(CMPs), porous organic cages (POCs), and hypercrosslinked polymers (HCPs),have also been employed as fillers of MMMs for pervaporation separation of liquid mixtures.Wuetal.[139] prepared covalent organic frameworks (COF-LZU1)/PDMS MMMs fornbutanol/water separation, which exhibited highern-butanol/water sorption selectivity and demonstrated anti-trade-off effects between permeation flux and separation factor.Based on the PV performance of COFs/PDMS MMMs for butanol recovery, it is reasonable to assume that the incorporation of COFs into PDMS may be a promising way to improve PV performance for ethanol recovery.Huangetal.[140] prepared polyphosphazene nanotubes(PZSNTs)/PDMS MMMs for ethanol recovery and found that the selectivity increased and permeation flux increased by the incorporation of PZSNTs.The organic–MOPs hybrid materials maintain the advantages of good solubility in most general solvents, easyadjusting affinity among molecules, fillers and polymers, and low diffusion resistance due to microporous properties, which may provide new possibilities for the development of MMMs with high separation efficiency.
Particlesizeandloading
Particle size and loading are crucial to PV performance of MMMs for ethanol recovery.It was generally reported that the PV performance of MMMs would be enhanced with the augment of particle loading within a certain range, since high filler loading may provide more fast and continuous transport pathways contributed by the porous fillers.In general, the separation factor reached the maximum value with a certain particle loading, and then decreased by increasing particle loading furthermore due to the non-selective interfacial voids created by the particle aggregation and poor compatibility between fillers and polymer chains.In laboratory research, a thick selective layer of MMMs is usually used to overcome the uncertainty of separation factor caused by interface defects.However,a thinner selective layer without nonselective defects is necessary for the industrial application of MMMs to achieve both high membrane selectivity and permeability.Both large particle size and high particle loading would bring severe challenges to the preparation of ultrathin MMMs without defects.Minimizing particle size is theoretically possible to make much thinner defect-free membranes, while the tendency of particles to agglomerate has an inverse relationship to the particle size[24].Moermansetal.[16] first reported the incorporation of nano-sized zeolites into PDMS membranes and applied in the pervaporation of ethanol–water mixtures.The nano-sized zeolites showed much improved pervaporation results compared with the micron-sized silicate membranes.This was probably related to the mesopores due to voids between the nano-sized zeolites,which was possibly acting as freeways for the preferentially absorbed ethanol.Vaneetal.[113] found that membranes made with micron-sized (2.4 μm) ZSM-5 exhibited higher ethanol separation performance than those made with submicrometer-sized(0.35 and 0.70 μm) silicalite-1 because of their tendency to form silicalite-1 aggregates, especially for particle loadings of 50%(mass) or higher.The effect of particle size on PV performance of MMMs should be further studied systematically to balance the membrane thickness and particle loading.
Particledispersionmethods
In the initial stage of MMMs investigation,fillers were generally physically incorporated into PDMS matrix.It is often difficult to achieve a homogeneous dispersion only by simple mechanical stirring.In practice, the fillers are usually dispersed in polymer dope directly or in another solvent with thoroughly stirring and ultrasonication.The sonication process can be carried out using an ultrasonic bath or a probe-type sonicator.Vane’s work [113] verified that the effectiveness of probe-type sonicators on particle dispersion was obviously better than that of ultrasonic baths.Zhangetal.[141] proposedin-situultrasonic strengthening assembly in which sonication was applied not only during mixing but during assembly to avoid secondary aggregation on supporting surface.It was found that the dispersion of nanofillers in PDMS was markedly improved.The results above suggested thatin-situassembly via sonication might be a better method to improve the dispersion of inorganic fillers.
Pre-crosslinkingofpolymermatrix
Pre-crosslinking of PDMS matrix is an effective method to conquer the fillers aggregation by virtue of polymer viscosity or freezing effect.And so, the PDMS components selection and crosslinking conditions are crucial to the membrane micro-structure and pervaporation performance.Zhouetal.[142] employed the pre-crosslinking of PDMS (RTV615, consisting of two components of prepolymer and crosslinker) polymer network to facilitate the uniform dispersion of silicalite-1 in PDMS with 67%(mass)particle loading.Silicalite-1/PDMS MMMs exhibited a high permeation flux of 5.520 kg∙m-2∙h-1with separation factor of 15.5 for ethanol recovery from aqueous solutions (5% (mass)) at 50 °C.Zhanetal.[143] initiated the pre-crosslinking of hydroxyl-terminated PDMS to fabricate ZSM-5/PDMS MMMs,which showed a high permeation flux of 2.011 kg∙m-2∙h-1and separation factor of 12.6 at 70°C with 30% (mass) particle loading.Leeetal.[144] prepared silicalite-1/PDMS MMMs by using hydrosilylation-based UV curable PDMS to overcome fillers aggregation, which resulted in an extremely high-loading silicalite-1/PDMS MMMs with uniform particle distribution via ‘‘freezing effect” towards fillers in polymer matrix.Precrosslinking of the silicone rubber has been proved to be beneficial to resist particle aggregation and achieve homogeneous particle dispersion,but did make membrane preparation process more difficult due to the shorter time available after pre-polymerization[113].
Interfacialcompatibilityandinteraction
To achieve better interfacial compatibility and more uniform particle dispersion,a variety of surface modification methods have been proposed and demonstrated: (1) physi-/chemical modification of particles[145,146];(2)building strong interaction between particles and polymer matrix.Chenetal.[145]reported that treating silicalite-1 by acid or/and under steam could eliminate the metallic impurities in the zeolite and perfect the crystalline structure of the zeolite, which made silicalite-1 was more selective to ethanol and the desorption of the ethanol from the zeolite was also easier.Our group [146] found that surface etching of ZSM-5 with HF acid improved zeolite-PDMS interfacial adhesion due to the intrusion of PDMS into micro-pores out of the ZSM-5 surface,which contributed to the much better sorption selectivity and separation factor than that filled with non-etched ones, with a little expense of permeability.
Except for the acid/heat treatment of inorganic fillers, surface modification with silanes or other organic molecules is the most widely used method for inorganic particles to improve the interfacial compatibility with polymeric matrix as well as to facilitate the particle dispersion.Several groups [38,147–150] grafted zeolite with silane coupling agent and prepared modified zeolite/PDMS composite membranes as shown in Fig.11.It was found that PDMS chains could entangle with silane chains and offer considerable interactions between zeolite particles and PDMS matrix to achieve homogeneous distribution.Our group [38] modified silicalite-1 with four kinds of chlorosilanes with different alkyl chains which significantly improved the integration with PDMS matrix and the membrane selectivity with little sacrifice of permeation flux due to the entanglement among silane chains and PDMS chains.Xuetal.[119]modified ZIF-90 with dodecylamine(DLA)and fabricate DLA-ZIF-90/PDMS MMMs, which exhibited enhanced morphology homogeneity and separation performance for ethanol recovery,contributed by the flexible inner channels of ZIF-90-DLA particles with enhanced adsorption selectivity, as well as the improved affinity between ZIF-90-DLA particles and PDMS matrix.
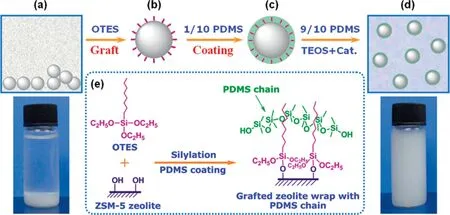
Fig.11. Schematic of incorporating ZSM-5 particles into PDMS matrix via surface modification [149].
Besides the surface modification, many researches focused on the modification of filler framework, such as ligand exchange and functionalization of MOFs [151,152].Yinetal.[151]modified zeolitic imidazolate framework-71 (ZIF-71) particles with four different ligands and prepared ZIF-71/PDMS MMMs for removal of alcohol from dilute aqueous solutions.The membranes modified with larger ligands demonstrated a constant decrease in alcohol and water permeability with a constant increase in alcohol/water selectivity,which might be ascribed to the narrowed pore size distribution in the modified ZIFs.
Compared with modification methods for increasing the physical interaction between fillers and polymer chains, building a firm bonding connection between fillers and polymer was proved to be a more effective way to improve the particle distribution and compatibility between particles and polymer matrix.Yietal.[150]prepared alkoxysilane modified silicalite-1/PDMS hybrid membranes,and found the chemical linking between the surface-modified silicalite-1 and PDMS substantially eliminated the nonselective voids inside the membrane, which effectively improved the separation factor of MMMs.Huetal.[153]prepared PDMS MMMs using micron-sized silicalite-1 as cross-linkers for butanol recovery from aqueous solution and found that both separation factor and butanol flux increased compared with that of pure PDMS membranes.Sietal.[57] proposed that silicalite-1 and PDMS were functionalized with methacrylate groups,and covalently bridged to fabricate silicalite-1/PDMS MMMs via an ultrafast-cured strategy.The MMMs exhibited an outstanding separation performance due to the two-phase covalent bridging and tailored network cavity,with a separation factor of 13.4 and total flux of 2.207 kg∙m-2∙h-1,respectively.
Zhuetal.[31]fabricated covalently linked ZIF-8@PDMS MMMs without interfacial defects for ethanol recovery, which exhibited the highest separation factor of 17.7 and a comparable total flux of 0.586 kg∙m-2∙h-1with 7% (mass) AZIF-8 loading (increased by 176.6 and 34.5%, respectively, in comparison with the pristine PDMS membrane).Wangetal.[154] reported a covalent crosslinking strategy to strengthen MOFs-PDMS interfaces and prepared trimethoxysilane (TMS) modified UiO-66/PDMS MMMs.High content UiO-66-TMS(up to 50%(mass))were loaded into PDMS while retaining good membrane formation ability,mechanical properties and pervaporation selectivity.
Our group[39,42]prepared two kinds of POSS/PDMS MMMs by chemical incorporation of POSS into PDMS matrix.It was found that chemical incorporation of POSS into PDMS matrix is an effective approach to achieve membrane homogeneity,tailor the microstructure of PDMS(such as free volume cavities and fractional free volumes),as well as enhance PV performance for ethanol recovery.The OPS-POSS cross-linked PDMS MMMs exhibited a separation factor of 16.4 and permeation flux of 0.253 kg∙m-2∙h-1with 7.5%(mass) V-POSS loading, increased by 130% and 260% compared with pure PDMS membrane, respectively.The V-POSS grafted PDMS MMMs showed a maximum separation factor of 17.7 and permeation flux of 0.536 kg∙m-2∙h-1with 5% (mass) V-POSS loading,increased by 130%and 260%compared with pure PDMS membrane,respectively.The results also suggested that POSS might act as a chemical linker between other fillers and polymer matrix due to its multi-functional properties for other PDMS based MMMs preparation.
Preparationmethods
Besides the intrinsic properties of fillers and polymer matrix,the preparation methods are also crucial to the micro-structure and pervaporation performance of MMMs.According to the literature reports [7], preparation methods of MMMs commonly includes physical blending, chemical hybridization,in-situsynthesis of particles in matrix,in-situpolymerization and bioinspired method as shown in Fig.8.Up to now, most ethanol permselective MMMs are still prepared by physical blending method and chemical hybridization due to their easiness to operate as mentioned above.To maintain better filler dispersion and compatibility with polymer matrix in MMMs, the methods ofin-situsynthesis of particles in matrix orin-situpolymerization are proposed.Maoetal.[155]reported theinsitufabrication of ZIF-8 nanoparticles in PDMS membrane via interfacial synthesis for enhanced ethanol/water separation as shown in Fig.12, which exhibited a superior pervaporation performance with relatively high permeation flux of 1.778 kg∙m-2∙h-1and comparable separation factor of 12.1.Thein-situfabrication method endowed the membranes with uniform dispersion of ZIF-8 nanoparticles and ultra-thin selective layer without interfacial defects.
In thein-situsynthesis of particles in matrix method, particle growth is confined to the polymer networks and the precise control over the formation of filler particles within polymer matrix is of critical importance to maximize the membrane performance.In thein-situpolymerization method,fillers are well-dispersed due to the isolation effect caused by the polymer networks generated within a confined space.The limitation of thein-situpolymerization method is restriction of the polymer chains intercalation.The methods ofin-situsynthesis of particles in matrix andin-situpolymerization are quite limited due to the difficulties in choosing proper systems and feasible preparation process as well as the control over the reaction and the reaction rate.Therefore, there are only a few studies on such PV membranes[7],and further investigation should be devoted to the development of universal membrane preparation systems and methods.
Bioinspired method means learning from nature and getting inspirations from living organisms’ advanced structures and functions [7].Inspired by the phenomena and principle of biomineralization in nature, the method of inducing inorganic precursors to be mineralized by biological or synthetic molecules in vitro can generate inorganic nanoparticles as versatile fillers in polymer matrix at mild conditions[156].Inorganic salts/alkoxide molecules are usually used as precursors and biological/synthetic molecules are used as inducers.The formation of inorganic particles can be regulated at the molecular level benefiting from the mutual interactions.Bio-adhesion is a ubiquitous phenomenon in nature.Inspired by the phenomena and principle of bio-adhesion,researchers have developed biomimetic adhesives such as polydopamine (PDA), with similar structure and function to the adhesive protein.Lietal.[157] reported a strategy to further enhance the hydrophobicity of ZIF-8, in which ZIF-8 particles were first coated by PDA to create chemically reactive surface and then modified with silane coupling agents.Benefiting from the PDA bioadhesion layer, the silane modification of ZIF-8 improved both the separation factor and butanol flux of MMMs simultaneously.Although the bioinspired methods have been widely used for membrane preparation, the application for ethanol permselective membranes preparation is still very limited.More attention should be paid to biomimetic methods such as bioinspired mineralization and bio-adhesion.

Fig.12. In-situ preparation procedure of ZIF-8@MMMs [155].
In summary,the pervaporation performance of MMMs for ethanol recovery is generally superior to that of pristine polymeric membranes, which is one of the most promising membrane structures to realize the industrial application of ethanol perm-selective membranes.Plenty of investigations concentrated on effects of various factors on pervaporation performance of MMMs, such as filler types, particle size, particle loading,interfacial compatibility,interfacial interaction.Further efforts should be devoted to the design of novel materials(including fillers and polymers)and construction of continuous transport pathways as well as the finely regulation of interfacial structure to achieve the synergistic effect of fillers and polymer matrix.
4.Emerging R&D on Ethanol Perm-selective Membranes
4.1.Bio-inspired materials and structures
The development of bioinspired materials and structures provides enlightening ideas for the construction of ethanol permselective membranes.On the one hand, membrane surface engineering could be achieved by making use of ‘‘lotus effect” or‘‘super-hydrophobicity” of the lotus leaf, which effectively improved the sorption selectivity and pervaporation performance[37].On the other hand, Interfacial interaction plays an important role in membrane performance and sustainability, and strong interface interaction can be easily realized in biological materials,such as biomimetic adhesives such as PDA,carbomer and hyaluronic acid,etc.[7].Furthermore,it was confirmed that the spreading direction of water and ethanol liquids could be tailored by designing 3D capillary ratchets(inspired by leaves of araucaria)that created an asymmetric and 3D spreading profile both in and out of the surface plane due to different liquids surface tensions.The finding inspired us that the novel 3D membrane structures with high separation efficiency might be designed based on the intrinsic liquid properties differences and bioinspired structures (3D capillary ratchets) rather than the traditional surface energy theory [158].
4.2.Interfacial engineering between fillers and polymer matrix
The interfacial design is critical in preparing high-performance MMMs, especially for the separation of larger sized ethanol from aqueous mixtures.From the perspective of micro-structure of MMMs, inorganic materials can be physically incorporated, or chemical grafted into/on polymer matrix as shown in Fig.13.Novel materials (including fillers and polymers) and preparation methods may provide the possibility for fine regulation of interface structure,in which building firm bonding interactions between filler and polymer is one of the most promising methods to strengthen the PV performance of MMMs.Moreover,the transport mechanism of permeating molecules through the interface of MMMs should be investigated furthermore to clarify the effect of fillers in MMMs on pervaporation performance.
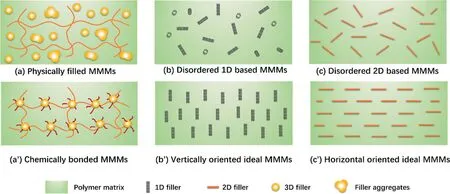
Fig.13. Emerging R&D on ethanol perm-selective membranes.
4.3.Orientation of hydrophobic 1D/2D filler in MMMs
It is well known that CNTs/GO can offer large and ultra-fast mass transfer channels for small molecules, but the intrinsic hydrophilic micro-environment of CNT/GO fillers and their disordered distribution restrict its applications for ethanol recovery.The investigations of CNT/GO filled MMMs for ethanol recovery can be carried out from the following aspects: (1) Surface modification of CNTs/GO materials to tailoring their physical and chemical micro-environment.On the one hand, the hydrophilic functional groups on the surface of CNTs/GO are not in favor to the preferential adsorption of ethanol, and so surface functionalization of CNTs/GO with hydrophobic groups or even hydrophobic porous nanoparticles [111,126] will be indispensable to improve the preferential adsorption and solution of ethanol; On the other hand, the hydrophobic modification of CNTs/GO can improve its solubility in common organic solvents and achieve homogeneous dispersion as well as strong interaction with polymer matrix,which provides more possibilities for the preparation of uniform and high-efficiency MMMs; Moreover, the hydrophobic interface at the carbon-nanotube wall or GO interlayer space might provide frictionless and rapid transport pathways for ethanol molecules.(2) The well-ordered arrangement of CNTs/GO in MMMs.Most of CNTs/GO fillers are disorderly distributed in polymer matrix, and the direction of the ultra-fast mass transfer channels provided by CNTs/GO are not consistent with transmembrane direction of small molecules.The ordered incorporation of 1D or 2D inorganic fillers into polymer matrix (vertically oriented or horizontal oriented, as shown in Fig.13) might provide much shorter continuous mass transfer channel for small molecules, which is expected to significantly improve the separation performance of the polymeric membrane.Xue’s group [159–161] fabricated vertically aligned and open-ended CNT/PDMS MMMs, which surpassed the CNT filling content limitation and broke the permeability-selectivity tradeoff with both parameters remarkably increasing (maximum 9 times) for bio-alcohol separation.And the density functional theory (DFT) calculations illustrated that the penetrant molecules preferentially passed through the CNT internal channels, rather than the PDMS chains and the CNT external wall.The research provided valuable inspiration for further nanoscale design of highly efficient channeled membranes for separation application.
5.Conclusions and Perspectives
This review summarizes the breakthroughs on ethanol permselective PV membranes from the perspectives of membrane materials, membrane macro-/micro-structures, transport mechanisms and evaluation of membrane performance.Although a large number of researches on ethanol perm-selective PV membranes have been developed to enhance PV performance in the past few decades, there is still a rather far way to achieve industrial applications due to the lack of major breakthroughs of pervaporation performance and practical membrane modules.Therefore, further research should be devoted to exploring high-performing, stable,and economic membranes and impelling the industrial application of ethanol perm-selective PV membranes.There are several strategies for the further development of ethanol perm-selective PV membranes.
(1) The physical and chemical microstructures of mass transfer channels should be synergistically tuned based on the intrinsic physi-/chemical differences between water and ethanol molecules[11]as well as the interactions among penetrants and membranes.It has been proved that membrane hydrophobicity, cavities size and their distribution in polymers, as well as porous microstructures in inorganic materials are key factors to the solutiondiffusion properties of ethanol perm-selective PV membranes.Therefore, it is very important to further study how to accurately control the physical and chemical properties of mass transfer channels so as to facilitate the ethanol molecules transfer.It is suggested that advanced characterization technology can be employed to probe the membrane microstructure, and molecular dynamics simulations will play a greater role in obtaining more information in molecular dimensions during pervaporation which can’t be achieved in experiments.
(2) The separation mechanism of pervaporation should be deeply investigated, and mass transport models should be developed furthermore to understand transport fundamentals and better guide membrane design.Take diffusion-solution model for example, Ordinarily, the diffusion selectivity of ethanol molecules is much lower than the sorption selectivity, and selective diffusion becomes the rate-controlling step in the pervaporation process of most ethanol perm-selective PV membranes.The diffusion selectivity should be increased effectively by constructing fast transfer channels suitable for the continuous and rapid diffusion of ethanol molecules and thus the membrane selectivity could be significantly improved.Plenty of researches focus on improving the solution selectivity so as to enhancing membrane selectivity,while the researches on improving the diffusion selectivity are still very limited.
(3) Further study should be devoted to the integration of PV process with fermentation process and membrane performance in real fermentation broths with long-term operational stability.The constituents of the real fermentation broth, such as sugars,organic acids proteins, salts, and microorganisms, would have destructive effects on the membrane performances due to their influence on the vapor pressures of water and ethanol.Moreover,fouling and biofouling due to their adsorption and accumulation on the membrane surface also depressed the membrane performance.Kamelianetal.[162] fabricated a two-layered activelayer superhydrophobic (TALS) silicalite-1/PDMS membrane, and applied inin-situethanol recovery from fermentation broth,which showed the maximum performances with a permeation flux of 1.88 kg∙m-2∙h-1and separation factor of 32.34.The outstanding performance was resulted from that the presence of the upper selective layer not only improved the antiadhesive feature but also enhanced the hydrophobicity and pervaporation performances.This research provided an inspiration for the membrane design and preparation with robust durability and high PV performance in real fermentation-pervaporation coupling process from the perspective of designing novel membrane macro-structure and micro-structure.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors greatly appreciate the financial support of Beijing Natural Science Foundation Commission-Beijing Municipal Education Commission Joint Foundation, China (KZ201910011012),National Natural Science Foundation of China (21736001,21776153, 21206001), Open Research Fund Program of Key Laboratory of Cleaner Production and Integrated Resource Utilization of China National Light Industry (CP-2020-YB7), and College Students Scientific Research and Undertaking Starting Action Project,China.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.12.010.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of lithium carbonate by microwave assisted pyrolysis
- Simulation and design of a heat-integrated double-effect reactive distillation process for propylene glycol methyl ether production
- Hyper-parameter optimization of multiple machine learning algorithms for molecular property prediction using hyperopt library
- High-efficiency and safe synthesis of tonalid via two Friedel-Crafts reactions in continuous-flow microreactors
- Improvement of synergistic effect photocatalytic/peroxymonosulfate activation for degradation of amoxicillin using carbon dots anchored on rod-like CoFe2O4
- Boosting the hydrogen storage performance of magnesium hydride with metal organic framework-derived Cobalt@Nickel oxide bimetallic catalyst
