Superhydrophobic and mechanically robust polysiloxane composite coatings containing modified silica nanoparticles and PS-grafted halloysite nanotubes
2023-01-30JieWangLingZhangChunzhongLi
Jie Wang, Ling Zhang,Chunzhong Li
Key Laboratory for Ultrafine Materials of Ministry of Education, Shanghai Engineering Research Center of Hierarchical Nanomaterials, Frontiers Science Center for Materiobiology and Dynamic Chemistry, School of Materials Science and Engineering, East China University of Science & Technology, Shanghai 200237, China
Keywords:Polysiloxane Superhydrophobic coating Mechanical properties
ABSTRACT Excellent mechanical properties are the prerequisite for the application of superhydrophobic polymer coatings.However,significantly improving the mechanical properties without affecting other properties such as hydrophobicity is a huge challenge.In this study,a superhydrophobic coating with excellent mechanical properties was prepared by spraying a mixture of polysiloxane resins based on three siloxane monomers,hexadecyltrimethoxysilane(HDTMS)modified nano-SiO2 particles(SiO2-HDTMS)and polystyrene-grafted halloysite nanotubes(HNTs-PS).SiO2-HDTMS dispersed homogeneously in polysiloxane coatings and the water contact angle of corresponding coating exceeding 150°, achieving superhydrophobicity.The SiO2-HDTMS/HNTs-PS/polysiloxane composite coatings showed excellent abrasion resistance with the water coating contact angle remaining above 150°after 90 abrasion cycles,indicating that HNTs-PS can significantly improve the mechanical properties of the coating without affecting the hydrophobic properties of the coating.The achieved coating also exhibited excellent antifouling and acid and alkali corrosion resistance.This work provides a convenient and ecologically friendly method to prepare superhydrophobic polysiloxane composite coating with excellent mechanical properties,which is promising in the application of anti-fouling,anti-corrosion,and oil–water separation etc.
1.Introduction
The superhydrophobic surface has received widespread attention in recent years due to its application in anti-fouling, selfcleaning, anti-corrosion, anti-frosting, drag reduction, and oil–water separationetc[1–8].Superhydrophobic surface generally refers to a surface with a water contact angle (WCA) above 150° and a sliding angle (SA) less than 10°.At the beginning, people discovered that the surfaces of certain animals and plants were hydrophobic and the most famous of which is the ‘‘lotus effect”.In the research, it was found that the key factors that determine the hydrophobicity of the material are the surface structure and surface energy of the materials [9].
Superhydrophobic surfaces are often rough and have a micro–nano hierarchical structure.Therefore, adding fillers into the coating substrate to form micro–nano structures and increase surface roughness is a common method of obtaining superhydrophobic surfaces.Silica is one of the commonly used fillers for preparing hydrophobic coatings with excellent performance [10–16].Keetal.[17] used octadecyltrichlorosilane to graft on the surface of the silica particles.The polydimethylsiloxane(PDMS)/m-SiO2composite coating was obtained by using the drop-coating method.The particles’ dispersion and their compatibility with PDMS were improved by surface modification.And the WCA and SA of the superhydrophobic coating were 155° and 6°, respectively.Additionally, reducing the surface energy is another effective strategy to improve the hydrophobic performance.The easiest way to reduce surface energy is to modify the coating matrix or filler with low surface energy substances, such as perfluorodecyltriethoxysilane (PTES), hexadecyltrimethoxysilane (HDTMS) and octadecyl trichlorosilane (OTS)etal.Inreality, these two strategies are often combined to achieve the best results.Gaoetal.[18] successfully prepared rod-shaped nano-zinc oxide crystal arrays on a substrate using a hydrothermal method.Subsequently, the silicon dioxide was coated on the surface of zinc oxide as a transition layer, PTES was used to modify it,and finally a superhydrophobic coating was obtained on a glass substrate or a polyethylene terephthalate(PET)substrate.
However, most of the superhydrophobic materials have poor mechanical properties.Their surface microstructures are formed merely by stacking individual particles and thus vulnerable to be damaged by external forces.Undoubtedly,the reduction of surface roughness caused by the destruction of surface microstructure will result in the loss of superhydrophobicity.Therefore, the improvement of mechanical properties is very important for the maintenance of superhydrophobic properties of coatings [19–29].Halloysite nanotubes(HNTs)with unique nanotube-like structures act like glass fibers,thus significantly improving the abrasion resistance of the coatings [30,31].Due to the remarkable efficacy and low cost, HNTs have become one of the most popular fillers for improving the mechanical properties of polymer.Wangetal.[32]prepared fluorinated HNTs/SiO2hybrid nanoparticles with good hierarchical structure and biphobicity through Stöber method and thiol-ene click reaction.Then a durable superhydrophobic coatingwith excellent mechanical properties was prepared due to the addition of HNTs.Zhangetal.[33] prepared polymethylmethacrylate(PMMA)-g-HNTs/epoxy acrylate composites by ultraviolet (UV) curing.Compared with pure epoxy acrylate, the mechanical properties of PMMA-g-HNTs/epoxy acrylate composites had been significantly improved.The impact resistance of the coatings had been increased by about 150%; and the abrasion of composites was reduced by 83.75%.However, thiol-ene click and UV curing are relatively complicated processes.
Herein, a novel coating with superhydrophobic properties and excellent wear resistance was prepared [34–39] by one-step spraying.Nano-SiO2particles modified with hexadecyltrimethoxysilane (SiO2-HDTMS) and halloysite nanotubes grafted with polystyrene (HNTS-PS) were incorporated into a polysiloxane matrix.The effect of filler content on the hydrophobicity and mechanical properties of composite coatings had been studied.The polysiloxane composite coating with 20% (mass)SiO2-HDTMS still maintained superhydrophobicity after 50 abrasion cycles on sandpaper with particle size of 25 μm under a load of 200 g.And the polystyrene surface of HNTS-PS was hydrophobic, so the HNTS-PS could significantly improve the coatings’ wear resistance without affecting the hydrophobicity.When the ratio of HNTS-PS-5 to SiO2-HDTMS was 1:2, the WCA of the composite coating was as high as 156° along with the SA of 5° and could maintain superhydrophobicity after 90 abrasion cycles.In addition, the coating had excellent chemical stability, the water contact angles of the coating were still above 145° after 6 h of acid corrosion or alkali corrosion.Therefore, the composite hydrophobic coating could be applied in the field of oil–water separation with quick separation capacity.
2.Materials and Methods
2.1.Materials
3-Glycidoxypropylmethyldimethoxysilane (GPDMS,98%),phenyltrimethoxysilane (PTMS, 98%), dimethoxydimethylsilane(DMDS,98%),hexadecyltrimethoxysilane(HDTMS),styrene,azobisisobutyronitrile, and methacryloxypropyltrimethoxysilane (MPS)were supplied by Adamas Reagent Co.Ltd.(Switzerland).Silica nanoparticles was purchased from Quechen Silicon Chemical Co.Ltd.(China).3-Aminopropyltriethoxysilane (APTS, 98%) was obtained from Sinopharm Chemical Reagent Co.Ltd.(China).Polyvinylpyrrolidone(PVP,Mw=10,000)was supplied from Shanghai Lingfeng Co.Ltd.(China).Tetraethyl silicate (TEOS) was purchased from Shanghai Titan Co.Ltd.(China).Halloysite (HNTs)was purchased from Lingshou County (China).All the chemicals were used as received.
2.2.Modification of nano-SiO2 by coupling agent
SiO2(1 g)and ammonia(5 ml)were ultrasonically dispersed in 50 ml of ethanol-aqueous (3:1) solution to form uniform solution.Then HDTMS(1 g)was added into the mixture,and the system was homogenized by stirring for 12 h.After reaction, washing with ethanol and drying, the SiO2-HDTMS particles were obtained.
2.3.Preparation of HNTs-PS
HNTs (5 g) and ammonia(20 ml) were ultrasonically dispersed in ethanol(100 ml)to form uniform solution.TEOS(2.5 g)and MPS(2.5 g)were added into the above solution,and the mixed solution was stirred for 24 h at 60 °C.After reaction, the modified halloysites were recorded as HNTs-TEOS-MPS.
HNTs-TEOS-MPS (0.3 g), PVP (0.001 g), styrene monomer (1 g)and azodiisobutylcyanide(0.02 g)were added in 200 ml of ethanol.The system was stirred at 80 °C for 12 h.The solution was separated by centrifugation after reaction, the HNTs-PS was obtained and dried at 50 °C, which was recorded as HNTs-PS-1.A series of samples were prepared in the same way.The dosage of styrene monomer was 3, 5, 7, 9 g and the samples were denoted as HNTs-PS-X, whereXrepresented the amount of styrene.
2.4.Preparation of polysiloxane
0.02 mol DMDS, 0.02 mol PTMS, 0.01 mol GPDMS, 1 g of ethanol, 2 g of deionized water were mixed in a 100 ml three-neck flask.Added ammonia to the mixture to a pH of 8–9.Afterwards,polysiloxane was obtained after stirring for 10 h at 60 °C.
2.5.Preparation of composite coatings
SiO2-HDTMS/polysiloxane coatings:A series of composite coatings with different SiO2-HDTMS content were prepared.Polysiloxane (0.1 g), SiO2-HDTMS (0.011, 0.025, 0.043, and 0.067 g) and 3.5 ml ethanol were mixed equably by stirring.The mass content of silica was 10%, 20%, 30% and 40%.Then, APTS(0.01 g) was added as the curing agent.After uniform mixing,the coatings were prepared by spraying and cured at 60 °C for 12 h.The nozzle diameter of the spray gun used was 0.3 mm, the pressure was 0.35 MPa and the spray gun was spaced 10 cm apart from the glass plate.
SiO2-HDTMS/HNTs-PS-5/polysiloxane coatings:Based on the above experiments, the mass content of silica was fixed at 20%, a series of composite coatings with different HNTs-PS-5 content were prepared.The mass ratio of HNTs-PS to SiO2-HDTMS was 1:1, 1:2, 1:3, so the total mass fractions of HNTs-PS-5 and SiO2-HDTMS was 33.3%, 27.3% and 25.0% respectively.Then SiO2-HDTMS/HNTs-PS-5/polysiloxane coatings were fabricated with the same method as SiO2-HDTMS/polysiloxane coatings.The preparation process was shown in Fig.1(a).
2.6.Characterization methods
Infrared spectrometer (FTIR, Nicolet 5700, Thermo Fisher, USA)was used to test the surface functional groups of nano-silica modified by silane coupling agent and HNTs before and after PS modification.The sample was prepared by the tablet method, and the test range was 400–4000 cm-1.HNTs modified by styrene with different quality was tested by thermogravimetric analyzer(TGA,Diamond TG/DTA, PE, USA).The quality of the samples was 4–12 mg,drying moisture before testing.The nitrogen atmosphere was used in the test,and the heating rate was 10 °C∙min-1, the temperature range was 25–800 °C.Used transmission electron microscope(TEM, JEM-1400, JEOL, Japan) to observe the dispersion state of three different silane coupling agents modified nano-silica and different quality styrene modified HNTs.The samples were prepared separately by the fishing method,and the micro-grid copper mesh was dried at 50 °C.

Fig.1. The Schematic diagrams: (a) modification of SiO2, (b) modification of HNTs, and (c) the preparation process of composite coatings.
The water contact angle of the prepared coating was tested by contact angle tester (HARKE-SPCAX3, Germany).The liquid used was deionized water with a volume of 5 μl.The hydrophobic coating was tested by a self-made wear tester,and the size of the coating sample was 6.3 cm × 6.3 cm.The test process was as follows:the sandpaper (particle size was 25 μm) was fixed, the load was 200 g, then the parallel sandpaper moves the coating, the moving distance was 10 cm, the moving speed was 1 cm∙s-1, and then reciprocating.One back and forth was a cycle,and after each cycle,the sample was rotated 90°,and then tested for the next cycle.The quality change of the coating was recorded,and the change of surface morphology after wear was observed.The hydrophobic coatings were completely immersed in HCl solution with pH=1 and NaOH solution with pH=14 to explore the acid and alkali resistance.Separation efficiency was defined as the percentage of the volume of oil after separation to the volume of oil before separation.Flux was defined as the volume of oil phase passing through per unit area of hydrophobic nylon cloth per unit time,the diameter of the effective circular hydrophobic nylon cloth was 38.50 mm in this experiment.
3.Results and Discussion
3.1.Characterization of modified fillers
The dispersion of SiO2and SiO2-HDTMS in ethanol solution were shown in Fig.2.It was clear to see that unmodified SiO2tended to aggregate and was difficult to disperse while SiO2-HDTMS showed better dispersion, which indicated that the modification of SiO2by coupling agent could inhibit the agglomeration of SiO2.It could be obviously seen from Fig.3 that the surfaces of HNTs-PS-1 and HNTs-PS-3 had very thin polystyrene coating layers compared with pure HNTs.When the amount of styrene reached 5 g, the surface of HNTs-PS-5 had obvious polystyrene coating.When the amount of styrene reached 7 g, it was found that the polystyrene began to form micron-sized polystyrene particles.And the halloysite nanotubes were adhered to the surface of the polystyrene particles.This phenomenon became more obvious when the amount of styrene reached 9 g.Through the comprehensive evaluation of the coating rate and the degree of agglomeration,HNTS-PS-5 with uniform coating was chosen for subsequent experiments.
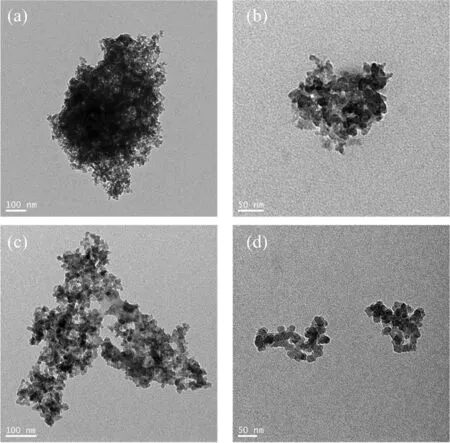
Fig.2. TEM images of (a) and (b) SiO2, (c) and (d) SiO2-HDTMS.
SiO2-HDTMS and HNTs-PS were characterized by infrared spectroscopy, and the outcomes were illustrated in Fig.4.As could be seen in Fig.4(a),the absorption peaks at 3431 and 1623 cm-1were derived from the stretch and bend vibrations of —OH respectively,which were mainly derived from the hydroxyl groups on the surface of silica.The strong peak at 1069 cm-1was derived from Si—O.At 2925 and 2854 cm-1, there were obvious —CH2— absorption peaks in the infrared spectrum, which were derived from the long carbon chain on the coupling agent; it indicated that the silane coupling agent was successfully grafted to the surfaces of SiO2particles.The grafting of silane coupling agent reduced silica particles’ surface energy and the trend of agglomeration.

Fig.3. The TEM images of (a) HNTs, (b) HNTs-PS-1, (c) HNTs-PS-3, (d) HNTs-PS-5, (e) HNTs-PS-7, and (f) HNTs-PS-9.
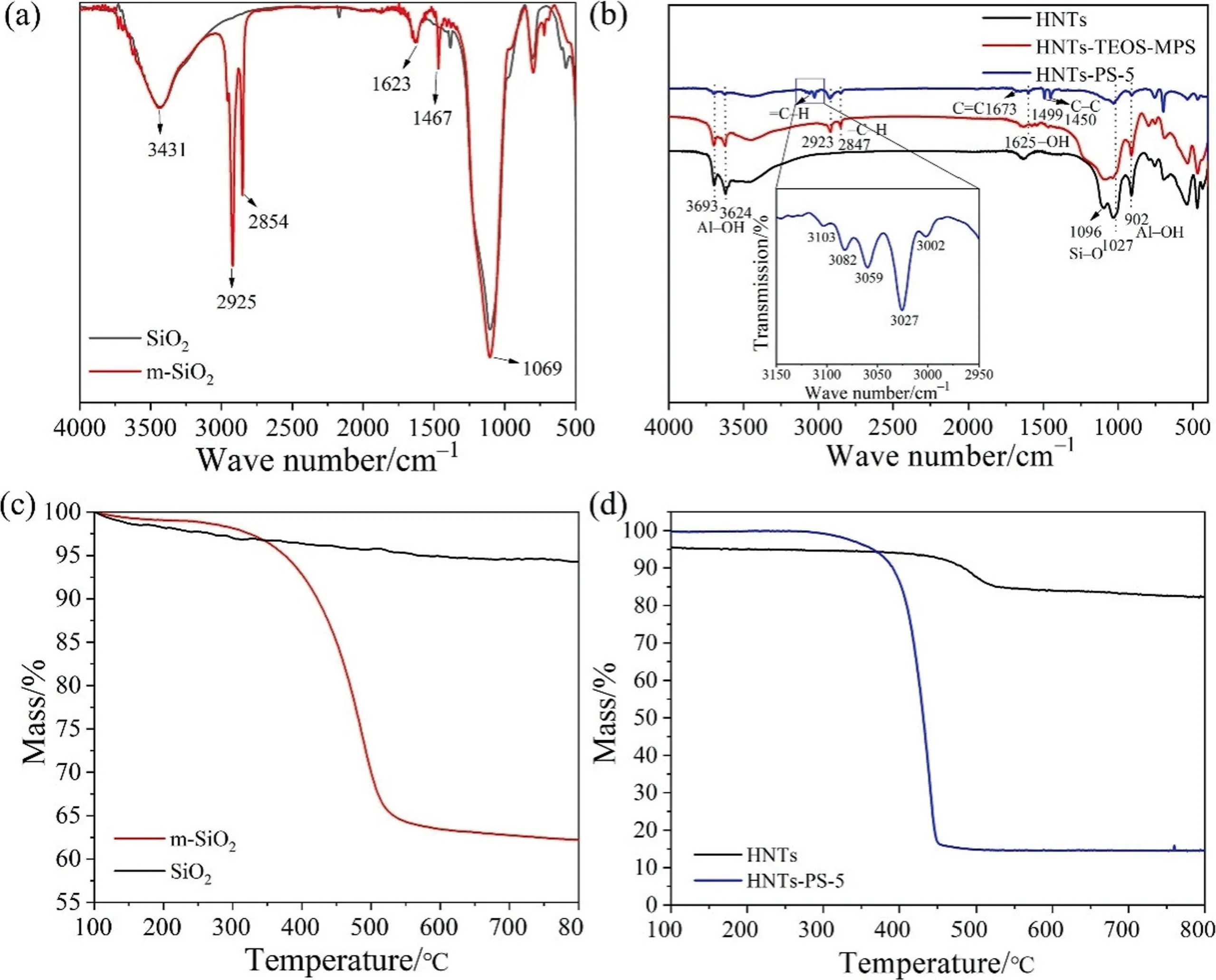
Fig.4. The FTIR spectra and TGA of (a, c) SiO2 and m-SiO2, (b, d) HNTs and HNTs-PS-5.
It could be seen from Fig.4(b) that the main composition of HNTs was aluminosilicate with some functional groups mainly including Al—OH, —OH and Si—O.The corresponding absorption peaks were located at 3693, 3624, 1625 and 1027 cm-1respectively.The infrared spectrum of HNTs-TEOS-MPS showed obvious absorption peaks of —CH2—, which were located at 2923 and 2847 cm-1.It was attributed to the carbon chain structure of MPS.For HNTs-PS-5, the absorption peak of =C—H in polystyrene could be clearly seen from the spectrum, which was located at 3002, 3027, 3059, 3082 and 3103 cm-1respectively.At the same time, the absorption peak of C=C bond could also be seen in the infrared spectrum,which was mainly located in 1673 cm-1.While the absorption peak of C—C bond in polystyrene appeared in 1499 and 1450 cm-1.These proved that polystyrene had been successfully grafted onto the surface of HNTs.
According to Fig.4(c), the residual rates of untreated SiO2and SiO2-HDTMS after calcination at 800 °C were 94.5% and 60.5%respectively.This indicated that there were 34% (mass) coupling agents in SiO2-HDTMS.It could be seen from Fig.4(d) that the residual rates of untreated HNTs and HNTS-PS-5 after calcination at 800 °C were 87.0% and 46.5% (mass) respectively.The difference of residual rate was due to the decomposition of PS at 400–500 °C.The results showed that SiO2and HNTs were modified successfully.
3.2.Characterization of composite coatings
3.2.1.SiO2-HDTMS/polysiloxanecoatings
The morphology of hydrophobic coatings with different SiO2-HDTMS contents were shown in Fig.S1 in Supplementary Material.When the mass fraction of filler was 10%, the surface of the glass substrate was still partially uncovered.When the mass fraction of SiO2-HDTMS increased to 20%, the whole glass substrate was completely coated, the surface roughness of the coating was obviously improved.When the mass fraction of SiO2-HDTMS reached 30%–40%, the difference of surface roughness was no longer significant due to the high filler content.
The wettability and wear resistance of the coatings were tested.The hydrophobic properties of the coatings were shown in the Fig.5(a).It could be seen that the WCA of the coatings raised with the increase of SiO2-HDTMS content.Compared to a pure polysiloxane coating without SiO2-HDTMS,the WCA of the coating with 10%(mass)SiO2-HDTMS was increased by 19%.And when the mass content of SiO2-HDTMS was 20%,the WCA of the coating exceeded 150°,achieving superhydrophobic.It was ascribed to the increase in surface roughness due to the completely coated SiO2-HDTMS.
In order to characterize the mechanical properties of the coating,the wear resistant property of the coatings was tested by sandpaper abrasion test (as shown in Fig.5(d)) and the corresponding results were shown in Fig.5(b) and (c).It could be seen from Fig.5(b) that when the mass content of SiO2-HDTMS was 10%,the WCA of the coating increased slightly with the increase of abrasion cycles.This was because when the initial contact angle was low, the surface roughness of the coating was low, and the roughness of the coating would increase after being worn.When the mass content of SiO2-HDTMS was 40%, the water contact angles of the coatings decreased with the increase of abrasion cycles as a whole.This was because that the original multi-level structure had been destroyed, resulting in a decrease in the roughness of the coating surface.However, it was worth noting that when the mass content of SiO2-HDTMS filler was 20%, the water contact angle of the coating remained almost unchanged exceeding 150°even after 50 abrasion cycles, maintaining superhydrophobicity.As shown in the Fig.5(c), the mass loss of the coating with 20%SiO2-HDTMS increased with the increase of abrasion cycles, but the overall loss was small(only 0.0181 g after 50 abrasion cycles),which showed excellent wear resistance.The mechanism can be speculated as follows.The carbon chain on the surface of SiO2-HDTMS was long, its entanglement with the matrix provided certain mechanical strength.But when the content of SiO2-HDTMS filler further increased, the convex surface of the coating was loose.This made the micro–nano structure of the coating surface vulnerable to be damaged with the increase of abrasion cycles.So, when the mass content of modified silica was 20%, the coating had optimal overall performance.On this basis, modified halloysite was added to further enhance the wear resistant property of this coating with 20% (mass) SiO2-HDTMS.
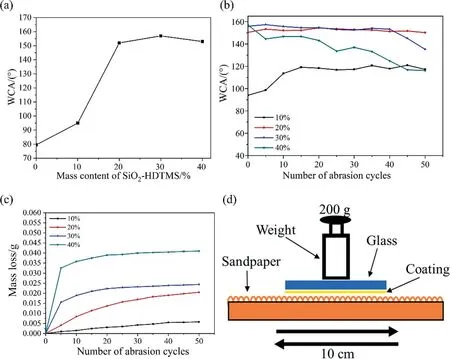
Fig.5. The wettability and wear resistance of SiO2-HDTMS/polysiloxane coatings: (a) water contact angles of coatings with different SiO2-HDTMS mass content, (b) water contact angles and (c) mass loss during 50 abrasion cycles, (d) the process of the sandpaper abrasion test.
3.2.2.SiO2-HDTMS/HNTs-PS-5/polysiloxanecoatings
The morphology of hydrophobic coatings with different contents of HNTs-PS-5 were presented in Fig.6.As shown in the picture Fig.6(a) and (b), the modified halloysites were embedded in the coating substrate, which could play a good strengthening effect; And when the content of modified halloysite was reduced,no obvious modified halloysite was seen in Fig.6(c)–(f).All three coatings had high roughness and exhibited similar surface morphology, compared with the coating without modified halloysite.It showed that the addition of HNTS-PS-5 did not cause obvious changes in the surface topography of the coatings.

Fig.6. Hydrophobic coatings contained different HNTs-PS-5 content: (a–b) 1:1, (c–d) 1:2, (e–f) 1:3 (HNTs-PS-5:SiO2-HDTMS).
The wettability and wear resistance of the SiO2-HDTMS/HNTs-PS-5/polysiloxane coatings were tested.The hydrophobic properties of the coatings were shown in the Table 1.The water contact angles of the hydrophobic coatings with different HNTs-PS-5 content all exceeded 150°,which were 157.7°,155.9°,and 153.8°,indicating that all three coatings had superhydrophobicity.It could be seen from Fig.7 that while maintaining excellent superhydrophobicity,the three coatings all showed excellent wear resistant property, with a mass loss of 0.0182, 0.0119 and 0.0122 g respectively after 50 abrasion cycles.Compared to the coating without HNTs-PS-5, when the ratios of HNTs-PS-5 to SiO2-HDTMS were 1:2 and 1:3, the coatings’ mass loss was reduced by 34.3% and 32.6%respectively.When the ratio was 1:1, the wear resistant property was not improved due to the total filler content in the coating was too high, which weakened the wear resistant property.When the ratios of HNTs-PS-5 to SiO2-HDTMS was 1:1 and 1:2,the superhydrophobicity was still maintained after 90 abrasion cycles.This indicated that HNTs-PS-5 could significantly improve the mechanical properties of the coatings.In conclusion, when the ratio of HNTs-PS-5 to SiO2-HDTMS was 1:2, the coating had the best wear resistant property, the morphology of the coating after 100 wear cycles was shown in the Fig.S2 that there was still a lot of coating substrate on the glass substrate, this proved that HNTs-PS-5 was beneficial to the wear resistance of the coating.
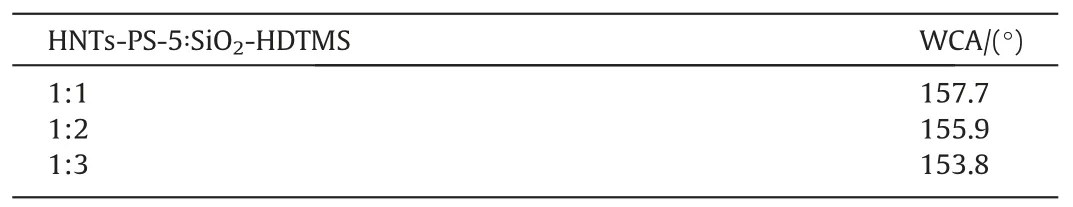
Table 1Water contact angles of the coatings with different mass fractions of HNTs-PS-5
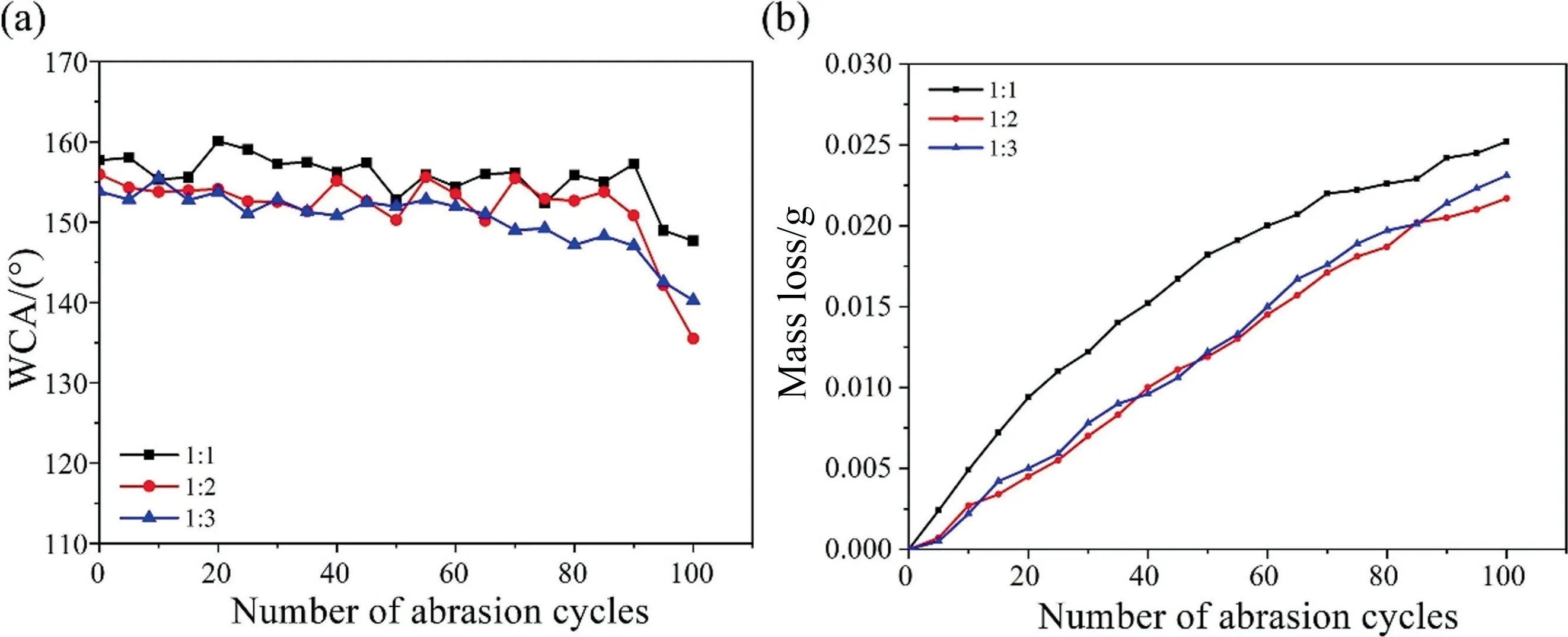
Fig.7. The wettability and wear resistance of SiO2-HDTMS/HNTs-PS-5/polysiloxane coatings: (a) water contact angles and (b) mass loss during 100 abrasion cycles.
3.2.3.Acid-basecorrosiontest
In order to evaluate the chemical stability of the SiO2-HDTMS/HNTs-PS-5/polysiloxane coatings under acid-base conditions, the acid and alkali resistance of three composite coatings had been tested,and the findings were shown in Fig.8.It could be concluded from the Fig.8 that three composite coatings had the similar chemical stability.In the whole acid resistance test,the composite coatings maintained superhydrophobicity, and the water contact angles all exceeded 150°.The excellent acid resistance of coatings was ascribed to the coating had micro–nano multistage structure,many peaks and valleys were formed on the surface, the air trapped in the valley could form a thin air layer.When the coating was immersed in the solution, the thin air layer could prevent the direct contact between the liquid and the coating.So,the solid–liquid contact area was greatly reduced and the liquid could only contact the peaks formed by the fillers with excellent acid resistance.In the alkali resistance test, the water contact angles of the three coatings all decreased slightly,and after 6 h of corrosion,the water contact angle still maintained about 145°, which was lower than the acid resistance.This was because silica particles could react with NaOH(pH=14)but not with HCl(pH=1).With the increase of soaking time, the multistage structure of the coating was destroyed in alkaline solution, so the coating gradually lost its superhydrophobicity.
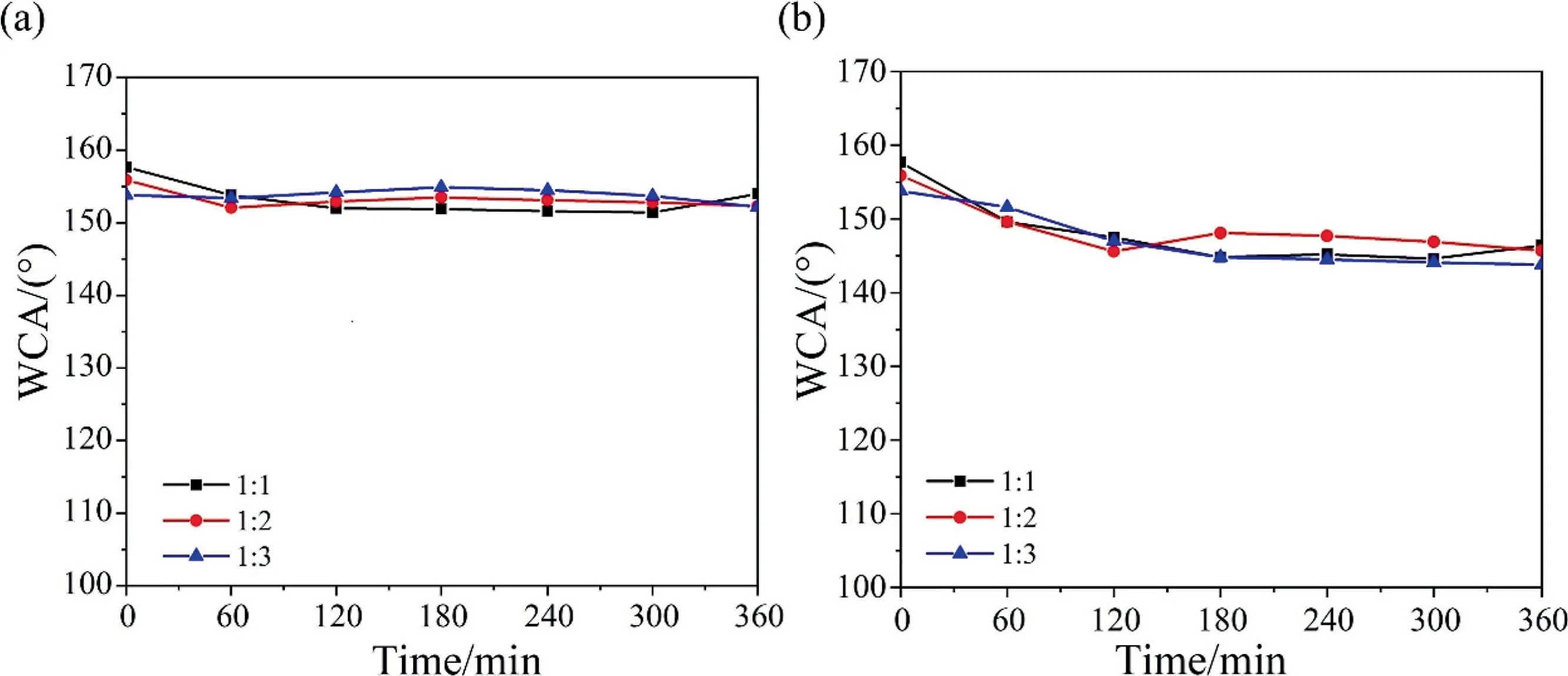
Fig.8. (a) Acid resistance and (b) alkali resistance of the SiO2-HDTMS/HNTs-PS-5/polysiloxane coatings.
3.2.4.Antifoulingproperty
As shown in Fig.9, the antifouling property of superhydrophobic coating with the ratio of HNTs-PS-5 and SiO2-HDTMS of 1:2 was tested.Fig.9(a)–(d) showed the process of removing dirt on the surface with clean water.It could be seen that the dust on the surface rolled down from the coating surface with water droplets and did not adhere to the coating surface.Fig.9(d) showed that the dust was completely removed and the cleanliness of the surface was restored,indicating that the coating has good antifouling property.
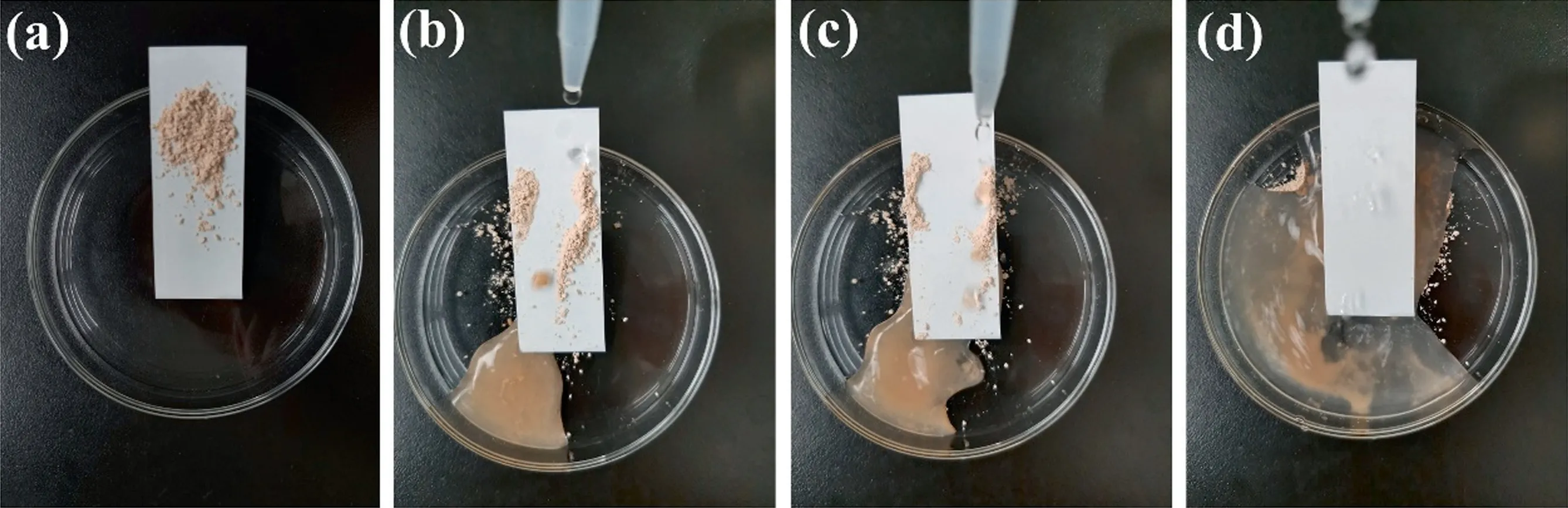
Fig.9. Antifouling property of the SiO2-HDTMS/HNTs-PS-5/polysiloxane coating.
3.3.Application of superhydrophobic coating—oil–water separation
In order to apply superhydrophobic coating in practice,hydrophobic nylon cloth was prepared by spraying superhydrophobic SiO2-HDTMS/HNTs-PS-5/polysiloxane coating on nylon fiber cloth(aperture was 13 μm)to realize the function of oil–water separation.As can be seen from Fig.S3,the original nylon fiber
cloth had a smooth surface, after spraying the hydrophobic layer,the surface of the nylon fiber cloth was uniformly covered with a layer of SiO2-HDTMS/HNTs-PS-5/polysiloxane coating, presenting multi-level hydrophobic structure, while the original pores were not blocked.
It could be seen from the comparison of Figs.S4(a) and (b), the original nylon fiber cloth was hydrophilic, and water droplets would spread and infiltrate on its surface.While in Figs.S4(c) and(d) the water droplets were spherical, showing excellent hydrophobic properties.In order to explore the oil–water separation ability of nylon fiber cloth covered with hydrophobic coating,the oil–water separation experiment was designed and the results were shown in Fig.10.Fig.10(a)was a simple oil–water separation device,composed of two filter cups,with a hydrophobic nylon fiber cloth placed in the middle,and the two filter cups were fixed with a strong spring clamp.Put 50 ml water (blue) and 50 ml dichloromethane in the graduated cylinder.The two liquids had a clear interface (Fig.10(b)).Then the 100 ml liquid was poured into the oil–water separation device quickly.The upper part of the blue water was isolated by the hydrophobic coating, while the dichloromethane was unaffected and completely passed through the coating after 23 s.The volume of separated oil was 48 ml (Fig.10(h)).After calculation, the separation efficiency of oil–water mixture was 96%.With the assistance of gravity only, the flux of the hydrophobic nylon fiber cloth was 6454 L∙m-2∙h-1.

Fig.10. The experiments of oil–water separation.
4.Conclusions
In summary, a wear-resistant polysiloxane superhydrophobic coating was prepared by incorporation of modified silica and halloysite nanotubes.FTIR and TGA indicated that HDTMS and PS was successfully grafted to the surface of nano-silica and HNTs respectively.Then the modified particles obtained were compounded with polysiloxane.
The addition of silica increased the roughness of the coating and improved the hydrophobicity of the coatings significantly.When the mass content of HDTMS was 20%, the SiO2-HDTMS/polysiloxane coatings showed superhydrophobicity and good wear resistance maintaining superhydrophobicity after 50 abrasion cycles.Furthermore, we had proved that the introduction of modified halloysite can significantly enhance the wear resistance of the superhydrophobic coating.The prepared SiO2-HDTMS/HNTs-PS-5/polysiloxane coating showed excellent superhydrophobic stability during abrasion test.The water contact angles of the coating could exceed 150° after 90 abrasion cycles.And it also showed excellent acid and alkali corrosion resistance.The composite superhydrophobic coating could be applied in the field of oil–water separation, which could separate oil and water rapidly and easily.It can be concluded that the composite coating has a promising development future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21878092, 21838003, 91834301 and 51621002),the Shanghai Scientific and Technological Innovation Project(19JC1410400), Program of Shanghai Academic Research Leader(19XD1401400), the Innovation Program of Shanghai Municipal Education Commission, the Fundamental Research Funds for the Central Universities (222201718002).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.12.017.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of lithium carbonate by microwave assisted pyrolysis
- Simulation and design of a heat-integrated double-effect reactive distillation process for propylene glycol methyl ether production
- Hyper-parameter optimization of multiple machine learning algorithms for molecular property prediction using hyperopt library
- High-efficiency and safe synthesis of tonalid via two Friedel-Crafts reactions in continuous-flow microreactors
- Improvement of synergistic effect photocatalytic/peroxymonosulfate activation for degradation of amoxicillin using carbon dots anchored on rod-like CoFe2O4
- Boosting the hydrogen storage performance of magnesium hydride with metal organic framework-derived Cobalt@Nickel oxide bimetallic catalyst
