Preparation of lithium carbonate by microwave assisted pyrolysis
2023-01-30ShenWangXiaokangPeiYongLuoGuangwenChuHaikuiZouBaochangSun
Shen Wang, Xiaokang Pei, Yong Luo, Guangwen Chu,*, Haikui Zou,Baochang Sun,*
1 State Key Laboratory of Organic-Inorganic Composites, Beijing University of Chemical Technology, Beijing 100029, China
2 Research Center of the Ministry of Education for High Gravity Engineering and Technology, Beijing University of Chemical Technology, Beijing 100029, China
Keywords:Lithium bicarbonate Pyrolysis process Traditional heating Microwave heating Product yield
ABSTRACT Investigations were conducted to purify crude Li2CO3 via direct carbonation with CO2 at atmospheric pressure and pyrolysis with both water bath heating method and microwave heating method.The reaction kinetics of LiHCO3 pyrolysis was studied and the effect of different operating conditions including initial concentration of LiHCO3 solution, pyrolysis temperature and stirring speed on the purity of Li2CO3 was investigated to obtain the optimal operating conditions.Results showed that the effect law is similar in the two pyrolysis processes.The purity of the Li2CO3 increases firstly and then decreases with the increase of the initial concentration of LiHCO3 solution and the stirring speed, while the purity of Li2CO3 first decreases and then increases with the increase of pyrolysis temperature.The product yield increases with the increase of initial concentration of LiHCO3 solution and pyrolysis temperature and is essentially unaffected by the stirring speed.Under the optimal operating conditions, the purity of Li2CO3 can reach up to 99.86%and 99.81%in water bath heating and microwave heating process,respectively.In addition,the pyrolysis rate of microwave assisted pyrolysis is 6 times that of water bath heating process,indicating that the microwave heating technology can significantly improve pyrolysis efficiency and reduce energy consumption.
1.Introduction
Lithium carbonate (Li2CO3), a basal lithium salt, exhibits broad applicability in glass manufacturing,ceramic production,medicine and metallurgy [1,2].It has been reported that the global demand for lithium carbonate was 2.26 × 105tons in 2016 [3], and will come up to 4 × 105tons in 2025 [4].Particularly, with the development of the battery, atomic energy and information industries,the consumption of high-purity lithium carbonate will account for 65% of total consumption by 2025 [5].Therefore, the efficient purification technologies for high-purity Li2CO3have drawn extensive attention in recent years.
The common techniques for Li2CO3purification include recrystallization,precipitation,electrolysis,and carbonationdecomposition [6–9].Among these,the carbonationdecomposition method is a promising approach with the features of high efficiency, simple process, low cost and low pollution[10,11].Liuetal.[12] have adopted this carbonationdecomposition method to prepare battery-grade lithium carbonate.The carbonation-decomposition process includes carbonation,decomposition, filtration, and dry,etc.First, the crude lithium carbonate mixed with water is converted to lithium bicarbonate solution by carbonation with CO2because lithium bicarbonate has higher solubility than lithium carbonate in water, as listed in Table 1.Then,the insoluble impurities can be removed by filtration before heating,while the soluble impurities can be removed by filtration during heating process at certain temperature.Hence,highpurity lithium carbonate can be preparedviafiltrating and heating LiHCO3solution.The main reactions are as follows:

Table 1Solubility of Li2CO3 and LiHCO3

Table 2Physical and chemical analyses of crude Li2CO3
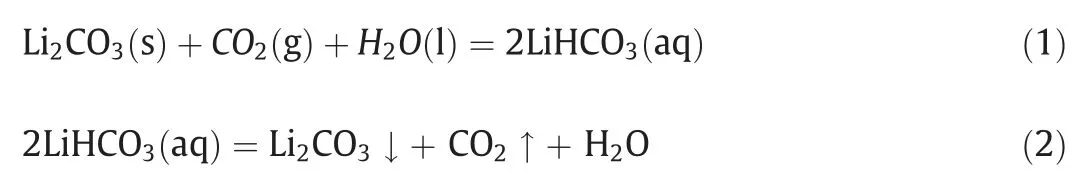
During this process, the decomposition process is the controlling step for the quality and performance of Li2CO3due to the solubility difference between different components in the LiHCO3solution [13].
Therefore,the accurate data of the pyrolysis process seems to be of great significance,and many reports have been devoted to investigating the decomposition kinetics.For example, Yietal.[14]reported the crystallization kinetics of the decomposition process in a constant batch reactor with no evaporation.This work has provided important kinetic data for the pyrolysis process.Liaoetal.[15] reported that the crystallization of lithium carbonate coincided with the second-order reaction kinetics, and obtained the apparent activation energy of the process.As can be seen from these previous studies,how to obtain the uniform temperature distribution and eliminate concentration change caused by evaporation during pyrolysis process is the key of dynamics research.
At present,the decomposition process commonly utilizes a conventional stirred tank with water bath heating,which exhibits relatively poor heat and mass transfer due to its relatively high transfer resistance, thereby affecting the crystal growth process and product purity.Microwave, with the advantages of rapid homogeneous bulk heating and environmentally benign, has drawn widespread attention[16–18].It has been applied in ceramics process, chemical synthesis and analysis, metallic materials synthesis,food drying[19–21].In addition,microwaves have been widely utilized as an effective heating route for the treatment of solid wastes to obtain high value-added products,such as resource recovery from oil sand,waste lubricating oil,waste plastics,and so on[22–24].Microwave,a kind of electromagnetic waves,with high frequencies ranging from 300 MHz to 300 GHz can cause rapid vibration of polar molecules, leading to the fast temperature rise and volumetric heating effect [25].Leetal.[26] prepared a Ytype zeolite by the hydrothermal method under the microwave irradiation, indicating that this process exhibits the advantages of short crystallization time and excellent product performance.Lietal.[27] extracted water-soluble fibroin from silkworm silk by microwave-assisted heating, and it was demonstrated that microwave heating can significantly reduce reaction time and energy consumption.Zhaoetal.[28] employed microwave energy to enhance the co-pyrolysis of polyethylene terephthalate wastes and biomass, and it was demonstrated that microwave-assisted pyrolysis technology is currently one of the most ideal alternative to enhance heat transfer and accelerate pyrolysis process.Lietal.[29] investigated the microwave-assisted co-pyrolysis of biomass with oil sands,and it was demonstrated that microwave is an efficient method to enhance the heat transfer for pyrolysis.
However,to the best of our knowledge,previous studies mainly focused on the carbonation process.The decomposition process,which is the controlling step for the quality and performance ofLi2CO3, has rarely been reported.Furthermore, the preparation of lithium carbonate by microwave assisted pyrolysis is still lacking.
Therefore, in this paper, high-purity Li2CO3was prepared by carbonation-decomposition using the industrial-grade Li2CO3,and the effect of different operating conditions including initial concentration of LiHCO3solution, pyrolysis temperature and stirring speed on the purity of Li2CO3was investigated in microwave reactor and stirring tank reactor (STR), respectively.Meanwhile,the crystallization kinetics of Li2CO3from LiHCO3solutions was studied by stirring tank reactor.The study on the crystallization kinetics of Li2CO3from LiHCO3solutions will provide theoretical basis for crystallizer design and the optimization of the process operation.The result with optimal operating conditions will provide a reference for industrial applications.
2.Experimental
2.1.Materials and equipment
Industrial grade lithium carbonate (99.02%) was purchased from Henan Detai Chemical Factory.The physical and chemical analyses were listed in Table 2.The solution pH was measured by PHS-3C pH Meter.The experimental temperature in the flask was controlled by a super-thermostat(with error±1 K)and microwave pyrolysis experiments were performed in the microwave reactor.Scanning electron microscope (SEM) analysis was performed onto a S4700 to observe the morphologies of lithium carbonate.The XRD pattern was conducted by a powder X-Ray diffraction (XRD-6000, Shimadzu, Japan).All solutions were prepared with deionized water (conductivity at 18.5 mΩ∙cm).It can be seen from Table 3 that as the temperature increases, the thermodynamic solubility product of lithium carbonate gradually decreases, indicating that the solubility of lithium carbonate decreases at high temperatures.

Table 3Ksp of Li2CO3 at different temperatures

Table 4Comparison of pyrolysis experiments between STR and MW reactor
2.2.Experimental procedure
The crude lithium carbonate and deionized water were mixed with CO2to induce the reaction in flask which was sealed and condensed throughout the experiment to maintain the volume of the solution.Then the produced slurry was filtered to remove insoluble impurities and obtain a solution of lithium bicarbonate.Subsequently, lithium bicarbonate solution was stirred continuously by a magnetic stirrer during which the dissolved CO2was stripped from slightly acidic solutions to achieve the critical supersaturation of Li2CO3,and then the Li2CO3was precipitated out.The experimental process is shown in Fig.1.
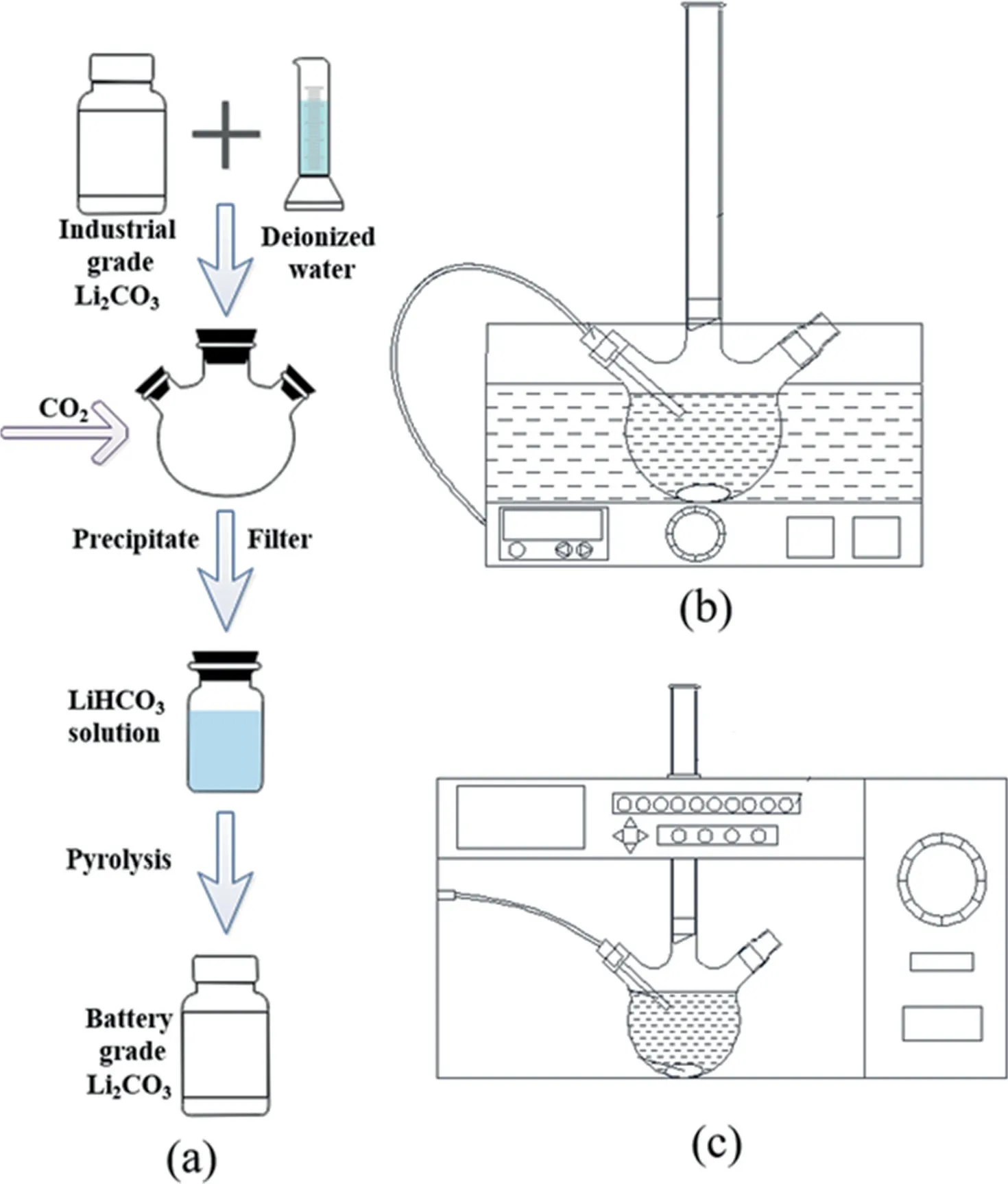
Fig.1. Preparation procedure of battery grade lithium carbonate from industrial grade lithium carbonate.(a) Experimental procedure.(b) Pyrolysis in the STR.(c)Pyrolysis in the MW reactor.
The crystals of lithium carbonate were separated by filtration at negative pressure and subsequently dried in an oven for 8 h at80 °C.The concentration of Li2CO3and the impurities were analyzed by ICP-MS.The product morphologies and particle size were observed and analyzed by scanning electron microscopy (SEM).In addition, the yield was obtained by calculating the mass ratio of lithium carbonate before and after the reaction.The formula for calculation is as follows:

where:Yis the yield of the Li2CO3(%),m1is the total mass of the product(g),C1is the purity of the Li2CO3(%),C2is the concentration of lithium ions in the solution before the reaction(g∙ml-1),V3is the volume of the solution before the reaction (ml).
3.Results and Discussion
3.1.Reaction kinetics
The reaction process of preparing battery grade lithium carbonate includes the carbonization of crude lithium carbonate(Eq.(1))and pyrolysis of lithium bicarbonate solution (Eq.(2)).The latter mainly involves the following equations:

As the pyrolysis reaction progresses, the lithium carbonate gradually reaches a supersaturation state, subsequently producing the nucleus of the lithium carbonate precipitate which will grow into lithium carbonate.It has been verified that higher supersaturation leads to more nucleus in the solution [30].Therefore, the supersaturation of lithium carbonate is crucial to the formation of lithium carbonate precipitation.
The main factors affecting the supersaturation of lithium carbonate include the initial concentration of LiHCO3solution, pyrolysis temperature and stirring speed.This work mainly focused on these three aspects and each parameter was investigated using the STR and MW reactor.
The crystallization kinetics can provide theoretical data for crystallization process analysis and optimization, crystallizer design and operation.They have been studied by some scholars,while due to the different stirring effects in the experiment will affect the escape of CO2and the formation of lithium carbonate precipitation.It is difficult to get the intrinsic kinetic correlation of pyrolysis reaction.Therefore, this work applied the highstrength mechanical agitation to equalize the concentrations of all substances in the solution and eliminate the effect of diffusion.The pyrolysis reactions were carried out at 70, 80, and 90 °C,respectively.Besides,the reaction rate was obtained by measuring the concentration of lithium ion in the solution at different times.Fig.2 shows the change of Li+concentration over time.
The empirical correlation equation was proposed as Eq.(8).After fitting the data, the equation was obtained as Eq.(9).Fig.3 shows the effect of Li+concentration on reaction rate in the STR.
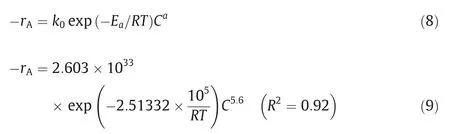
It can be seen from the empirical correlation that the macroscopic activation energy of the pyrolysis reaction is high, and the reaction rate is greatly affected by the concentration of the reactants in the solution with an index of 5.6.
3.2.Preparation of lithium carbonate by water bath heating
3.2.1.EffectofLiHCO3concentration
The prepared LiHCO3solution and deionized water were mixed in different ratios to obtain different concentrations of LiHCO3solution, and then pyrolysis reaction was conducted under fixed conditions.Fig.4 shows the effect of LiHCO3solution concentration on the mass concentration of lithium carbonate and impurity concentration in STR.Fig.5 shows the effect of LiHCO3solution concentration on the yield in STR.
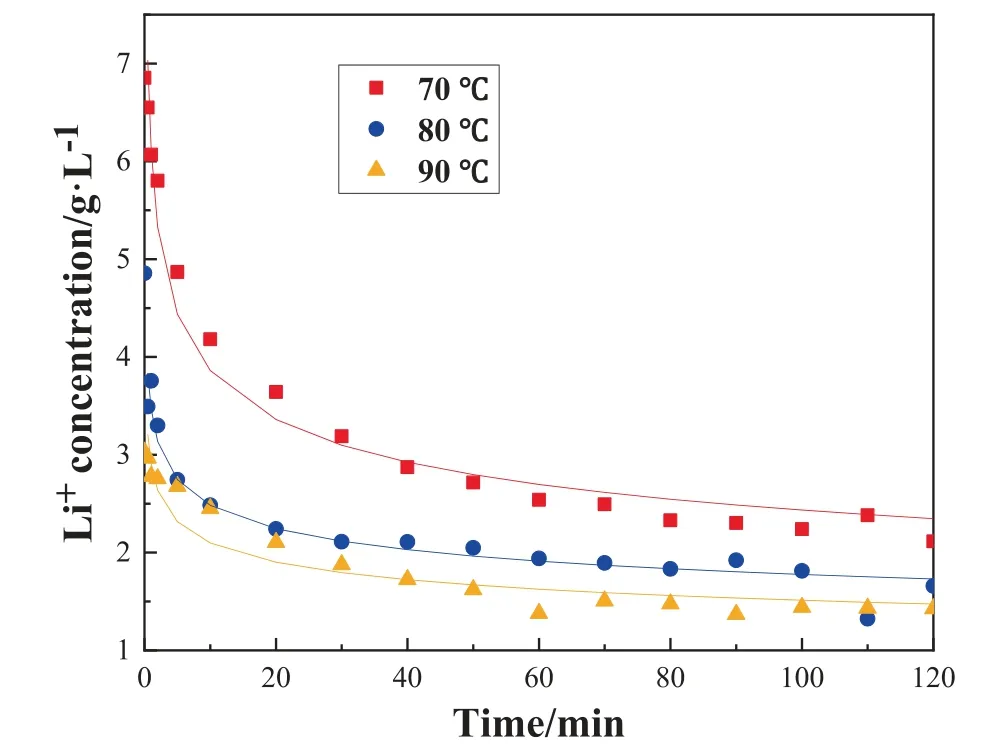
Fig.2. Change of Li+ concentration over time in the STR.

Fig.3. Effect of Li+ concentration on reaction rate in the STR.
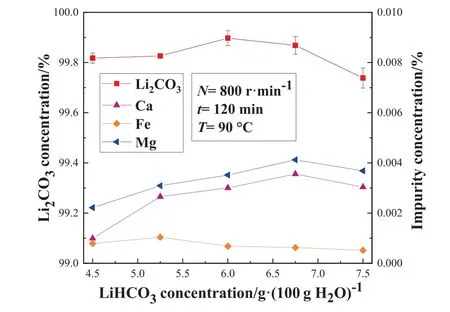
Fig.4. Effect of LiHCO3 solution concentration on the mass concentration of Li2CO3 and impurity concentration in the STR.
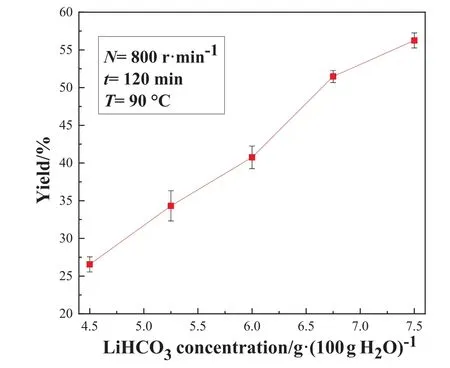
Fig.5. Effect of LiHCO3 solution concentration on the Li2CO3 yield in the STR.
It can be seen from Fig.4 that the mass fraction of Li2CO3increases firstly and then decreases with the increase of the LiHCO3concentration.The reasons for this phenomenon are as follows:on the one hand, the STR adopts water bath heating which has a relatively poor heat transfer efficiency, and the decomposition of LiHCO3belongs to an endothermic reaction, which leads to the uneven temperature distribution of the solution in the STR, thus affecting the precipitation of Ca2+and Mg2+; on the other hand,when the concentration of the LiHCO3solution increases, the concentration of other impurity ions in the solution also gradually increases.Consequently, even after purification, a certain amount of impurity ions remains on the crystal surface, thus affecting the purity of the product.
It is observed from Fig.5 that the product yield gradually increases with the concentration of LiHCO3solution,and the highest yield is close to 56%.The reason can be interpreted as follows:as the concentration of LiHCO3increases, the concentration of lithium ion in the solution increases, and the solubility of lithium carbonate is fixed at a certain temperature, thereby increasing the supersaturation of lithium carbonate.Thus, the increase of the concentration of LiHCO3solution is conducive to the crystallization of the product and the increase of the yield.
3.2.2.Effectofpyrolysistemperature
The pyrolysis reaction was carried out at 70, 75, 80, 85, and 90 °C, and other experimental conditions were fixed.Fig.6 shows the effect of temperature on the mass concentration of lithium carbonate and impurity concentration in the STR.Fig.7 shows the effect of temperature on the yield in the STR.
As shown in Fig.6, the mass fraction of lithium carbonate decreases first and then increases with the increase of the pyrolysis temperature.The possible reasons include two aspects.On the one hand, as the pyrolysis temperature increases, the solubility of MgCO3in the solution gradually increases, leading to high purity of the product, while the solubility of CaCO3is the opposite that it gradually decreases as the temperature increases in the solution,resulting in a gradual decline in the purity of the product.On the other hand, an increase in the pyrolysis temperature leads to an increase in the rate of pyrolysis reaction, causing some of the impurity ions to precipitate on the surface of the crystal, thus affecting the purity of the product.
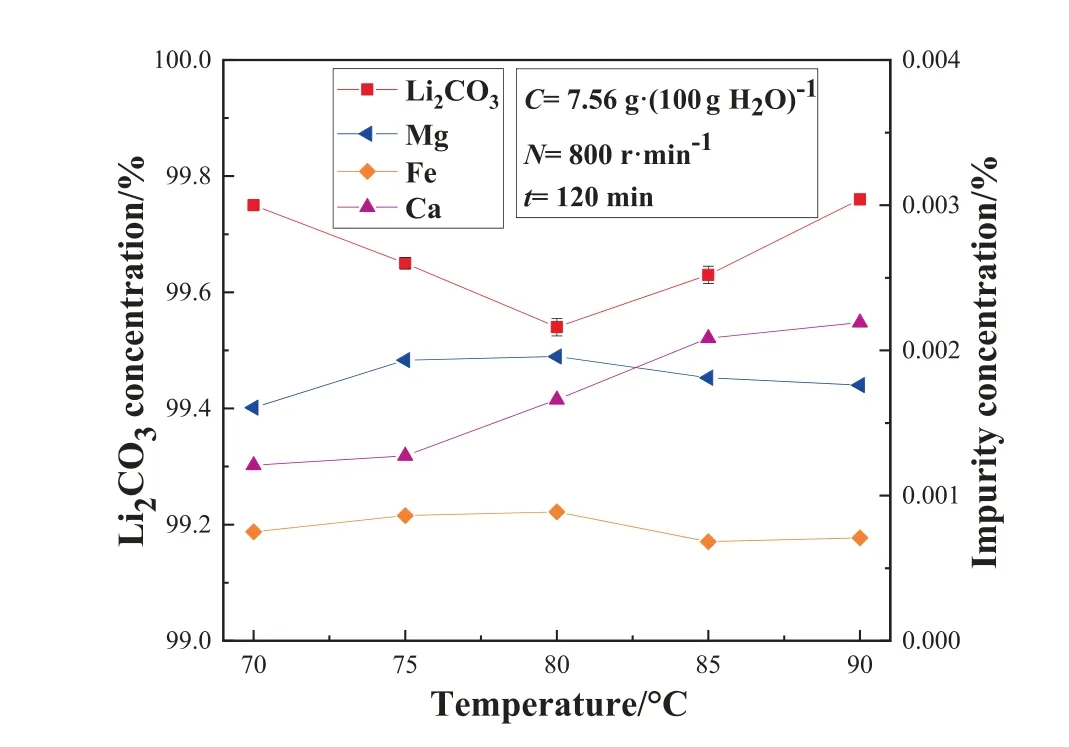
Fig.6. Effect of pyrolysis temperature on the mass concentration of Li2CO3 and impurity concentration in the STR.
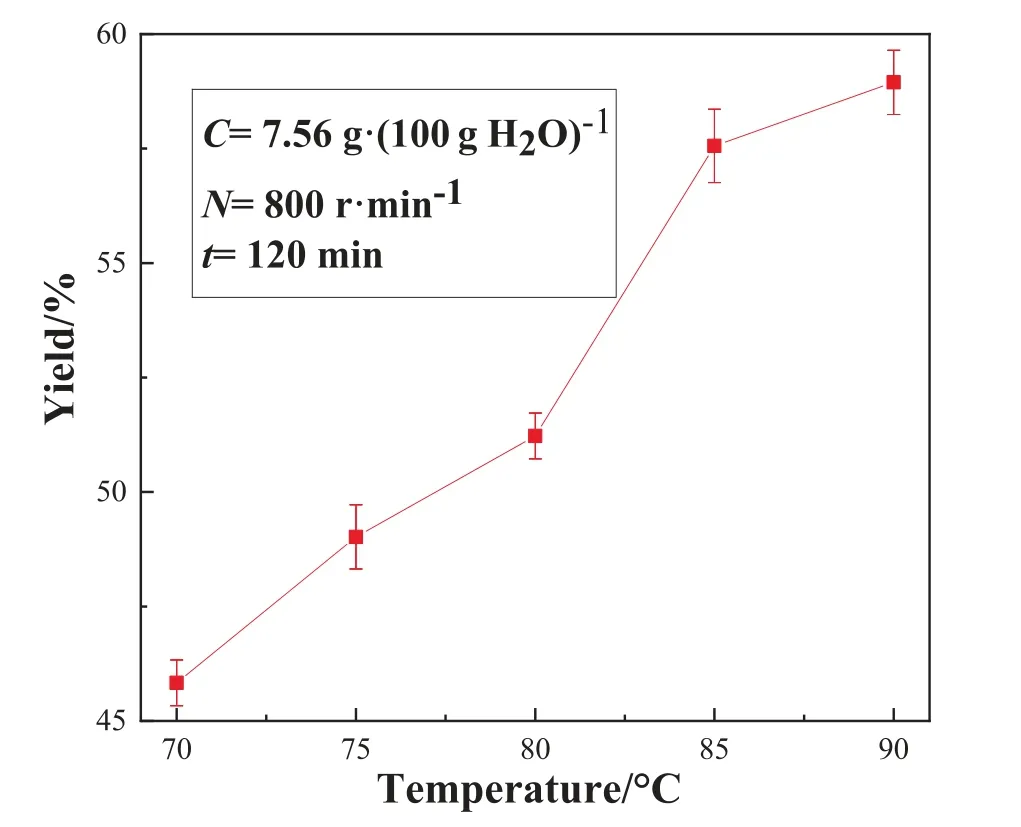
Fig.7. Effect of pyrolysis temperature on the Li2CO3 yield in the STR.
It can be seen from Fig.7 that the product yield gradually increases with the increase of temperature, and the highest yield reaches nearly 58%.The reasons can be interpreted as follows: on the one hand,as the pyrolysis temperature increases,the solubility of lithium carbonate decreases, resulting in a gradual increase in the supersaturation of lithium carbonate in the solution; one the other hand, the elevation of temperature accelerated the diffusion of the ions to the surface and the lattice of the crystals which in turn accelerated the crystal growth.
3.2.3.Effectofstirringspeed
The pyrolysis reaction was carried out at 200,400,600,800,and 1000 r∙min-1while fixing other experimental conditions.Fig.8 shows the effect of stirring speed on the mass concentration of lithium carbonate and the concentration of some impurities in STR.Fig.9 shows the effect of stirring speed on the yield in the STR.
It can be seen from Fig.8 and Fig.9 that the mass fraction of lithium carbonate increases first and then decreases with the increase of the stirring speed.At 400–800 r∙min-1, the purity of the product lithium carbonate remains essentially the same.The stirring speed has no effect nearly on the yield, which remains at about 56.5%.The reasons that may affect the purity of the product include two aspects.For one thing, the temperature inside the solution tends to be uniform as the stirring speed gradually increasing, and the precipitation process can be controlled.For another, the increase of the stirring speed causes the crystal to be broken,so that the impurity ions are adsorbed and precipitated along the surface of the crystal.As a result, the purity of the product is affected.

Fig.8. Effect of stirring speed on the mass concentration of Li2CO3 and impurity concentration in the STR.
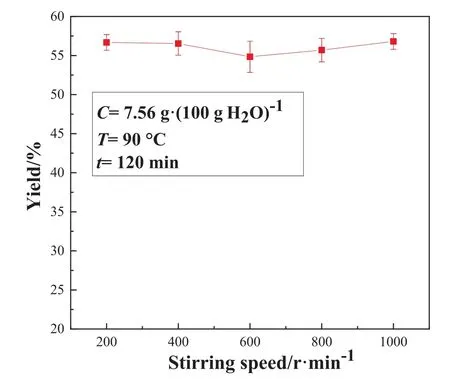
Fig.9. Effect of stirring speed on the Li2CO3 yield in the STR.
3.3.Preparation of lithium carbonate by microwave assisted pyrolysis
3.3.1.EffectofLiHCO3concentration
The prepared LiHCO3solution and deionized water were mixed in different ratios to obtain different concentrations of LiHCO3solution, and then pyrolysis reaction was conducted under fixed conditions.Fig.10 shows the effect of LiHCO3solution concentration on the mass concentration of lithium carbonate and impurity concentration in the microwave reactor.Fig.11 shows the effect of LiHCO3solution concentration on the Li2CO3yield in the microwave reactor.It can be seen from Fig.10 and Fig.11 that the change of the mass fraction of lithium carbonate, impurity concentration and yield is similar to the trend in the STR.
As shown in Fig.10, the mass fraction of lithium carbonate increases first and then decreases with the increase of the concentration of LiHCO3solution.The reason why the purity of the product is affected may be that the increase of LiHCO3concentration leads to an increase in the concentration of carbonate ions in the solution,and the solubility of CaCO3and MgCO3is fixed at a certain temperature, which leads to the precipitation of Ca2+and Mg2+on the surface of the crystal, thereby affecting the purity of the product.
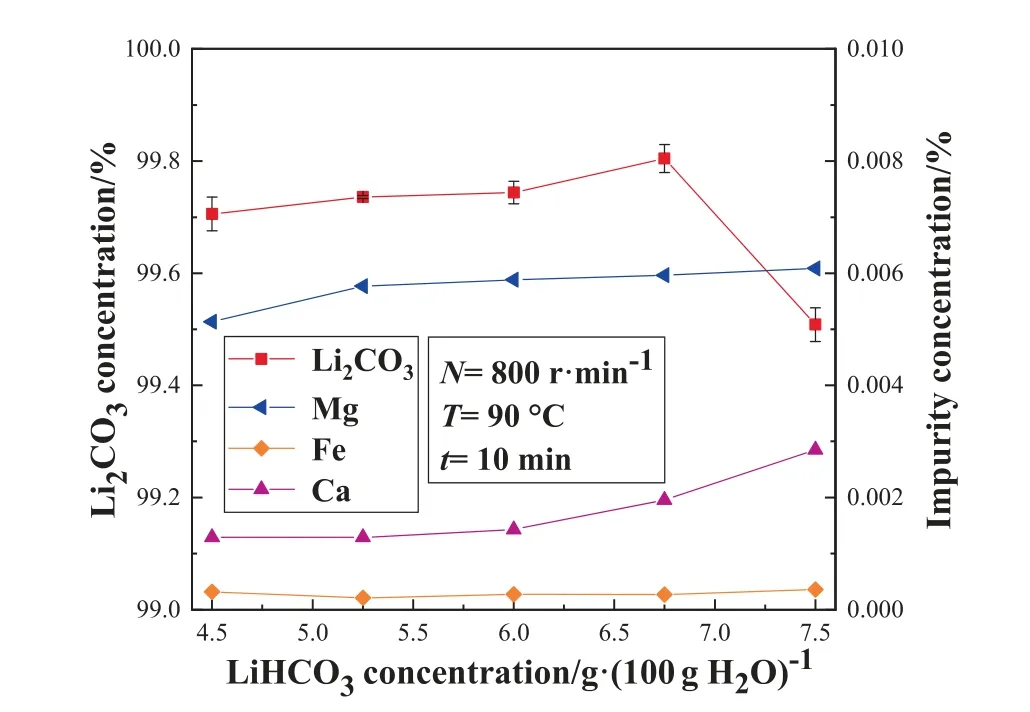
Fig.10. Effect of LiHCO3 solution concentration on the mass concentration of Li2CO3 and impurity concentration in the MW reactor.
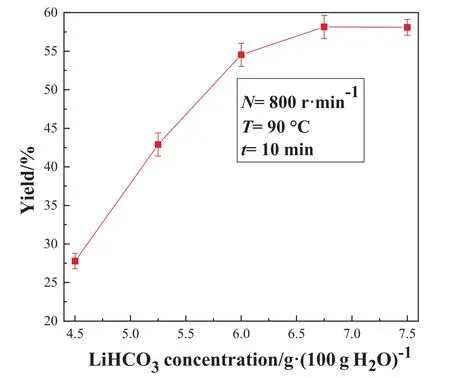
Fig.11. Effect of LiHCO3 solution concentration on the Li2CO3 yield in the MW reactor.
Fig.11 displays that the product yield gradually increases with the concentration of LiHCO3solution,and the highest yield is about 58%.The causes of this phenomenon may be that the concentration of Li+in the solution increases as the concentration of LiHCO3increases, but the solubility of Li2CO3is fixed at a certain temperature.Consequently, the supersaturation of lithium carbonate will increase gradually with the concentration of LiHCO3.
3.3.2.Effectofpyrolysistemperature
The pyrolysis process in 70, 75, 80, 85, and 90 °C was investigated when fixing other experimental conditions, respectively.Fig.12 shows the effect of temperature on the mass concentration of lithium carbonate and impurity concentration in the microwave reactor.Fig.13 shows the effect of temperature on the yield in microwave reactor.It can be seen from Fig.12 and Fig.13 that the change of the mass fraction of lithium carbonate,impurity concentration and yield is similar to the change in STR.
As shown in Fig.12, the mass fraction of lithium carbonate decreases first and then increases with the increase of the pyrolysis temperature.The possible reasons include two aspects.On the one hand, as the pyrolysis temperature increases, the solubility of MgCO3in the solution gradually increases, which improves the purity of the product.On the contrary, as the temperature increases, the solubility of CaCO3gradually decreases, resulting in a gradual decline in the purity of the product.On the other hand,an increase in the pyrolysis temperature leads to an increase in the rate of pyrolysis reaction,causing some of the impurity ions to precipitate on the surface of the crystal,thus affecting the purity of the product.

Fig.12. Effect of pyrolysis temperature on the mass concentration of Li2CO3 and impurity concentration in the MW reactor.

Fig.13. Effect of pyrolysis temperature on the Li2CO3 yield in the MW reactor.
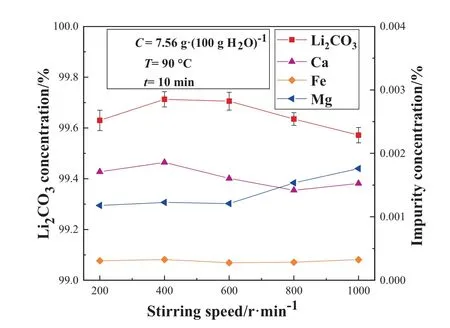
Fig.14. Effect of stirring speed on the mass concentration of Li2CO3 and impurity concentration in the MW reactor.

Fig.15. Effect of stirring speed on the Li2CO3 yield in the MW reactor.
It is observed from Fig.13 that the product yield gradually increases with the increase of the solution concentration, and the highest yield reaches 58%.The reasons can be interpreted as follows: on the one hand, as the pyrolysis temperature increases,the solubility of lithium carbonate decreases,resulting in a gradual increase in the supersaturation of lithium carbonate in the solution;one the other hand,the elevation of temperature accelerated the diffusion of the ions to the surface and the lattice of the crystals which in turn accelerated the crystal growth.

Fig.16. XRD patterns of the prepared Li2CO3 by the two methods.
3.3.3.Effectofstirringspeed
The pyrolysis reactions were carried out at 200, 400, 600, 800,and 1000 r∙min-1while fixing other experimental conditions respectively.Fig.14 shows the effect of stirring speed on the mass concentration of lithium carbonate and some impurities in the microwave reactor.Fig.15 shows the effect of stirring speed on the yield in the microwave reactor.
It can be seen from Fig.14 and Fig.15 that the stirring speed has a great influence on the purity of lithium carbonate and the concentration of the impurity elements Ca2+and Mg2+.As the stirring speed increases, the purity of the lithium carbonate first increases and then decreases.At 400–600 r∙min-1, the purity of the product lithium carbonate remains essentially the same.The stirring speed has no effect nearly on the yield, which remains at about 57%.The reasons that may affect the purity of the product include two aspects.On the one hand, with the gradual increase of the stirring speed, the hot spots generated by the microwave inside the solution decrease, the temperature tends to be uniform,and the ion precipitation process is controlled.On the other hand,the increase of the stirring speed causes the crystals to break, and the impurity ions are adsorbed and precipitated on the surface with the crystals, hence, the purity of the product is affected.
3.4.Comparison of the STR and MW + STR
Fig.16 displays the XRD patterns of the prepared Li2CO3by the two methods.It can be found that the XRD patterns of both products are in good agreement with the standard XRD pattern of Li2CO3.
Table 4 shows that the comparison of pyrolysis experiments between the STR and microwave reactor under the optimal operating conditions.It can be seen that both the two methods can obtain battery-grade lithium carbonate.In addition,the pyrolysis process using microwave heating can significantly increase the reaction rate and reduce energy consumption.At the same time, the product yield obtained by microwave heating is also higher than that of STR.The purity of the product obtained in MW + STR is slightly lower than that obtained in STR with water bath heating.This phenomenon may be attributed to more nuclei quickly formed caused by the rapid temperature rise in MW+STR relative to STR,leading to the larger total specific area of the yielded particles in MW+STR,which is easier to adsorb more impurity ions to the surface and the lattice of the crystals.Nevertheless, microwave heating, a simple green process with high efficiency, still shows great potential in the preparation of Li2CO3.
Fig.17 shows that the SEM images of lithium carbonate between the STR and MW reactor respectively.The morphology of the product is columnar.In addition,the particle size of lithium carbonate obtained by microwave heating method is more uniform than that of lithium carbonate obtained by water bath heating method, indicating that the microwave heating technology exhibits the advantage of excellent product performance.
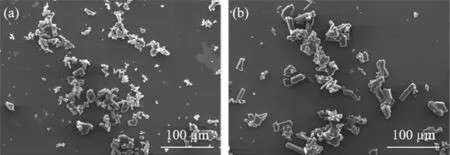
Fig.17. SEM images of lithium carbonate.(a) In the STR.(b) In the MW reactor.
4.Conclusions
The carbonation decomposition method can effectively purify Li2CO3.Columnar lithium carbonate was obtained using the carbonization decomposition method.The preferred operating conditions for the water bath heating process are 6.05 g/100 g H2O of initial concentration, 90 °C of pyrolysis temperature, and 400 r∙min-1of stirring speed.The preferred operating conditions for the microwave pyrolysis process are 6.80 g/100 g H2O of initial concentration, 90 °C of pyrolysis temperature, and 400 r∙min-1of stirring speed.Under the optimal operating conditions, the purity of Li2CO3can reach up to 99.86%and 99.81%in water bath heating and microwave heating process,respectively.Under the conditions of guaranteed yield and product quality, the use of microwave heating method can significantly improve reaction efficiency and reduce energy consumption.At the same time, the saturated lithium carbonate solution after the completion of the reaction can be used for recycling,and the utilization rate of Li can be effectively improved.
Nomenclature
C1purity of the Li2CO3, %
C2concentration of Li+in the solution before the reaction,g∙ml-1
m1total mass of the product, g
V3volume of the solution before the reaction, ml
Yyield of the Li2CO3, %
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos.U1607114, 21878009, 21725601).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Improvement of synergistic effect photocatalytic/peroxymonosulfate activation for degradation of amoxicillin using carbon dots anchored on rod-like CoFe2O4
- A breathing A4 paper by in situ growth of green metal–organic frameworks for air freshening and cleaning
- Simulation and design of a heat-integrated double-effect reactive distillation process for propylene glycol methyl ether production
- Hyper-parameter optimization of multiple machine learning algorithms for molecular property prediction using hyperopt library
- High-efficiency and safe synthesis of tonalid via two Friedel-Crafts reactions in continuous-flow microreactors
- Boosting the hydrogen storage performance of magnesium hydride with metal organic framework-derived Cobalt@Nickel oxide bimetallic catalyst
