High-efficiency and safe synthesis of tonalid via two Friedel-Crafts reactions in continuous-flow microreactors
2023-01-30YangHanYuanyuanLiuShiweiWangXuehuiGeXiaodaWangTingQiu
Yang Han, Yuanyuan Liu, Shiwei Wang, Xuehui Ge, Xiaoda Wang,Ting Qiu
Engineering Research Center of Reactive Distillation, Fujian Province Higher Education Institutes, College of Chemical Engineering, Fuzhou University, Fuzhou 350108, China Qingyuan Innovation Laboratory, Quanzhou 362801, China
Keywords:Microreactor Continuous synthesis Process intensification Tonalid Friedel-Crafts reaction
ABSTRACT Tonalid,an important fragrance ingredient with widespread application,was synthesized via two Friedel-Crafts reactions, which were catalyzed by AlCl3.The traditional tonalid production was conducted in batch stirring tank reactors,suffering from low production capacity and the safety hazard of temperature runaway.To solve these problems, the continuous-flow technologies were developed for the highefficiency and intrinsically safe synthesis of tonalid in microreactors.Catalyst AlCl3 was neatly homogenized in proper solvents by forming complex with reactant, which was a necessary step for the continuous synthesis in microreactors.Several reaction conditions, including reactant molar ratio, catalyst concentration, temperature, and microchannel hydrodynamic diameter, were investigated for the two Friedel-Crafts reactions in microreactors.At optimized conditions, the yields of the two Friedel-Crafts reactions were 44.15%and 97.55%,respectively.In comparison with the batch reactors,the reaction times of these two reactions could both be reduced by nearly two thirds in microreactors at the similar yield.
1.Introduction
Musk is widely used as a fragrance ingredient in high-grade perfumes, cosmetics, soaps, laundry detergents, and so on [1].The productivity of natural musk is very low, because it is produced in the body of animals[2].In order to satisfy the huge market demand, synthetic musks have been produced to replace the natural ones [3–5].Nitro musk was one of the earliest and largest synthetic musks used, accounting for about half of the total amount of synthetic musk in the world market now [6].However,its carcinogenicity to human made many countries prohibit its uses in the products contacting with skin [7–9].Tonalid, a kind of synthetic polycyclic musk with the chemical name of 7-acetyl-1,1,3,4,4,6-hexamethyl-1,2,3,4-tetrahydronaphthalene (AHMT), is an outstanding substitute to nitro musk because of its excellent fragrance, stable chemical properties, and safety [10].It has become one of the most important commercial synthetic polycyclic musks with the worldwide consumption of 5000–10000 tons per year [11].
The most widely used AHMT synthesis route contained two steps with 1,1,3,4,4,6-hexamethyl-1,2,3,4-tetrahydronaphthalene(HMT) as intermediate, as shown in Fig.1(a) and (b) [12–16].The first step was a Friedel-Crafts alkylation reaction conducted in an appropriate solvent withp-cymene (p-CMB) and 2,3-dimethyl-1-butene (2,3-DMB-1) as raw materials,tert-butyl chloride (TBC) as hydrogen absorbent, and anhydrous aluminum chloride (AlCl3) as catalyst, as shown in Fig.1(a).The second step was a Friedel-Crafts acylation reaction, in which HMT reacted with acetyl chloride (ACE) in aliphatic hydrocarbon or halogenated aliphatic hydrocarbon solvents under the catalysis of AlCl3, as illustrated in Fig.1(b).These two reactions were quenched by hydrolyzing the catalyst AlCl3with water.Since both the synthesis and quenching steps were strongly exothermic reactions and conducted in batch stirring tank reactors (BSTRs), there were problems aplenty in the industrial process of AHMT production.Firstly, in order to avoid the great release of reaction heat,reactants were added drop by drop into the BSTRs containing solvent and catalyst, which resulted in the very low production efficiency.Secondly, the heat released from the AlCl3hydrolysis reaction could not be removed in time from BSTRs due to their poor heat transfer performance,which brought potential safety hazards of temperature runaway or the overuse of quenching water.

Fig.1. Reaction routes for the synthesis of (a) HMT and (b) AHMT.
Microreactor(MR)has received increasing attentions as a novel chemical process intensification technology in recent years[17–20].Due to the small characteristic dimension of 100–1000 μm,MRs possess highly efficient heat and mass transfer performances in comparison with the conventional BSTRs [21–28].In addition, the operation in the MR was highly controllable and intrinsically safe [29–32].In view of these advantages, MRs were applied to enhance several kinds of rapid exothermic reactions such as oxidation [33,34], nitration [35,36], diazotization [37,38],and so on [39,40] to improve the yield and selectivity.The work reported by Yoshida’s group [41,42] realized the main-product yield of 92%,becoming a successful example for the intensification of Friedel-Crafts reaction by MRs.Heilandetal.[43] developed a microfluidic system, seamlessly integrating microflow and microbatch synthesis to explore asymmetric iminium-catalyzed Friedel-Crafts alkylations, facilitating the drug development or medical diagnostics.Fangetal.[44] prepared α-Fe2O3and CaCO3nanoparticles and employed them to catalyze the Friedel-Crafts reaction in continuous flow reactors,forming an efficient and green pathway for the continuous Friedel-Crafts acylation in industry.It has been proved that MR is suitable for the industrial applications of fast exothermic Friedel-Crafts reaction [42–44].Even so, the example of conducting the Friedel-Crafts reaction in MRs had been limited until now.Based on the previous researches on MRs, it could be expected that continuous MRs was an effective equipment to solve the bottleneck problems of industrial AHMT production process.In virtue of the excellent heat transfer performance of MR, AHMT production process could be changed from the inefficient batch operation mode of drop-by-drop to the continuous one.The production capacity could readily be improved to a commercial scale at high yield without the increase of risk.
In this study,we developed a continuous micro-chemical system for AHMT synthesis.Firstly,the catalyst AlCl3was homogenized to avoid microchannels blocking.Secondly, the reaction conditions were optimized for the synthesis of intermediate HMT and product AHMT in MRs to improve the total yield.Finally,the performances of MR and BSTR were compared for these two reactions.
2.Experimental
2.1.Chemicals
p-Cymene (p-CME, >99.0% (mass)),tert-butyl chloride (TBC,>99.0%(mass)),cyclohexane(CHE,>99.5%(mass)),and acetyl chloride (ACE, >99.0% (mass)) were purchased from Aladdin Industrial Co.Ltd., China.Dichloroethane (DCME, >99.2% (mass)),dichloroethane (DCEE, >99.2% (mass)) and anhydrous aluminum chloride (AlCl3, >99.0% (mass)) were purchased from Shanghai Macklin Biochemical Co., Ltd, China.2,3-Dimethyl-1-butene (2,3-DMB-1,>99.2%(mass))was provided by Dalian Chemphy Chemical Co., Ltd, China.Cold sodium chloride solution (10.0% (mass)) was precooled in a low-temperature thermostatic bath (PSL-2000,Tokyo Rikakikai Co., Ltd, Japan) at 5 °C, working as a quenching solution.
2.2.Apparatus and procedure
The MR systems for continuous synthesis of HMT and AHMT are shown in Fig.2(a) and (b), respectively.Both these two systems consisted of two T-shaped micromixers(M1 for the mixing of reactants and catalyst and M2 for the injection of quenching solution),two precooling microchannels (C1 and C2), a reaction microchannel(R1),and a quenching microchannel (Q).Microreactor systems with different inner hydrodynamic diameters of 1, 2, and 3 mm were used in the experiment.
Before the continuous synthesis of HMT, the reactant and catalyst phases were prepared at 15 °C, respectively.2,3-DMB-1, TBC,and somep-CME were mixed as reactant phase.Solvent, catalyst AlCl3, and a part ofp-CME were mixed in a certain molar ratio to prepare a homogeneous catalyst phase.Reactant and catalyst phases were injected into the reaction channel R through the micromixer M1 by two syringe pumps (TYD01, Lead Fluid Co.,Ltd), respectively.A thermostatic water bath was used to control the reaction temperature.In order to quench the reactions, cold sodium chloride solution was pumped into the microchannel by another syringe pump through the micromixer M2.The realization of quenching reaction in microreactors was another important improvement of the traditional production.After optimization,the sodium chloride solution temperature was set as 5 °C and its volume flow rate was equal to that of the quenched mixture.Al(OH)3precipitate and HCl gas were generated after the decomposition of AlCl3in water.The generation of HCl gas improved the mass and heat transfer performance in microchannel by intensifying the flow disturbance,making the quenching process safer.The gas–liquid two-phase flow was generated due to the formation of HCl gas,avoiding the occurrence of microchannel blocking, which was caused by the formation of precipitate Al(OH)3.The intermediate HMT formed was purified by recrystallization after vacuum distillation and used as one of the raw materials in the continuous synthesis of AHMT.
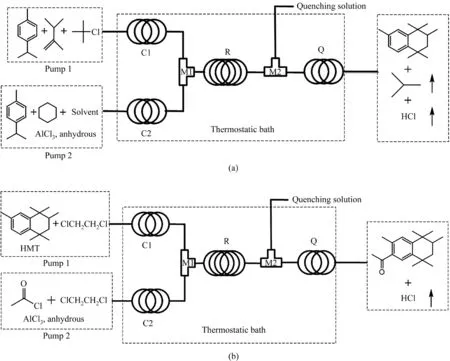
Fig.2. Schematic diagram of the experimental setup for continuous (a) HMT and (b) AHMT synthesis in microreactors.C1 and C2: precooling microchannel, R: reaction microchannel, Q: quenching microchannel, M1 and M2: T-micromixer.
The production process of AHMT was similar to that of HMT,except the preparation of reactant and catalyst phases, and the purification of product AHMT.Intermediate HMT was predissolved in solvent as reactant phase.Catalyst AlCl3was slowly added into a three-mouth round bottom flask pre-filled with solvent, and then reactant ACE was dropwise added at -5 °C to prepare catalyst phase.Both the additions of AlCl3and ACE were conducted with the liquid in the flask intensely stirred.The product AHMT was purified by recrystallization after the solvent was separated using a rotary evaporator.
2.3.Analytical method
All the samples were analyzed by a gas chromatography (Shimadzu GC-2014, Japan) equipped with a capillary column and a flame ionization detector.The capillary column had a length of 30 m, film thickness of 0.25 mm, and internal diameter of 0.25 mm.The injection and detector temperature were 280 °C and 340°C,respectively.Temperature program was set as follows.The initial 50°C ramped up at a rate of 5°C∙min-1to 60°C,where it was held for 1 min.Then, ramped up at a rate of 40 °C∙min-1to 190 °C, where it was held for 2 min.Finally, ramped up at a rate of 40 °C∙min-1to 280 °C, where it was held for 2 min.Nitrogen with mass purity of 99.999% was used as a carrier gas with a flow rate of 3 ml∙min-1.2 μl sample was injected into the gas chromatography at a time.Butyl acetate was used as an internal standard to determine the constituent content.
The conversion (C(2,3)-DMB-1) and yield for HMT (Y(HMT))synthesis were calculated using the following equations

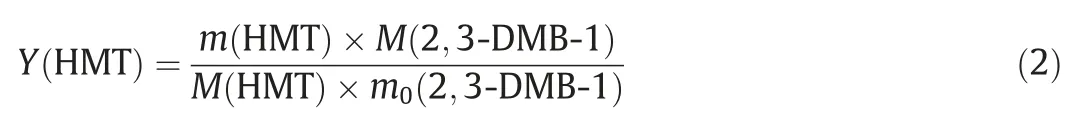
m(2,3-DMB-1) andm(HMT) were the mass of 2,3-DMB-1 and HMT in the sample, respectively.The subscript 0 represented the initial state.M(2,3-DMB-1) andM(HMT) were the molecular weight of 2,3-DMB-1(84.16 g∙mol-1)and HMT(216.36 g∙mol-1),respectively.The yield of HMT was calculated based on 2,3-DMB-1 because excessivep-CME and TBC were used.
The conversion for HMT (C(HMT)) and yield for AHMT (Y(AHMT)) synthesis were calculated using the following equations
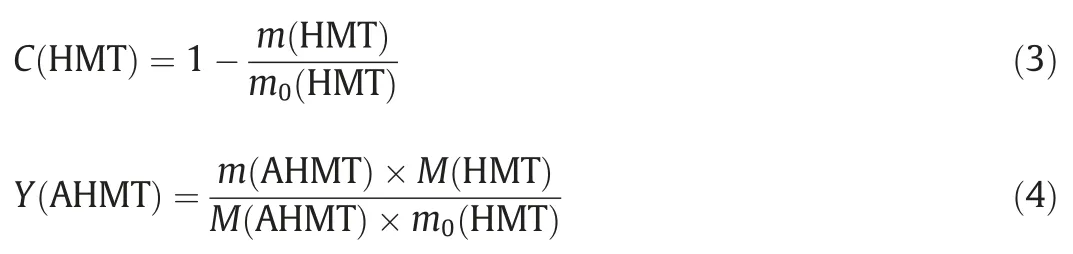
m(HMT) andm(AHMT) were the mass of HMT and AHMT in the sample.M(AHMT) was the molecular weight of AHMT(258.41 g∙mol-1).The yield of AHMT was calculated based on HMT because excessive ACE was used.
3.Results and Discussion
3.1.Synthesis of intermediate HMT
3.1.1.Frombatchtocontinuoussynthesis
HMT synthesis was generally carried out in the inert solvent of CHE to dilute the heat of reaction[16].However,the catalyst AlCl3was essentially insoluble in CHE, resulting in the formation of liquid–sold two-phase reaction system [16].It was not suitable to conduct such a heterogeneous reaction in MRs,since the catalyst solid would block microchannels bringing safety hazards.Therefore, an appropriate solvent should be chosen to realize the homogenization of reaction system for the continuous and safe operation of HMT synthesis in MRs.
Although the chlorinated hydrocarbons and catalyst AlCl3both have a Cl group,neither of the chlorinated hydrocarbons DCME and DCEE could completely dissolve the catalyst AlCl3, as shown in Fig.3(a) and (b).According to the mechanism of HMT synthesis,the catalyst AlCl3and reactantp-CME formed a catalytic active complex during the reaction [16].Using a suitable solvent to dissolve this complex is another method to homogenize the catalyst.The complex couldn’t be completely dissolved when CHE or the reactantp-CME were used as solvents, as shown in Fig.3(c) and(d), respectively.However, the chlorinated hydrocarbons DCME and DCEE dissolve the complex well, as shown in Fig.3(e) and(f).More detailed experiment results showed that thep-CME:AlCl3molar ratio was required to be higher than 1.5:0.15 for the formation of fluidized catalyst complex, and then the DCME (or DMEE):AlCl3molar ratio should be higher than 1.5:0.15 for the homogenization of complex.
Although the complex dissolved more readily in the polar solvents such as DCME and DCEE than in the nonpolar solvent such as CHE, the alkylation activity of aluminum chloride is strong in the polar solvent, which might be more active to cause side reactions including alkyl rearrangement and alkyl transfer reactions[45].In addition,p-CME carbocation intermediates are electrically dispersed, and they are more stable in nonpolar solutions than in the polar solvent [46,47].In conclusion, in order to weaken the polarity of the reaction environment,the mixture of polar and nonpolar solvents was used to dissolve the catalyst complex.The molar ratio of polar solvent to nonpolar solvent was controlled precisely,since excess nonpolar solvent could lead to the precipitation of catalyst complex.The experiment results showed that as the solvent molar ratio of CHE to DCEE was less than 1.5:1.5,the catalyst complex could be dissolved completely in the mixed solvents, as shown in Fig.3(g) and (h).
The homogenized mixture,which contained catalyst AlCl3,reactantp-CME,and solvent,was referred to as catalyst phase 1 in this work.The mixture of reactantsp-CME, 2,3-DMB-1 and TBC was referred as reactant phase 1.p-CME was an excess reactant in the HMT synthesis.Some of thep-CME was used as co-solvent to dissolve AlCl3.The remainingp-CME was added into reactant phase 1, to reduce the volume difference between catalyst phase 1 and reactant phase 1.Based on the experiment conditions at which catalyst AlCl3could be homogenized, HMT synthesis was carried out in the MR system illustrated in Fig.2(a).The effects of different solvents on HMT synthesis are listed in Table 1.The comparisons of No.1 with 2 and No.3 with 4 show that solvent type had subtle effects on the conversion and yield when the polar solvent was used.DCEE was chosen as solvent for the continuous HMT synthesis in MRs, since it was safer than DCME in industrial application due to its higher boiling point and lower volatility.By comparing No.2 with 4,it could be found that the HMT selectivity improved from 38.78% to 53.76% with the conversion increased from 74.62% to 81.28% after the nonpolar solvent CHE was added.This was consistent with our prediction that nonpolar solvent could suppress the side reactions and increase the stability of the reaction intermediate carbocation.The comparison of No.4 with 5 shows that further increasing the amount of CHE was not very helpful to the improvement of yield and selectivity.In the following, HMT synthesis experiments were carried out at the AlCl3:p-CME (in catalyst phase):DCEE:CHE molar ratio of 0.15:1.5:1.5:1.5.

Fig.3. States of catalyst AlCl3 in different solvents for HMT synthesis.(a)DCME=0.35 mol,(b)DCEE=0.35 mol,(c)p-CME+CHE=0.15 mol+0.35 mol,(d)p-CME=0.50 mol,(e) p-CME + DCME=0.15 mol + 0.35 mol, (f) p-CME + DCEE=0.15 mol + 0.35 mol, (g) p-CME + CHE + DCME=0.15 mol + 0.20 mol + 0.15 mol, (h) p-CME + CHE + DCEE=0.15 mol + 0.20 mol + 0.15 mol.Common conditions: temperature=15 °C, stirring rate=300 r∙min-1, AlCl3=0.015 mol.
3.1.2.Optimizationofreactionconditions
Fig.4 shows the effects of the TBC:2,3-DMB-1 molar ratio of on HMT synthesis.When the TBC:2,3-DMB-1 molar ratio was increased from 1.0:1 to 1.1:1, the conversion of 2,3-DMB-1 gradually increased from 78.43%to 80.38%with the HMT yield increased from 42.02% to 44.15%.That was because increasing the TBC:2,3-DMB-1 molar ratio could promote the reaction to move forward to improve the HMT yield.TBC decomposed quickly into hydrogen chloride HCl and isobutylene under the catalysis of AlCl3during the reaction,even though the other reactants were not added.After the total consumption of TBC, the main reaction stopped, but the side reaction such as 2,3-DMB-1 self-polymerization continued under the catalysis of AlCl3and HCl.As a result, with the TBC:2,3-DMB-1 molar ratio increased from 1.1:1 to 1.4:1,the 2,3-DMB-1 conversion continuously increased while the HMT yield kept almost unchanged, as shown in Fig.4.Therefore, the suitable TBC:2,3-DMB-1 molar ratio was 1.1:1 for the HMT synthesis in MRs.

Fig.4. Effects of the molar ratio of TBC to 2,3-DMB-1 on HMT synthesis.Reaction conditions: 2,3-DMB-1:p-CME molar ratio in reactant phase=1:0.5, AlCl3:p-CME:DCEE:CHE molar ratio in catalyst phase=0.15:1.5:1.5:1.5,temperature=15°C,flow rate of 2,3-DMB-1=0.065 ml∙min-1, residence time=20 min, microchannel diameter=2 mm.
Fig.5 shows the influences of the molar ratio of AlCl3to 2,3-DMB-1 on HMT synthesis.With the increase of the AlCl3:2,3-DMB-1molar ratio from 0.11 to 0.15, the reaction rate became higher resulting in the improvement of 2,3-DMB-1 conversion from 70.05% to 80.15% and HMT yield from 34.10% to 44.21%.The conversion continued to increase when the molar ratio exceeded 0.15, but the HMT yield was roughly unchanged.It indicated that the side reaction of 2,3-DMB-1 self-polymerization was also accelerated under high catalyst concentration.Finally, the molar ratio of AlCl3to 2,3-DMB-1 was determined to be 0.15:1.
Fig.6 shows the effects of reaction temperature on HMT synthesis.With the reaction temperature increased from 5 °C to 15 °C,both the conversion and yield improved, since the reaction was accelerated by increasing the temperature.When the temperature exceeded 15℃, the HMT yield decreased significantly with the increase of reaction temperature, while the 2,3-DMB-1 conversion improved slightly.It indicated HMT was converted into other byproducts at the temperature higher than 15℃.Therefore, 15 °C was the most suitable reaction temperature for HMT synthesis.
Fig.7 shows the effects of microchannel diameter on HMT synthesis.With the increase of the microchannel diameter from 1 mm to 2 mm, the 2,3-DMB-1 conversion and HMT yield remained unchanged.However, when the microchannel diameter was increased from 2 mm to 3 mm, the yield of HMT decreased from 44.5% to 41.88% with the conversion of 2,3-DMB-1 remained at about 80.27%.The heat transfer rate decreased with the increase of microchannel diameter due to the decrease of specific surface area.Reaction heat released by HMT synthesis,which is a strongly exothermic Friedel-Crafts alkylation reaction, could not be timely removed from the microreactor with larger hydrodynamic diameter.The heat was accumulated in the microreactor,resulting in the increase of reaction temperature and the transformation of HMT to other byproducts.Therefore, the suitable inner diameter of the microreactor was 2 mm for HMT synthesis.
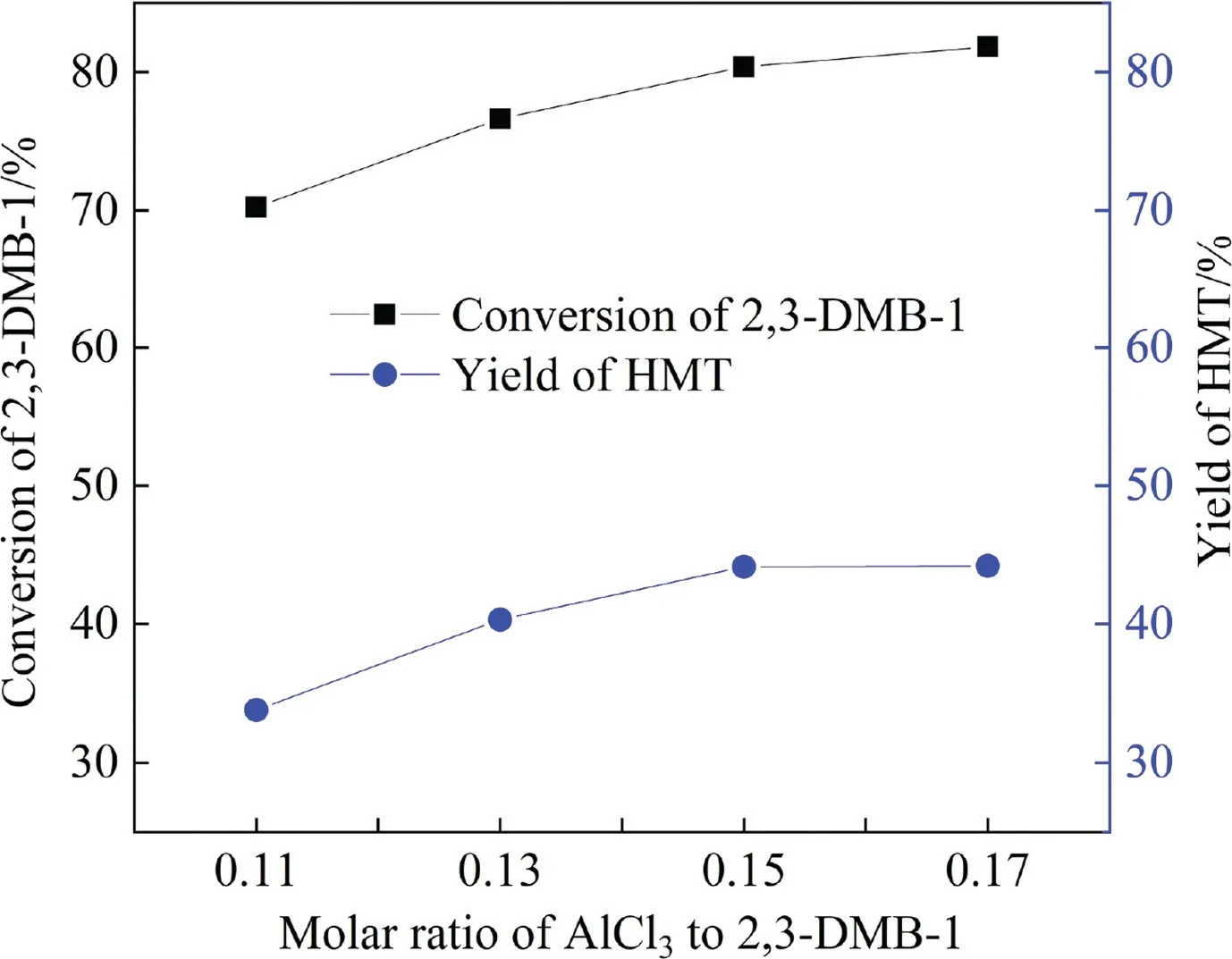
Fig.5. Effects of the molar ratio of AlCl3 to 2,3-DMB-1 on HMT synthesis.Reaction conditions: 2,3-DMB-1:TBC:p-CME molar ratio in reactant phase=1:1.1:0.5, p-CME:DCEE:CHE molar ratio in catalyst phase=1.5:1.5:1.5, temperature=15 °C,flow rate of 2,3-DMB-1=0.065 ml∙min-1, residence time=20 min, microchannel diameter=2 mm.
In addition, the molar ratios of 2,3-DMB-1 to DCEE andp-CME were investigated.They had slight effects on the HMT synthesis in the range investigated,as shown in Tables S1–S3 in the Supplementary Material.In this experiment,the optimized 2,3-DMB-1:p-CME:DCEE:CHE molar ratio was 1:2:1.5:1.5 with 75%p-CME distributed in the catalyst phase.
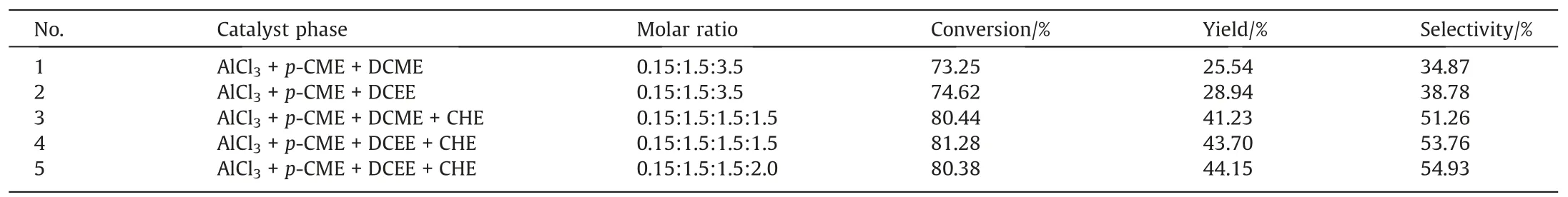
Table 1Effects of different solvents on HMT synthesis

Fig.6. Effects of reaction temperature on HMT synthesis.Reaction conditions:2,3-DMB-1:TBC:p-CME molar ratio in reactant phase=1:1.1:0.5, AlCl3:p-CME:DCEE:CHE molar ratio in catalyst phase=0.15:1.5:1.5:1.5, flow rate of 2,3-DMB-1=0.065 ml∙min-1, reaction time=20 min, microchannel diameter=2 mm.
Fig.10 shows that there is an optimal catalyst AlCl3concentration for the highest AHMT yield.With the increase of AlCl3:HMT molar ratio from 1:1 to 1.2:1,the HMT conversion and AHMT yield showed an increasing trend.With the further increase of catalyst concentration, the main reaction was terminated in a relatively short time.At the same residence time,the time for the conversion of main product AHMT to byproducts became longer with the increase of catalyst concentration.As a result, AHMT yield decreased from 97.5% to 96.3% with the increase of AlCl3:HMT molar ratio from 1.2:1 to 1.6:1.Finally, the molar ratio of AlCl3to HMT was determined to be 1.2:1.
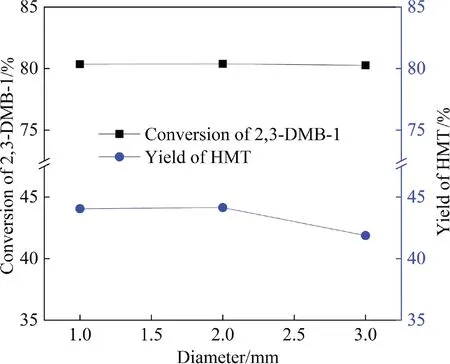
Fig.7. Effects of microchannel diameter on HMT synthesis.Reaction conditions:2,3-DMB-1:TBC:p-CME molar ratio in reactant phase=1:1.1:0.5, AlCl3:p-CME:DCEE:CHE molar ratio in catalyst phase=0.15:1.5:1.5:1.5,temperature=15°C,flow rate of 2,3-DMB-1=0.065 ml∙min-1, residence time=20 min.
3.2.Synthesis of product AHMT
3.2.1.Frombatchtocontinuoussynthesis
In order to realize the continuous synthesis of AHMT in MRs,the catalyst AlCl3also needed to be homogenized in the reaction mixture.Although the catalyst AlCl3could not be completely dissolved by pure halogenated hydrocarbon solvents, it could be homogenized after the reactant ACE was added into the solvents of DCME and DCEE, as shown in Fig.8(a) and (b), respectively.Experiment results indicated that the minimum molar ratio of ACE:AlCl3was 1.5:1.2 for the fluidization of AlCl3, and then the DCME (or DCEE):AlCl3molar ratio should be higher than 6:1.2 for the homogenization of the fluidized AlCl3.A plausible explanation was that ACE and AlCl3form a complex, which dissolved easier in the halogenated hydrocarbon solvents.The effects of solvents type on the continuous AHMT synthesis were investigated in the MR system illustrated in Fig.2(b) with the experiment results listed in Table 2.The conversion and yield were 99.66% and 93.03%,respectively,which were almost unaffected by solvent types.Since DCEE was safer than DCME in industrial application due to its higher boiling point and lower volatility, it was used as solvent for the AHMT continuous synthesis in MRs.As a result, catalyst AlCl3was homogenized by DCEE with reactant ACE as co-solvent.This homogeneous liquid was named as catalyst phase 2 in this work.

Table 2Effects of different solvents on AHMT synthesis

Fig.8. States of catalyst AlCl3 in different solvents for AHMT synthesis.(a)DCME+ACE=0.3 mol+0.075 mol,(b)DCME+ACE=0.3 mol+0.075 mol.Common conditions: temperature=10 °C, stirring rate=300 r∙min-1, AlCl3=0.06 mol.
The melting point of the reactant HMT is about 65℃, so it also needed to be dissolved in the solvent DCEE to realize the continuous operation.The solvent containing HMT was named as reactant phase 2.The catalyst and reactant phases were pumped separately into the MR, in which ACE reacted with HMT.According to the experiments, the DCEE:HMT molar ratio should be larger than 4:1 for the complete dissolution of HMT.In this experiment, the molar ratio of HMT:ACE:DCEE was 1:1.5:10 with 40% DCEE distributed in the reactant phase.
3.2.2.Optimizationofreactionconditions
The purity of HMT had an obvious influence on the yield of AHMT, as shown in Fig.9.With the increase of HMT purity from 90% to 97%, AHMT yield significantly improved from 90.81% to 96.11%, while the HMT conversion increased slightly from 98.9%to 99.9%.It could be speculated that the impurities could induce the occurrence of unwanted side reaction in AHMT synthesis.The byproducts resulted in not only the increase of subsequent separation cost,but also the decline in AHMT quality.The recommended HMT mass purity is >97%, since AHMT yield was basically unchanged when HMT mass purity was higher than 97%.

Fig.9. Effects of HMT purity on AHMT synthesis.Reaction conditions: HMT:DCEE molar ratio in reactant phase=1:4, AlCl3:ACE:DCEE molar ratio in catalyst phase=1.2:1.5:6,temperature=10°C,flow rate of HMT=0.027 ml∙min-1,reaction time=25 min, microchannel diameter=1 mm.
Fig.11 shows the effects of reaction temperature on AHMT synthesis.With reaction temperature increased from 5 °C to 10 °C,both the HMT conversion and AHMT yield improved,which should be attributed to the increase of reaction rate.However, when the reaction temperature was further increased to 20 ℃, AHMT yield decreased to 95% with the almost completely conversion of HMT.It indicated that the side reaction in which AHMT participated was intensified at higher reaction temperature.Therefore, 10 °C was the most suitable reaction temperature for AHMT synthesis in MRs.
As shown in Fig.12, increasing microchannel diameter has an unfavorable effect on AHMT synthesis.AHMT yield decreased from 97.15% to 94.10% with the microchannel diameter increased from 1 mm to 3 mm, while HMT conversion decreased slightly.It could be attributed to the deterioration of mixing and heat transfer efficiencies with the increase in microchannel diameter.Since AHMT synthesis was a strongly exothermic Friedel-Crafts acylation reaction,the decrease in AHMT yield should be attributed mainly to the fact that reaction heat could not be moved away in time.The accumulation of heat in the system resulted in the increase in reaction temperature to accelerate the side reaction.AHMT synthesis was not conducted in the MRs with hydrodynamic diameter smaller than 1 mm, since it was difficult to product AHMT in such a small MR in industrial applications.The microchannel diameter of 1 mm was a suitable selection for AHMT synthesis after comprehensive consideration.
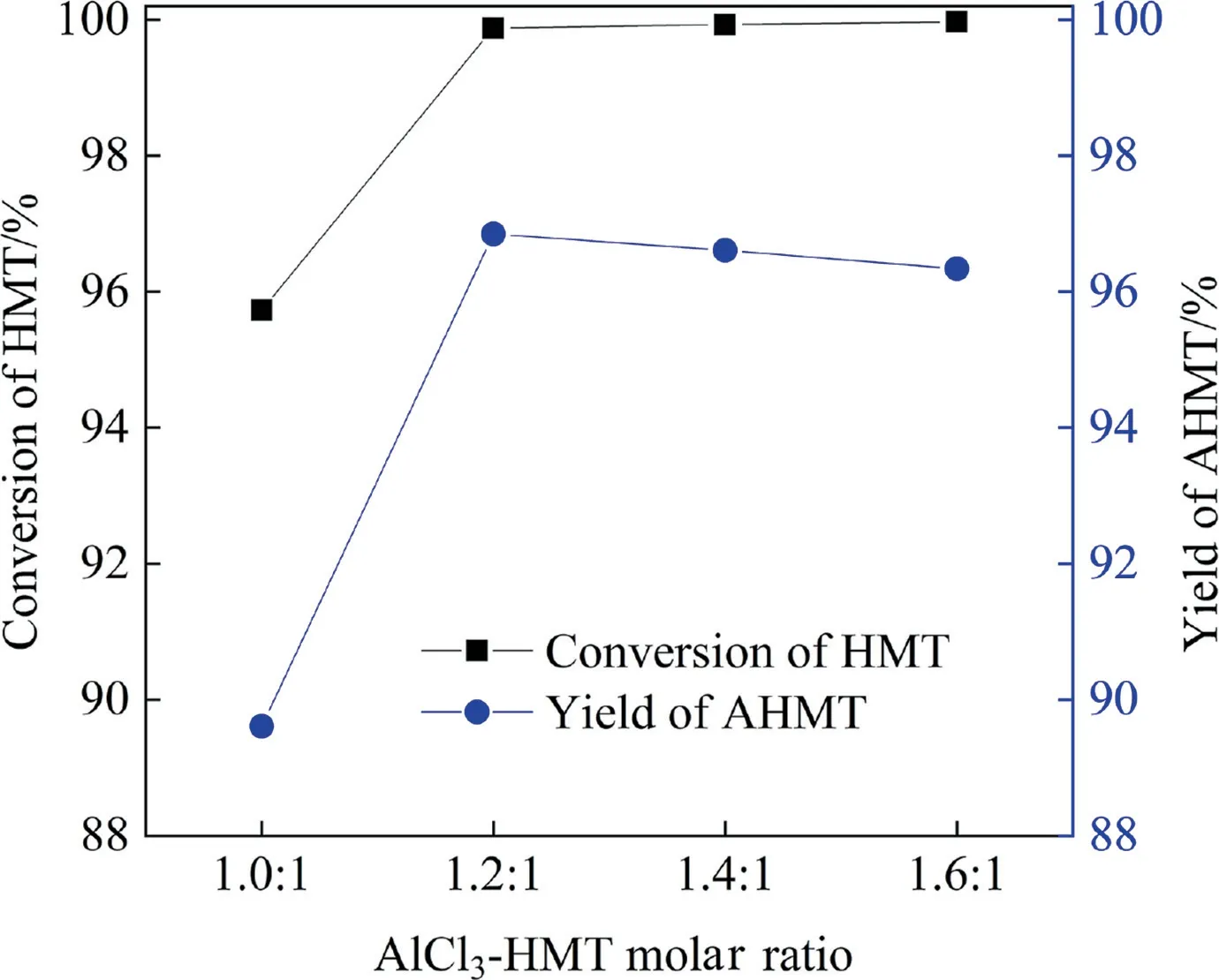
Fig.10. Effects of HMT-AlCl3 molar ratio on AHMT synthesis.Reaction conditions:HMT:DCEE molar ratio in reactant phase=1:4, ACE:DCEE molar ratio in catalyst phase=1.5:6, reaction temperature=10 °C, flow rate of HMT=0.027 ml∙min-1,reaction time=25 min, microchannel diameter=1 mm, HMT purity=97% (mass).

Fig.11. Effects of reaction temperature on AHMT synthesis.Reaction conditions:HMT:DCEE molar ratio in reactant phase=1:4, AlCl3:ACE:DCEE molar ratio in catalyst phase=1.2:1.5:6, flow rate of HMT=0.027 ml∙min-1, reaction time=25 min, microchannel diameter=1 mm, HMT purity=97% (mass).
Increasing solvent amount in catalyst phase could dilute the reaction heat to avoid the rise in temperature.We investigated the effects of HMT:DCEE molar ratio on AHMT synthesis by varying the solvent amount in catalyst phase.As listed in Table 3, AHMT yield increased from 93.55% to 96.85% with the complete conversion of HMT, when the DCEE (in catalyst phase):HMT molar ratio was increased from 4:1 to 6:1.As the molar ratio was further increased to 7:1,the yield varied slightly.Although using more solvent was advantageous to restrain the rise in temperature, more energy was required to recover the solvent.It was suggested that the DCEE (in catalyst phase):HMT molar ratio should be less than 6:1.

Table 3Effects of the molar ratio of HMT to DCEE in catalyst phase on AHMT synthesis

Fig.12. Effect of microchannel diameter on AHMT synthesis.Reaction conditions:HMT:DCEE molar ratio in reactant phase=1:4, AlCl3:ACE:DCEE molar ratio in catalyst phase=1.2:1.5:6,temperature=10°C,flow rate of HMT=0.027 ml∙min-1,reaction time=25 min, HMT purity=97% (mass).
The effects of ACE:HMT molar ratio was investigated in the range of 1.5:1 to 3.0:1, given that the minimum ACE:HMT molar ratio was 1.5:1 for the complete dissolution of catalyst AlCl3in the solvent.In this range,the ACE:HMT molar ratio had little effect on AHMT synthesis, as listed in Table S5 in the Supplementary Material.
3.3.Comparison between batch and microchannel reactors
In this section, the performances of BSTR and continuous MR were compared for the synthesis of HMT and AHMT.In order to make the comparison reliable, the batch and continuous operations were carried out at the same reactant molar ratio, catalyst concentration, and reaction temperature.For the batch operation in BSTRs,the catalyst phase was totally poured into the tank before the reactant phase was added drop by drop.The BSTR was immersed in a thermostatic water bath to control the reaction temperature.The liquid in BSTRs was stirred at a speed of 300 r∙min-1to realize well mixing.In order to investigate the effects of reaction time,the dropping rate was controlled by a syringe pump with the volume of reactant phase kept constant in each run.The reaction time became longer at low dropping rate, so conversion was higher.These two reactions were quenched by pouring 5 °C cold sodium chloride aqueous solution into the reaction mixture to hydrolyze the catalyst AlCl3.The HCl gas generated was absorbed by the NaOH aqueous solution.For the continuous operation in MRs, the catalyst and reactant phases were separately pumped into the microchannel by two syringe pumps.The reaction time in MRs was varied by changing the microchannel length.

Fig.13. Comparison between microchannel reactor and tank reactor.Common reaction conditions:2,3-DMB-1:TBC:p-CME molar ratio in reactant phase=1:1.1:0.5,AlCl3:p-CME:DCEE:CHE molar ratio in catalyst phase=0.15:1.5:1.5:1.5, temperature=15 °C.Special reaction condition for the microchannel reactor: flow rate of 2,3-DMB-1=0.065 ml∙min-1, microchannel diameter=2 mm.Special reaction condition for the tank reactor: stirring rate=300 r∙min-1, amount of 2,3-DMB-1=8.42 g.
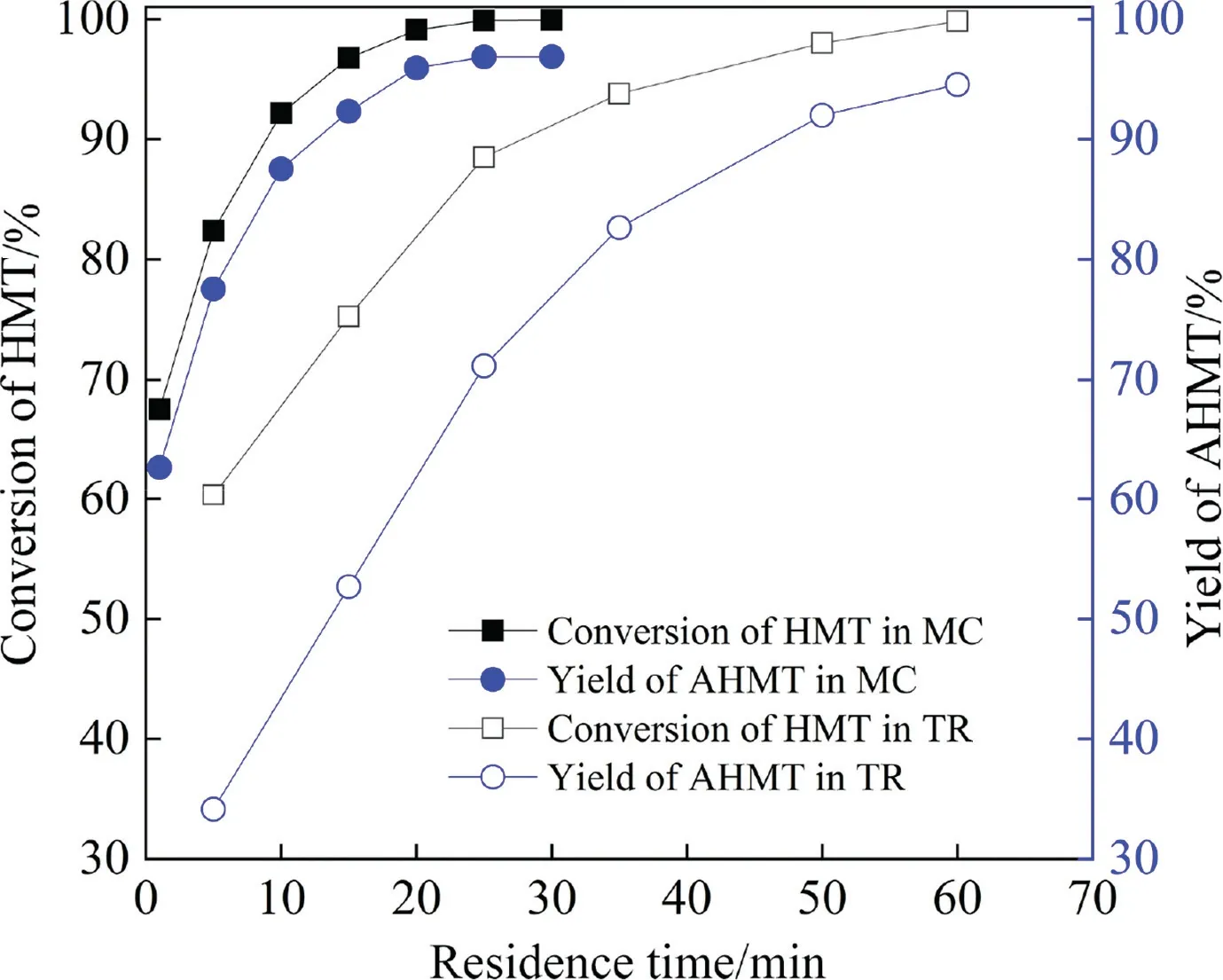
Fig.14. Comparison between microchennel and tank reactors for AHMT synthesis.Common reaction conditions:HMT:DCEE molar ratio in reactant phase=1:4,AlCl3:ACE:DCEE molar ratio in catalyst phase=1.2:1.5:6, reaction temperature=10 °C,HMT purity=97% (mass).Special reaction condition for microchannel reactor:liquid flow rate=0.027 ml∙min-1,microchannel diameter=1 mm.Special reaction condition for tank reactor: stirring rate=300 r∙min-1, amount of HMT=11.15 g.
The comparisons are given in Figs.13 and 14 for the HMT and AHMT synthesis, respectively.As shown in Fig.13, both the 2,3-DMB-1 conversion and HMT yield increased with the increase of reaction time, and reached up to 80.38% and 44.15% within 20 min in MRs, respectively.Although similar 2,3-DMB-1 conversion and HMT yield were also obtained in BSTRs,the reaction time tripled to 60 min.The superiority of MR was also observed for the AHMT synthesis.As shown in Fig.14,the highest HMT conversion of 99.99%and AHMT yield of 97.55%were reached within 20 min in MRs, while the reaction time required was 60 min in BSTRs.
The HMT and AHMT synthesis are Friedel-Crafts alkylation and acylation reactions, respectively.Both of them are strongly exothermic reactions.If the reaction heat could not be removed in time from the reaction system, it would be accumulated resulting in the improvement of reaction temperature.High reaction temperature was disadvantageous to the increase of yield for these two reactions, as shown in Figs.6 and 11.The reactant phase should be slowly dropped into the reactors when the HMT and AHMT synthesis were conducted in BSTRs, since the reaction heat could not be removed in time there.The heat-transfer capability of MRs is much better than that of BSTR.It meant that microreactors could undertake more production of HMT or AHMT over the same period without the increase of reaction temperature.Therefore,both of the HMT and AHMT synthesis could be processed quicker in MRs to improve the production capacity.In addition,the technological process will become safer due to the continuous operations.
4.Conclusions
In this work, AHMT was synthesized using highly efficient and safe continuous-flow technology with HMT as intermediateviatwo Friedel-Crafts reactions, which were conducted in two MRs,respectively.The catalyst AlCl3was tactfully homogenized with reactant as co-solvent, which was a necessary step for the continuous synthesis in MRs.In the HMT synthesis step, catalyst AlCl3and reactantp-CME formed a complex, which could be dissolved more readily.The mixture of DCEE and CHE was chosen as solvent.The polar solvent DCEE was used to dissolve the complex, while the nonpolar solvent CHE was used to suppress the side reactions.The molar ratio of 2,3-DMB-1 to DCEE,p-CME, CHE, and AlCl3was 1:1.5:2:1.5:0.15 in catalyst phase.At optimized operation conditions of TBC:2,3-DMB-1 molar ratio=1.1:1, reaction temperature=15 °C, microchannel diameter=2 mm, the conversion and yield of HMT synthesis could reach up to 80.38% and 44.15%, respectively.In the AHMT synthesis step, DCEE was selected to dissolve the catalyst AlCl3with reactant ACE as cosolvent.The minimum molar ratio of DCEE:ACE:HMT was 6:1.5:1 for the homogenization of AlCl3.The highest HMT conversion and AHMT yield were 99.99%and 97.55%,respectively,at the optimized HMT:ACE:DCEE:AlCl3molar ratio of 1:1.5:10:1.2, temperature of 10°C,and microchannel diameter of 1 mm.By comparing the performances of continuous MR and BSTR, it was found that the HMT and AHMT systhesis could both be conducted more efficient in MRs with the reaction time reduced by two thirds.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the financial support for this work from the National Natural Science Foundation of China (No.21706034),the Natural Science Foundation of Fujian Province (No.2021J01645),and the Key Program of Qingyuan Innovation Laboratory (No.00221004).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.09.028.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of lithium carbonate by microwave assisted pyrolysis
- A breathing A4 paper by in situ growth of green metal–organic frameworks for air freshening and cleaning
- Simulation and design of a heat-integrated double-effect reactive distillation process for propylene glycol methyl ether production
- Hyper-parameter optimization of multiple machine learning algorithms for molecular property prediction using hyperopt library
- Improvement of synergistic effect photocatalytic/peroxymonosulfate activation for degradation of amoxicillin using carbon dots anchored on rod-like CoFe2O4
- Boosting the hydrogen storage performance of magnesium hydride with metal organic framework-derived Cobalt@Nickel oxide bimetallic catalyst
