Rational design of Aspergillus flavus A5p1-immobilized cell system to enhance the decolorization of reactive blue 4 (RB4)
2023-01-30WenboYangQingyunLiShiqiGuoShijieSunAixingTangHaiboLiuYouyanLiu
Wenbo Yang, Qingyun Li,2,*, Shiqi Guo, Shijie Sun, Aixing Tang, Haibo Liu, Youyan Liu,2
1 School of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, China
2 Guangxi Key Laboratory of Biorefining, Nanning 530003, China
Keywords:Aspergillus flavus A5p1 Reactive blue 4 (RB4)Polyurethane foam (PUF)Immobilized cell Decolorization
ABSTRACT Anthraquinone dyes are a class of typical carcinogenic and hard-biodegradable organic pollutants.This study aimed to enhance the decolorization of anthraquinone dye by rationally designing an expected immobilized system.Reactive blue 4 (RB4) was used as a substrate model and a previous isolated dyedegrading strain Aspergillus flavus A5p1 was purposefully immobilized.Considering the effects of cell attachment and mass transfer, the polyurethane foam (PUF) with open pore structure was selected as the immobilization carrier.Results showed that the RB4 decolorization efficiency was significant enhanced after immobilization.Compared to the free mycelium system, the decolorization time of 200 mg∙L-1 RB4 was shortened from 48 h to 28 h by the PUF-immobilized cell system.Moreover, the PUF-immobilized system could tolerate RB4 up to 2000 mg∙L-1.In the packed bed bioreactor (PBBR),an average decolorization efficiency of 93.3% could be maintained by the PUF-immobilized system for 26 days.The decolorization process of RB4 was well described by the logistic equation and the degradation pathway was discussed.It was found that the higher specific growth rate of the PUF-immobilized cells was one of reasons for the enhanced decolorization.The good performance of the PUFimmobilized cell system would make it have potential application value for RB4 bioremediation.
1.Introduction
Synthetic dyes are extensively used in textile, paper, leather,plastic, cosmetic and other fields [1].Taking the textile industry as an example, the global dyes consumption reached about 7 × 105tons every year, and nearly 17% of resulting wastewater was discharged into the environment,thus posing a severe damage to the ecosystem [2–4].Bioremediation is widely accepted to be one of green and economical methods for dye decolorization[5,6].Many microorganisms,such asAspergillus[7],white-rotfungi[8],Bjerkanderaadusta[9] andPseudomonas[10] capable of decolorizing dye by adsorption or degradation have been documented.In practice, the biological systems are often susceptible to some adverse factors such as changeable operation conditions, toxic compounds and shock loading,leading to deteriorate or inefficient[11].Therefore,research efforts are ongoing to explore and develop effective measures for the treatment of dyes pollution [12].
The technique of immobilization is widely adopted to enhance the biological treatment, owing to its high efficiency and good resistance to the adverse impacts [13].There have been many reports on the decolorization by the immobilized cell system.Some common materials,such as coconut fiber[14],alginate[15],starch[16]and light expanded clay[17],etc.have been used as the immobilization carriers.The prominent advantages of the immobilized cell system are largely attributed to high biomass and good biomass retention, which plays a fundamental role in the bioremediation enhancement [18].Thus, it is obvious that carrier is a direct influence on for constructing such a kind of system.However, at present, the use of immobilized carrier mainly depends on extensive screening or experience in previous study.To reduce the blindness and increase success in carrier screening, more rational thinking is needed to construct the immobilized cell system.
Good physicochemical property and excellent biocompatibility are widely considered to be the basic requirements for the immobilization carrier.Significantly,carrier affinity with the cell and the matter of substrate mass transfer are central to the immobilized system, affecting the amount of immobilized biomass and its enhanced effect.Hence, the relationship between carrier and cell,substrate and cell should be considered when constructing the immobilized system.Polyurethane foam (PUF) with porous structure is one of commonly used carrier for cells immobilization[19].The applications of PUF to immobilize microorganisms for the treatment of environmental pollutants, such as cyanide [20],azo dyes [21,22], and domestic sewage [23] under sterile and non-sterile conditions have been reported.The porous structure of PUF makes it has huge specific surface area, and is favorable for the attached growth of microorganisms[24].More importantly,the PUF can be easily synthesized and its pore size can be controlled as needed.Integrated analysis shows that PUF would be a suitable material carrier to construct the desired immobilized cell system with high biomass and good mass transfer.
This study aimed to investigate the enhanced dye decolorization by the expected PUF-immobilized cell system.Anthraquinone dye, one of carcinogenic synthetic dyes, has become the preferred dye for industrial production owing to its good dyeing performance, easy preparation and low cost [25].Because of the combined resonance effects among the anthracene structures, the biodegradation of anthraquinone dyes is very difficult [1].Hence,reactive blue 4 (RB4), one of anthraquinone dyes, was used as a substrate model.The experiment of RB4 enhanced decolorization was conducted by immobilizing the dye-degrading strainAspergillusflavusA5p1 isolated previously[26].The decolorization performance of the PUF-immobilized cell system in batch experiments and packed bed bioreactor(PBBR)were assessed,respectively.Furthermore,the decolorization kinetics and the degradation pathway of RB4 were also discussed.
2.Materials and Methods
2.1.Dye-degrading strain and chemicals
The dye-degrading strain named A5p1, identified asAspergillus flavus, was one of decoloring strains previously isolated from the molasses wastewater and preserved in the China General Microbiological Culture Collection Center with the accession number CGMCC 4292 [26].It was found thatAspergillusflavusA5p1 had ability to decolorize a variety of dyes without aflatoxin production under broad environmental conditions.The polyurethane foams were purchased from Kaida New Material Co.,Ltd(Xinghua,China).The density and hardness of PUF were(21±1)kg∙m-3and(55±5)°with the pore size of 60 PPI(pores per linear inch)and 96%of open porosity.All other chemicals used in this study were of analytical reagent grade or high purity.The dye reactive blue 4 (Fig.1) was purchased from Shanghai Yuanye Biotechnology Co.,Ltd(Shanghai,China).

Fig.1. Chemical structure of reactive blue 4 (RB4).
2.2.Decolorization of RB4 by the immobilized cells
In order to obtain the maximum attached biomass for decolorization, the effects of different open pore sizes of PUF, namely 20 PPI,40 PPI,60 PPI and 80 PPI,on the immobilization were firstly investigated.The PUFs were machined to 1 cm3cubes and soaked in 70% ethanol for 24 h,then soaked twice in deionized water and dried to constant weight at 80°C in an electric thermostatic drying oven [27].The PUF samples were sterilized in an autoclave for 20 min at 121°C.The spore suspension was prepared by inoculatingAspergillusflavusA5p1 on the potato dextrose agar (PDA) and then cultured at 30 °C for 7 d in the incubator.The spores were eluted with 0.5% Tween 80 to make the spore suspensions with the concentration of 2.15 × 107spores∙ml-1and stored at 4 °C.

whereC0is the absorbance of RB4 at 0 h and Ctis the absorbance of RB4 atth.
2.3.Influence of the major factors on RB4 decolorization
The major influential factors on RB4 decolorization were investigated by single-factor experiment, including initial pH (4.0, 6.0,8.0,10.0),temperature(25°C,30°C,35°C,40°C),and RB4 concentration (100–2000 mg∙L-1).The decolorization experiments were conducted as mentioned above.All the samples were repeated at least in triplicate.Meanwhile,0.5 g PUFs were placed into the conical flasks as the control groups to investigate the RB4 adsorption by the carrier.Data were shown as the mean ± SD (error bars) of three replicates.
2.4.Construction and operation of the bioreactor
A packed bed bioreactor was constructed to continuously decolor RB4 as showed in Fig.2.The bioreactor was made of cylindrical borosilicate glass(internal diameter=9.4 cm;height=30 cm)with the theoretical total volume of 2000 ml.One of water outlets was designed at the height of 20 cm from the bottom of the PBBR,and a vent was set on the top for gas relief.The working volume of the bioreactor was approximately 1500 ml.The prepared PUFimmobilized cells with the biomass of (3.14 ± 0.13) g were placed on the net layer by layer in PBBR.The simulated wastewater was stored in an influent tank and flowed into the PBBR through a silicon tubing inserted into the bottom at the rate of 30 ml∙h-1viaa peristaltic pump.To maintain aerobic condition, air was supplied by an air compressor and filtered through a 0.45 μm filter before feeding into the bioreactor.The circulating hot water was pumped into the jacket of the bioreactor through a peristaltic pump to maintain a constant average temperature of (30±1)°C.The residual concentration of RB4 was analyzed by sampling the effluent in the storage tank.To better investigate the efficiency and reliability of the PBBR,the RB4 decolorization in the free mycelium bioreactor was also examined.

Fig.2. Schematic diagram of the PBBR.
2.5.Scanning electron microscope (SEM) analysis
The carrier of PUF and PUF-immobilized cells samples were treated by using 1% (vol) glutaraldehyde in 100 mmol∙L-1phosphate buffer (pH 7.4) for 12 h at room temperature.The samples were subsequently washed with distilled water and dehydrated by using acetone (50%–100%) serially [29].Finally, the samples were freeze-dried at -45 °C and then coated with gold powder for the analysis of SEM (Hitachi SU8220).
2.6.Analytical method
The residual concentration of RB4 was determined by spectrophotometric method using UV–Vis spectrophotometer at the maximum absorption wavelength of RB4 (λ=599 nm) [30].In addition, the activities of laccase (Lac), lignin peroxidase (Lip)and manganese peroxidase (Mnp) were determined at wavelengths of 420 nm, 310 nm and 465 nm, respectively [17,31,32].Prior to analysis, the samples were centrifuged at 8000 r∙min-1for 10 min.The biomass of free-mycelium (cell dry mass) was determined by drying at 80 °C in a vacuum oven to a constant mass.The biomass immobilized on/in the foam cubes was calculated according to the mass of the PUF before and after immobilization.
The degradation samples were vacuum filtered and the supernatant were extracted three times with 20 ml of ethyl acetate.The three fractions of ethyl acetate extracts were mixed, and then concentrated on rotary evaporator.Detection and identification of the degradation products were performed by ultra-high performance liquid chromatography quadrupole time of flight mass spectrometry (UHPLC-Q-TOF/MS).The flow rate and temperature of the drying gas were set to 15 L∙min-1and 150 °C, respectively,while the flow rate and temperature of the sheath gas were set to 12 L∙min-1and 350 °C, respectively.Positive polarity was assessed under the following conditions: capillary voltage:4000 V, injection volume: 20 μl, scannedm/zrange: 100–1700.External calibrations in negative modes were carried out using a pierce velos solution in the electrospray ionization (ESI) source.The samples were also analyzed by gas chromatogram and mass spectra(GC–MS)on HP-5MS column.The initial oven temperature of 50°C was maintained for 3 min,then raised to 250°C at a rate of 10 °C∙min-1with a hold for 7 min.Finally, the temperature was raised to 280 °C with rate of 10 °C∙min-1and held for 3 min.MS was operated in electron-impact (EI) mode with a scan range ofm/z25–900.
3.Results and Discussion
3.1.Decolorization of RB4 by the PUF-immobilized cells
There have been documented that most of biological decolorizations were mainly mediated by biosorption.For instance, a strain ofBacillussubtiliscould adsorb RB4viaamide, amino, phosphate and carboxyl groups [33].Romeroetal.[34] found that textile dye Grey Lanaset G.was decolorized through simultaneous adsorption and degradation byTrametesversicolorpellets.In the present study, RB4 was degraded during the growfth process ofAspergillusflavusA5p1.The attached growth of microorganisms is common in nature condition.It can be known,therefore,the property of carrier is closely related to the growth of attached cells[35,36].Yuetal.[37] used the PUF with three different pore sizes of 1.5 mm, 0.6 mm and 0.2 mm to immobilizeAspergillusnigerand found that the maximum citric acid yield was achieved by using the PUF of 0.6 mm.The pore size of 1.5 mm easily caused the strains falling off from the carrier in the case of rotation or stirring, while the pore size of 0.2 mm restricted the nutrition uptake by the internal strains.In our study, it was observed that the PUF with pore size of 60 PPI were filled with myceliumAspergillusflavusA5p1,i.e., the strain completely grew on/in the PUF, indicating a good affinity between the carrier and cells (Fig.3).Consequently,the PUF with pore size of 60 PPI was used for immobilization.
And you know what? She smiled then and a beautiful peacefulness washed over her once?stern countenance45. I grinned widely as she cordially nodded her head and continued down the street, slowly creeping out of my life as quickly as she had appeared. Yet I knew that her smile and gratefulness would always be imprinted46 upon my life and heart.
Time courses of RB4 decolorization by the PUF-immobilized cells and free mycelium were shown in Fig.4,respectively.It could be seen that RB4 of 200 mg∙L-1was completely decolorized to an undetectable level within 28 h by the PUF-immobilized cells.In contrast, the free mycelium required more than 48 h.Meanwhile,results of the control groups, namely without inoculum, showed that only 7% reduction was observed within 48 h, meaning the RB4 adsorption by the PUF and other abiotic factors were a small portion.The higher decolorization rate of PUF-immobilized cells could be ascribed to their network structures, which would facilitate mass transfer and thus benefit the growth ofAspergillusflavusA5p1 as well as the contact with RB4.However, in the system of free cells, mycelium aggregated into pellets and led to poor mass transfer, reducing both of the growth rate and the decolorization rate.In addition,results of the activities of laccase(Lac),lignin peroxidase (Lip) and manganese peroxidase (Mnp), proved to be the key enzymes of dye decolorization by fungi [1], could support above explanation (Table S1, Supplementary Materials).
3.2.Effects of the major factors on RB4 decolorization
Temperature exerts a significant influence on the growth of microorganism.As shown in Fig.5(a), the PUF-immobilized cells could efficiently degrade 300 mg∙L-1RB4 in the temperature range of 25–40 °C.The maximum degradation rate was achieved at the optimal incubation temperature of 30 °C.By comparison, the free mycelium degraded over 85.0% of 300 mg∙L-1RB4 at 30 °C, and the degradation rate decreased significantly when the temperature higher than 30 °C.The loss of cell viability and the related decolorization enzyme activity was one of reasons for the decrease of degradation at higher temperatures [38], whereas lower temperatures also led to the reduction of cell viability[39].When the temperatures were 25 °C and 40 °C, the degradation rate of PUFimmobilized cells were 1.49 times and 1.97 times higher than that of the free mycelium,respectively,exhibiting better adaptability to the change of temperature.

Fig.3. SEM of (a) Aspergillus flavus A5p1, (b) PUF and (c) PUF- immobilized cells.
pH is another important factor affecting the microbial activity.Fig.5(b) depicted the degradation trend of RB4 in the pH range of 4.0–10.0.Similarly, the degradation rates of RB4 by the PUFimmobilized cells were higher than that of the free mycelium.For an example, 96.2% of 300 mg∙L-1RB4 was degraded by the PUF-immobilized cells at pH 6.0 for 36 h, whereas 60% was degraded by the free mycelium.When the initial pH value was increased to10.0, the decolorization efficiency of RB4 by the PUFimmobilized cells was 90% after 48 h, while only 48.6% was achieved by the free mycelium after 72 h.The good performance of PUF-immobilized cells was consistent with that of documented results.Talluretal.[40] found that a stable degradation efficiency of cypermethrin was obtained at pH 5.0–9.0 for the PUFimmobilized cells compared to other immobilized cells of polyacrylamide, sodium alginate and agar.
The tolerance to high concentrations of dye can reflect the effectiveness and reliability of the biological system to some extent.Fig.5(c) showed that the specific decolorization rates of the PUFimmobilized cells were generally higher than that of the free mycelium.In particular, the differences in the decolorization became obvious with the increase of RB4 concentration.When the concentration of RB4 was increased to 1500 mg∙L-1,the decolorization rate of PUF-immobilized cells was 8.40 mg∙(L∙h)-1,which was 2.3 times higher than that of the free mycelium [3.65 mg∙(L∙h)-1].Even the concentration of RB4 was increased to 2000 mg∙L-1,the decolorization rate of PUF-immobilized cells could maintain of 7.92 mg∙(L∙h)-1, while the free ones decreased to 2.95 mg∙(L∙h)-1.The decolorization rates of PUF-immobilized cells and free ones reached the maximum of 8.40 and 5.64 mg∙(L∙h)-1at 1500 and 300 mg∙L-1, respectively.Chaudharietal.[41] reported that aerobic bacterial granules were able to tolerate to the concentration of RB4 up to 1000 mg∙L-1with the maximum decolorization rate of 6.16 mg∙(L∙h)-1.However,when the dye concentration was over 1200 mg∙L-1, the decolorization rate decreased significantly to 0.56 mg∙(L∙h)-1.So to speak,the good tolerance to high dye concentrations of the PUF-immobilized cells will be helpful to improve the efficiency and adaptability of the biological system.
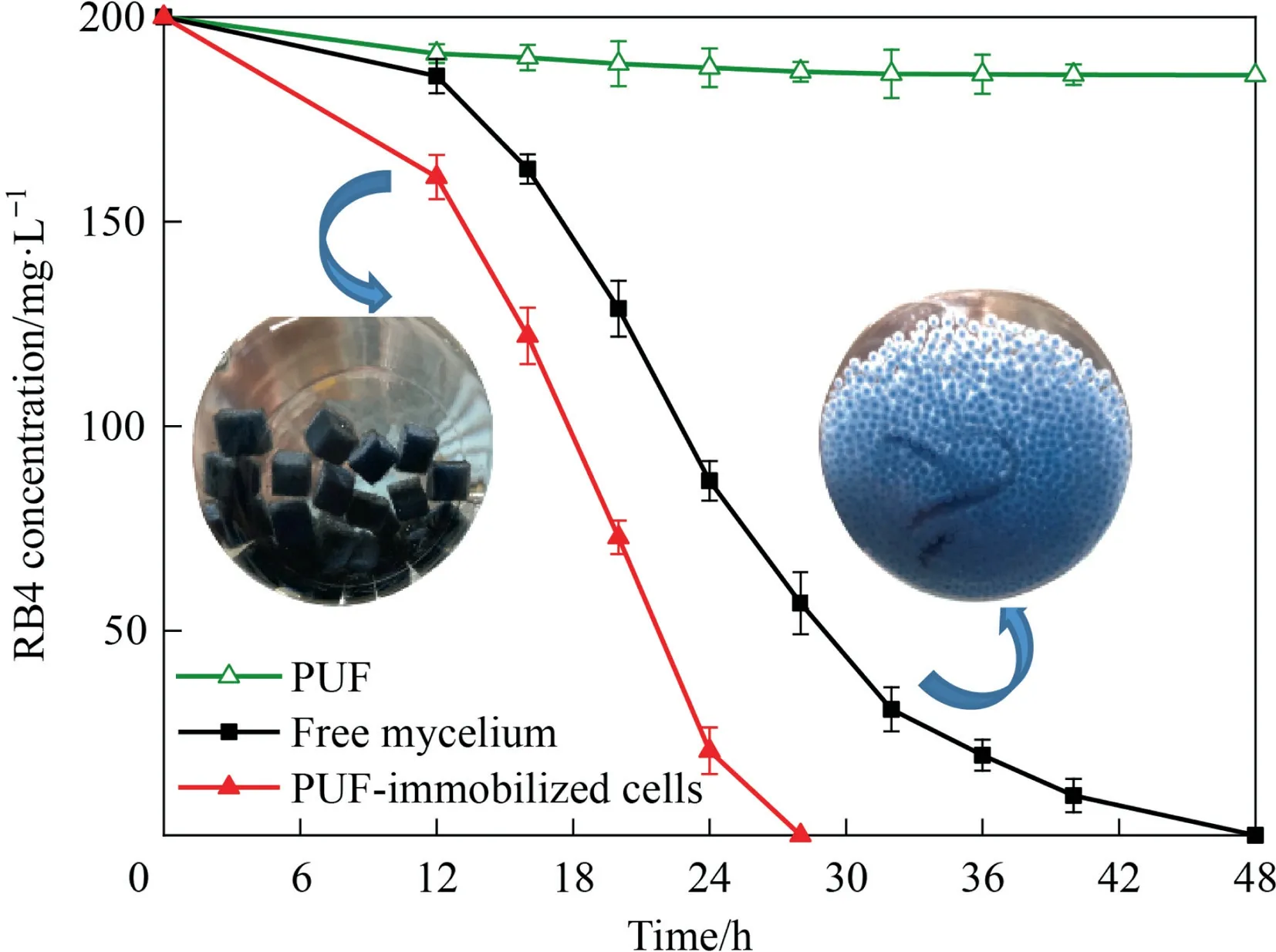
Fig.4. Decolorization time curve of RB4 at 30 °C, pH 6.0 and 150 r∙min-1.
3.3.Decolorization kinetics study
Logistic equation, an empirical model that accounted for the process kinetics and efficiency [42,43], could be used to describe the decolorization process and growth ofAspergillusflavusA5p1,respectively.Logistic equation in its commonly used form was showed as following:

whereXandDwere the biomass and the decolorization or removal percentage at a specific moment of the culture time(t),respectively.D0andDmwere the initial and maximum decolorization percentage,respectively.X0andXmwere the initial and maximum biomass concentrations, respectively.μDis the maximum specific decolorization rate, and μmis the maximum specific growth rate.
As shown in Fig.6, the decolorization of RB4 was similar to the trend of theAspergillusflavusA5p1 growth.The experimental data were in good agreement with the logistic model.It was clear that the decolorization efficiency increased with the growth ofAspergillusflavusA5p1.The decolorization efficiency reached its maximum within 36 h,meanwhileAspergillusflavusA5p1 grew to the stationary phase.The parameters obtained by fitting to the logistic model were shown in Table 1.The maximum specific decolorization rate μDand specific growth rate μmof PUF-immobilized cells were 0.3675 h-1and 0.3977 h-1, respectively.Correspondingly, the maximum decolorization efficiency was approximately 93.5%.With respect to the free mycelium, the maximum specific decolorization rate μDand specific growth rate μmwere 0.3114 h-1and 0.2957 h-1, respectively.The maximum decolorization efficiency was approximately 75.4%.Similar results were obtained in a study on the immobilizedBacillussp.M3 [44].The specific growth rate of PUF and alginate beads immobilizedBacillussp.M3 were 0.296 d-1and 0.247 d-1, respectively, which was roughly double the free cells’ (0.179 d-1).Accordingly, the higher specific growth rate of PUF-immobilized cells might be one of reasons for the enhanced decolorization.
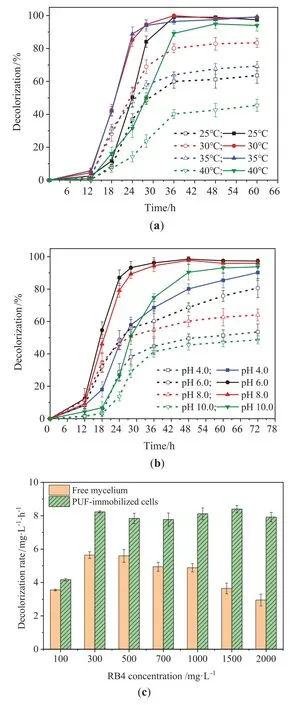
Fig.5. Effects of the major factors on RB4 decolorization.(a) Temperature, (b) pH.The dotted line represents free mycelium, and the solid line represents the PUFimmobilized cells.(c) RB4 concentrations.
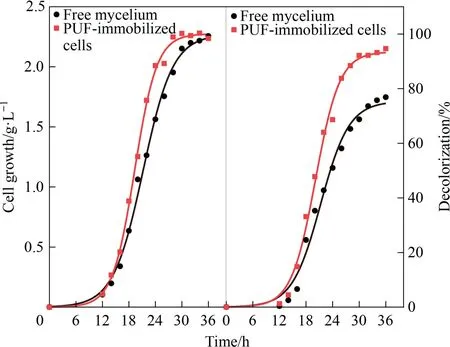
Fig.6. Time curves of cells growth and RB4 decolorization(symbols: experimental data; solid line: Logistic model).
3.4.Decolorization of RB4 in the packed bed bioreactor
Based on the above results, the continuous decolorization of RB4 in a PBBR was tested.As shown in Fig.7, the running of the PBBR with PUF-immobilized cells appeared a relatively stable trend.The average decolorization efficiency was 93.3% during 26 days’ running.In contrast, the decolorization efficiency of the PBBR with free suspended cells first increased,and then decreased slowly.It was found that the maximum decolorization efficiency of 95.1%was achieved on the fifth day of running,speculating a effect of adsorption by the free mycelium.On the 26th day, the decolorization efficiency was reduced to 46.1%.Obviously,the good performance of PUF-immobilized cell bioreactor could be mainly ascribed to the maintenance of high cells density,effectively reducing the loss of cells flowed out with the effluent.In addition, the proliferation ofAspergillusflavusA5p1 would be also helpful to improve the performance of the bioreactor.Recently, a spongessubmerged anaerobic baffled reactor for the treatment of azo dye-contaminated wastewater was constructed and could steadily operate for 180 days [6].Therefore, the PUF-immobilized cell bioreactor with high efficiency and stability would have a bright prospect for a long-time operation.
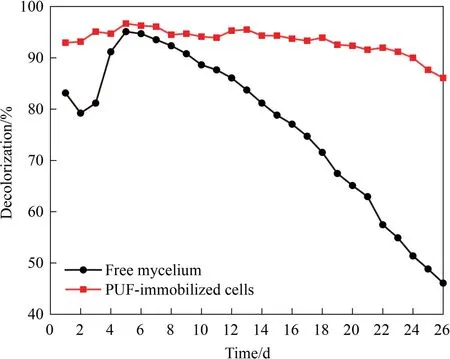
Fig.7. Decolorization of RB4 in the PBBR at a flow rate of 30 ml∙h-1.
3.5.Degradation pathway of RB4
The degradation products of RB4 were analyzed through UHPLC-Q-TOF/MS and GCMS respectively.According to the results of UHPLC-Q-TOF/MS(Table S2)and GCMS(Table S3),the degradation pathway of RB4 byAspergillusflavusA5p1 was proposed as showed in Fig.8.It has been documented that hydroxyl radical plays a key role in fungal degradation [45].RB4 was hydrolyzed with the action of hydroxyl radical to form 1-amino-4-hydroxy-9,10-dioxoanthracene-2-sulfonic acid and intermediate product I.The 1-amino-4-hydroxy-9,10-dioxoanthracene-2-sulfonic acid was transformed into dibutyl phthalate, butoxyacetic acid and styreneviadesulfurization and redox reactions.Dibutyl phthalate was confirmed as an intermediate product of Disperse Blue 2BLN degradation byAspergillussp.XJ-2.Subsequently, it was oxidized to ethyl propionate and entered the TCA cycle [46].The Nposition of intermediate product I was attacked by the hydroxyl radical to form p-hydroxytoluene and 2-amino-4,6-dichloro-1,3,5-triazine after dechlorination and desulfurization.However, the presence of 2-amino-4,6-dichloro-1,3,5-triazine was not detected,which might be be further degraded into small molecule compounds.Gözmenetal.[47] found that the intermediate product I of RB4 was transformed into 4-nitrophenol, hydroquinone and pbenzoquinone after oxidation, and then further transformed intosmall molecules of oxalic acid, acetic acid and carbon dioxide.Alietal.[48] drew a similar conclusion that the opening-ring of 2-a mino-4,6-dichloro-1,3,5-triazine was catalyzed by peroxidase to generate small molecules.Recently, a non-toxic final product of cyanuric acid was detected during RB4 degradation byTrameteshirsuteD7[17].Therefore,results preliminary showed thatAspergillus flavusA5p1 could degrade RB4 into less toxic substances, effectively reducing the impact on the ecological environment.

Fig.8. The proposed degradation pathway of RB4 by Aspergillus flavus A5p1.

Table 1Parameters obtained by the logistic model that characterized cells growth and RB4 decolorization
4.Conclusions
In the present study,the enhanced decolorization of RB4,one of anthraquinone dyes, was investigated by immobilizingAspergillus flavusA5p1.Considering the relationship between microorganism and the immobilization carrier, the PUF pore size of 60 PPI was rationally selected as the immobilization carrier.The PUFimmobilized cell system exhibited high decolorization efficiency,and was well-adapted to the change of temperature, pH and RB4 concentration compared with the free mycelium system.Furthermore, the continuous decolorization of RB4 in PBBR with PUFimmobilized cells could maintain an average decolorization efficiency of 93.3% at a flow rate of 30 mL∙h-1for 26 days.The higher specific growth rate ofAspergillusflavusA5p1 after immobilization was mostly contributed to the enhanced decolorization of RB4.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was funded by the National Natural Science Foundation of China (21066001), the Scientific Research Foundation of Guangxi University (XJZ130360) and the Innovation and Entrepreneurship Training Program for Undergraduate of Guangxi University (202010593174).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.11.028.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of lithium carbonate by microwave assisted pyrolysis
- Simulation and design of a heat-integrated double-effect reactive distillation process for propylene glycol methyl ether production
- Hyper-parameter optimization of multiple machine learning algorithms for molecular property prediction using hyperopt library
- High-efficiency and safe synthesis of tonalid via two Friedel-Crafts reactions in continuous-flow microreactors
- Improvement of synergistic effect photocatalytic/peroxymonosulfate activation for degradation of amoxicillin using carbon dots anchored on rod-like CoFe2O4
- Boosting the hydrogen storage performance of magnesium hydride with metal organic framework-derived Cobalt@Nickel oxide bimetallic catalyst
