Enhancement effect of Mn doping on Co3O4 derived from Co-MOF for toluene catalytic oxidation
2023-01-30JuanLeiPengWangShuangWangJinpingLiYongpingXuShuyingLi
Juan Lei, Peng Wang, Shuang Wang,, Jinping Li, Yongping Xu, Shuying Li
1 Department of Environmental and Safety Engineering, Taiyuan Institute of Technology, Taiyuan 030018, China
2 College of Environmental Science and Engineering, Taiyuan University of Technology, Jinzhong 030600, China
3 Dalian Syme Bioengineering Technology Limited Company, Dalian 045099, China
4 Shanxi Key Laboratory of Gas Energy Efficient and Clean Utilization, Taiyuan University of Technology, Taiyuan 030024, China
Keywords:Toluene Catalytic oxidation Co-MOF Doping Co-Mn
ABSTRACT The design of Co-Mn composite oxides catalysts derived from MOF is significant for catalytic combustion of toluene.Here, a series of M-CoaMnbOx, with enhanced catalytic properties compared with that of MCo3O4, were successfully prepared through pyrolysis of Mn-doped Co-MOF.The as-synthesized MCo1Mn1Ox (Co:Mn=1:1) exhibits an optimal catalytic activity with 90% toluene conversion reached at 227 °C, which benefits from the increase of Co3+, Oads and the synergistic effect between Mn and Co.According to the analysis of the in situ diffuse reflectance infrared Fourier transform spectroscopy,toluene could be degraded easier on M-Co1Mn1Ox with lower activation energy than M-Co3O4.The main intermediate products are benzaldehyde, benzoic acid, anhydride, and maleate species.Those findings reveal the value of Mn doping for improved activity of toluene oxidation on MOF derived Co3O4, which provide a feasible method for the construction of toluene-oxidation catalysts.
1.Introduction
With strong photochemical reactivity and high possibility for O3generation, toluene is usually regarded as a representative target pollutant of volatile organic compounds (VOCs) [1,2].Catalytic degradation is a valuable and effective technology for the elimination of toluene, based on the superiority of energy conservation and environment protection [3,4].The core of this technology is the construction of catalysts with high activity and stability.Metal oxides are emerging as promising catalysts on account of the lowcost and resistance to poisons[5–7], among which Co3O4is one of the most outstanding oxide.The abundant Co3+in Co3O4catalyst is generally considered to be the active site for toluene combustion[8–12].Recently, Co-MOF derived Co3O4have attracted widespread attention for their improved physicochemical properties,which are proved to be beneficial for toluene oxidation [13–16].It is certified that Co-MOF is definite a promising precursor of porous Co based metal oxides for toluene combustion.
The catalytic activity of Co-MOF derived Co3O4for toluene degradation,however,is still not encouraging.It has been reported that mixed metal oxide could display a superior toluene catalytic activity thanks to the synergistic effect between metals [17–19].Based on this,doping with other metal oxides may be an effective measure for the properties improvement of Co3O4.Mnx+with polymorphism can interact with other metal for a synergistic effect and be combined with O2-in compounded metal oxides,thus forming Lewis acid base pairs that are beneficial to toluene oxidation[20,21].So Mn is believed to be the preferred doping metal for Co3O4.Wang and his co-workers synthesized CoMnxOywith strong interaction between Co3O4and MnCo2O4.5, providing ample oxygen vacancies and reactive oxygen species for promoting catalytic oxidation of toluene [22].
Mn-Co binary oxides can be synthesized by pyrolysis of Co-Mn-MOF or Mn-doped Co-MOF, which thus brings two following advantages: 1) taking advantages of CoOxderived from Co-MOF;2) the synergistic effect for toluene combustion resulted from the interaction between CoOxand MnOx.Luo and his coworkers [23]obtained a series of MOF-MnaCoboxides with uniform metal dispersion adopting Co-Mn-MOF as precursors.The MOF-Mn1Co1displayed an excellent performance for toluene degradation which closely associated with abundant Mn4+, Co3+, Oads/Olattand strong redox properties.Nevertheless, the synthesis of bimetallic MOF was not a universally applicable method for any two metals [24].Ren and his companions [25] synthesized ZIF-67insituon α-MnO2, then obtained α-MnO2@Co3O4viathe thermolysis of α-MnO2@ZIF-67.The α-MnO2interacted with Co3O4on the interface,thus resulted the improved catalytic performance of α-MnO2@Co3O4for toluene combustion.Zhao’s team [26] constructed Co-Mn combined oxides with special structure, doubleshell and hollow,viacalcination of Mn-doped ZIF-67.The introduced Mn are mainly distributed in the outer shell of the catalyst,which can enhance the mobility of oxygen for better toluene oxidation activity.Apparently, some work had been done for the construction of Co-based metal oxides, taking Co-MOF as the precursor.However, almost all the relevant studies focused on ZIF-67, a classic MOF that is easy to be synthesized.Our previous work showed that Co-MOF with different morphology and composition could affect the catalytic property of the derived Co3O4and the N—O ligands of ZSA-1 could facilitate the generation of Co3+and Oadson the ZSA-1-Co3O4for an outstanding toluene catalytic activity [15].
Herein, we successfully constructed Co-Mn metal oxides with different original molar ratio of Co:Mnviaemploying Mn-doped ZSA-1 as precursor.Co-Mn metal oxides showed an improved performance for toluene combustion in comparison with the nodoped M-Co3O4.The structural characteristics of the catalysts were explored by several characterization methods.Furthermore, the reaction path of toluene combustion was traced by a in situ infrared characterization technology.
2.Experimental
2.1.Preparation of ZSA-1 and M-CoaMnbOx catalysts
The ZSA-1 (Co-MOF) [27] was synthesized based on a reported method.
A certain amount of 50% Mn(NO3)2aqueous solution was measured with a pipette and put into a beaker.Then 25 ml anhydrous ethanol was added and the solution was dispersed with ultrasound until uniform.After that,0.2 g of ZSA-1 was mixed into the solution to adjust the original Co:Mn (molar ratio) to be 0.5, 1, 2 and 3,respectively, according to the productive rate of Co3O4derived from ZSA-1.Stirring for 15 min, the suspension was separated by centrifugalization.The obtained Mn-doped ZSA-1 was then washed for 3 times with anhydrous ethanol, dried at 60 °C overnight.
The as-synthesized Mn-doped ZSA-1 with different Co:Mn molar ratio were pyrolyzed at at the same calcination conditions with that of Co3O4derived from ZSA-1 in our previous work [14].According to the Co:Mn molar ratio,the obtained M-CoaMnbOxcatalysts were named as M-Co0.5Mn1Ox, M-Co1Mn1Ox, M-Co2Mn1Oxand M-Co3Mn1Ox, respectively.The actual concentration of Co and Mn were measured by ICP-OES analysis.The corresponding Co:Mn of M-Co0.5Mn1Ox, M-Co1Mn1Ox, M-Co2Mn1Oxand MCo3Mn1Oxwere 1:2.2, 2.3:1, 3.0:1 and 4.1:1, respectively.
The M-Co3O4, as a reference in this manuscript, was derived from ZSA-1 directly in our previous work [14].The preparation method of M-Co3O4was consistent with that of M-CoaMnbOx, just without Mn-doping.
2.2.Characterization of the catalysts
XRD: a X-ray diffractometer of Bruker D8 Advance was used to determine the crystal phase structure of catalysts,with Cu Kα radiation (30 kV, 15 mA, λ=0.15418 nm), the scan speed was 8°∙min-1.SEM and EDS: the scanning electron microscope with energy dispersive spectrometer of Hitachi SU8010 was adopted for observing the morphology and element distribution of samples.TEM: transmission electron microscopy tests was performed by JEM-2010 analysis device, the linear resolution of which was 0.14 nm.N2adsorption–desorption isotherms: the pore structure and BET specific surface area of the samples were obtained with a Micromeritics TriStar II 3020 instrument.H2-TPR: before temperature-programmed reduction by hydrogen, the catalyst was preprocessed at 150 °C with Ar flow for 1 h, then the test was performed by using a Micromeritics AutoChem II 2920 chemisorption equipment.Raman: the Raman spectrometer used in this experiment was produced by Renishaw in the UK and the model is InVia.At room temperature, the incident wavelength was 514 nm, the exposure time was 60 s, 50 m slit width was selected, and the scanning range was 50–950 cm-1.XPS: a device of Escalab250 by Thermo Fisher Scientific K-Alpha was selected for X-ray photoelectron spectroscopy test.ICP-OES: the element contents analysis was carried out with inductively coupled plasma-optical emission spectroscopy of the AES: Varian (720-ES) instrument.DRIFTS:insitudiffusion reflectance infrared Fourier transform spectrophotometer of VERTEX 80v was used to track the degradation path of toluene over M-Co1Mn1Ox, the test conditions were consistent with our previous work [14].
2.3.Catalytic test
A fixed bed reactor, in which the main part is a stainless steel tube(internal diameter is 6 mm),was employed for the evaluation of catalytic oxidation activity of toluene over as-synthesized catalysts.With a flow of air, the gaseous toluene was carried out with an ice-bath bubbling device, together with another flow of air for attenuation, keeping the concentration of toluene at 1000 μl∙L-1with total flow rate of 66.7 ml∙min-1.0.2,0.1 and 0.05 g of catalyst(250–380 μm) was employed for a GHSV of 20000, 40000 and 80000 ml∙g-1∙h-1, respectively.The gas chromatograph (GC-6890A), in which the gasification chamber temperature is 160 °C,was adopted online for the determination of toluene concentration.After stabilizing for 15 min at each certain reaction temperature,the data was recorded by the FID detector for 6 times.T10%,T50%,T90%andT100%of toluene were calculated for the evaluation of catalyst activity.
3.Results and Discussion
3.1.Structure characterization
Fig.S1 displays the XRD spectrums of the M-CoaMnbOxcatalysts.As shown in the figure, the X-ray diffraction peaks of MCo1Mn1Ox, M-Co2Mn1Oxand M-Co3Mn1Oxare all agree with those of the standard Co3O4(PDF-#-43-1003), and M-Co3O4sample[14,28].However, compared with M-Co3O4, all peaks width of Co-Mn metal oxides catalysts are wider the peak strength are weaker, meaning the smaller particle size of the M-CoaMnbOxcatalysts.The particle size of the catalysts were computed and showed in Table 1.Obviously,the M-CoaMnbOxown smaller particle size than that of M-Co3O4.The characteristic diffraction peaks of MnOxare not detected, indicating three possible scenarios: 1)Mn is uniformly distributed in the Co-Mn metal oxides catalysts;2)the XRD peaks of MnOxare covered by peaks of Co3O4;3)MnOxis amorphous [29].

Table 1Pore structure and relative content of surface elements of samples
The SEM and EDS were applied to visually observe the morphologies of the catalysts and the distribution of Co, Mn, and O of M-Co1Mn1Ox.Obviously as shown in Fig.S2, the M-CoaMnbOxwhich partially kept the octahedra morphology of the ZSA-1,were formed with the aggregation of nanoparticles.However,our previous work found that M-Co3O4could retain the octahedral skeletonstructure of ZSA-1completely [15,27], meaning the pore structure of the M-CoaMnbOxcatalysts were changed, which may provide more vacancies for the participation of catalysis [30,31].From the EDS scanning patterns,Mn is evenly distributed on the surface of M-Co1Mn1Ox,further confirming the successful doping of Mn in the Co-Mn metal oxides catalysts.
HRTEM was used to explore the interior structures of the MCoaMnbOx.Fig.1 illustrates the lattice plane of the catalysts.As for Co3O4,0.243,0.286 and 0.467 nm spacing of lattice corresponding to the (3 1 1), (2 2 0) and (1 1 0) crystal planes, respectively[10,32].The coexist of (3 1 1) and (2 2 0) indicated the existence of {1 1 0} plane.The distribution of the crystal planes in (b), (e)and (h) of Fig.1 demonstrate the presence of {1 1 0} surfaces on the M-CoaMnbOxcatalysts [10].The lattice distance of 0.492,0.308 and 0.220 nm in Fig.1(c) were respectively attributed to Mn3O4and MnO2, further verifying the unquestionable doping of Mn in the M-Co1Mn1Oxcatalyst[25].We can draw similar conclusions from Fig.1(f) and (i) for M-Co2Mn1Oxand M-Co3Mn1Ox,respectively.In a word, {1 1 0} planes still exist on the Mn-doped Co-Mn metal oxides catalysts compared with M-Co3O4[14,15].According to previous studies,compared with other crystal planes,more Co3+and oxygen vacancies can be provided by the {1 1 0}plane, thus resulting it’s high reactivity [33–35].Moreover, the existence form of Mn are Mn3O4and MnO2, proving the fact that the valence of Mn are +2, +3 and +4.
The N2adsorption–desorption isotherms of the M-CoaMnbOxdisplayed H3-type hysteresis loop in Fig.S3(a), which reveals that the M-CoaMnbOxcatalysts are all mesoporous structure with typical type IV isotherm[28].As shown in Table 1,Co-Mn metal oxides catalysts own higher specific surface area compared with that of M-Co3O4, but with smaller average pore size and pore volume.The BJH pore size distribution of the catalysts in Fig.S3(b) illustrates a denser distribution of pore size before 10 nm of the MCoaMnbOx,which is inconsistent with the M-Co3O4of a wider pore size distribution.One possible explanation for this phenomenon is that the final M-CoaMnbOxnanoparticles are smaller than that of M-Co3O4, which is proved in the XRD analysis.Therefore when the M-CoaMnbOxnanoparticles stacking for the irregular pore structure of the catalysts, the gap between the small particles became smaller, forming a smaller pore diameter.The MCoaMnbOxwith relatively higher specific surface area may provide more active sites for toluene oxidation [29].
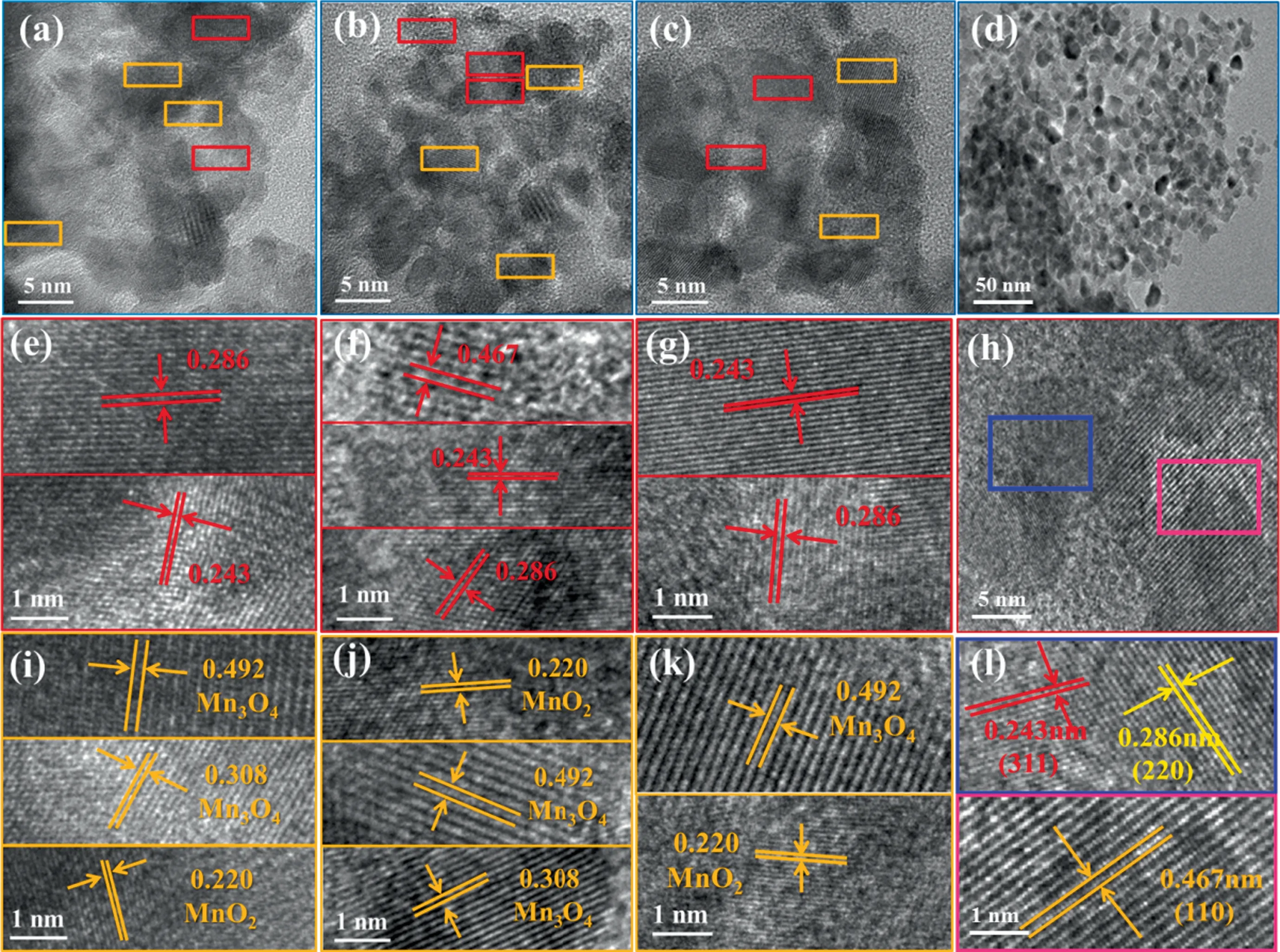
Fig.1. HRTEM images of (a), (e) and (i) M-Co1Mn1Ox; (b), (f) and (j) M-Co2Mn1Ox;(c), (g) and (k) M-Co3Mn1Ox; (d), (h) and (l) M-Co3O4.
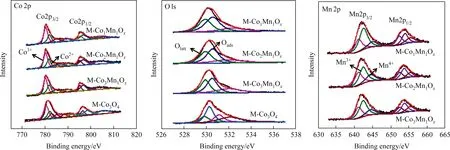
Fig.2. XPS patterns of all catalysts.
XPS characterization was carried out for further exploring the element compositions and valence state distribution of the MCoaMnbOxcatalysts.XPS peak software was used for the fitting of the peak splitting of Mn 2p and O 1s.As exhibited in Fig.2 Co 2p, the two major peaks at 781 eV and 796 eV can be divided for several peaks, among which 780.1 eV corresponds to Co3+and 781.5 eV is attributed to Co2+[10,36,37].The relative content of Co3+/Co2+of M-CoaMnbOxcatalysts are summarized based on the fitting peak area of Co 2p3/2and displayed in Table 1,together with the data of M-Co3O4for comparison.The numerical order for Co3+/Co2+is that M-Co1Mn1Ox(1.96) > M-Co3Mn1Ox(1.87) > MCo2Mn1Ox(1.83) > M-Co3O4(1.77).Intuitively, doping of Mn can promote the formation of more Co3+in the catalyst, which is beneficial for toluene oxidation.
Two main peaks at 653.9 eV and 642.2 eV are detected of Mn 2p for M-CoaMnbOxcatalysts,represent Mn 2p1/2and Mn 2p3/2.Peaks around 641.0 eV (Mn2+), 642.5 eV (Mn3+) and 644.2 eV (Mn4+)imply+2,+3 and+4 are the main valences state of Mn[38,39].This finding is a stronger proof for the result of HRTEM analysis.The Mn3+/Mn4+of each catalyst are also summarized in Table 1: MCo1Mn1Ox(3.81)>M-Co3Mn1Ox(2.53)>M-Co2Mn1Ox(2.07).Studies have shown that the reversible valence state exchange between cations,Co3+/Co2+(Co2+⇋Co3++e-)and Mn4+/Mn3+(Mn3+⇋Mn4++e-),can provide some oxygen-activated electrons in the catalytic reaction,so as to promote the catalytic reaction[25].The strong interaction between Mn3+and Co3+(Mn3++ Co3+→Mn4++ Co2+) can further promote electron transfer and oxygen circulation [38].In addition, in the surface of catalyst, more oxygen vacancies are needed to maintain electron balance due to the presence of Mn3+(low-valent Mn).This suggests that the higher relative atomic ratio of Mn3+/Mn4+corresponding to more oxygen vacancies.As a result,compared with M-Co3Mn1Oxand M-Co2Mn1Ox, M-Co1Mn1Oxwith highest Mn3+/Mn4+can contribute more oxygen vacancies for toluene combustion.
For the O 1s XPS spectra, peak at 529.9 eV represents Olattsurface lattice oxygen, while peaks at 530.8 and 531.2 eV are attributed to Oads-surface adsorbed oxygen of M-CoaMnbOxcatalysts [9,10,37].The value of Oads/Olattof the catalysts are ordered as follow: M-Co1Mn1Ox(1.32) > M-Co3Mn1Ox(1.24) > MCo2Mn1Ox(1.21) >M-Co3O4(1.17).The surface adsorbed oxygen(Oads) has been reported to be the reactive oxygen species of toluene oxidation, the more the Oadsare, the faster the reactive oxygen species circulation will be [29].As a result, the MCo1Mn1Oxmay displays an excellent toluene catalytic performance.
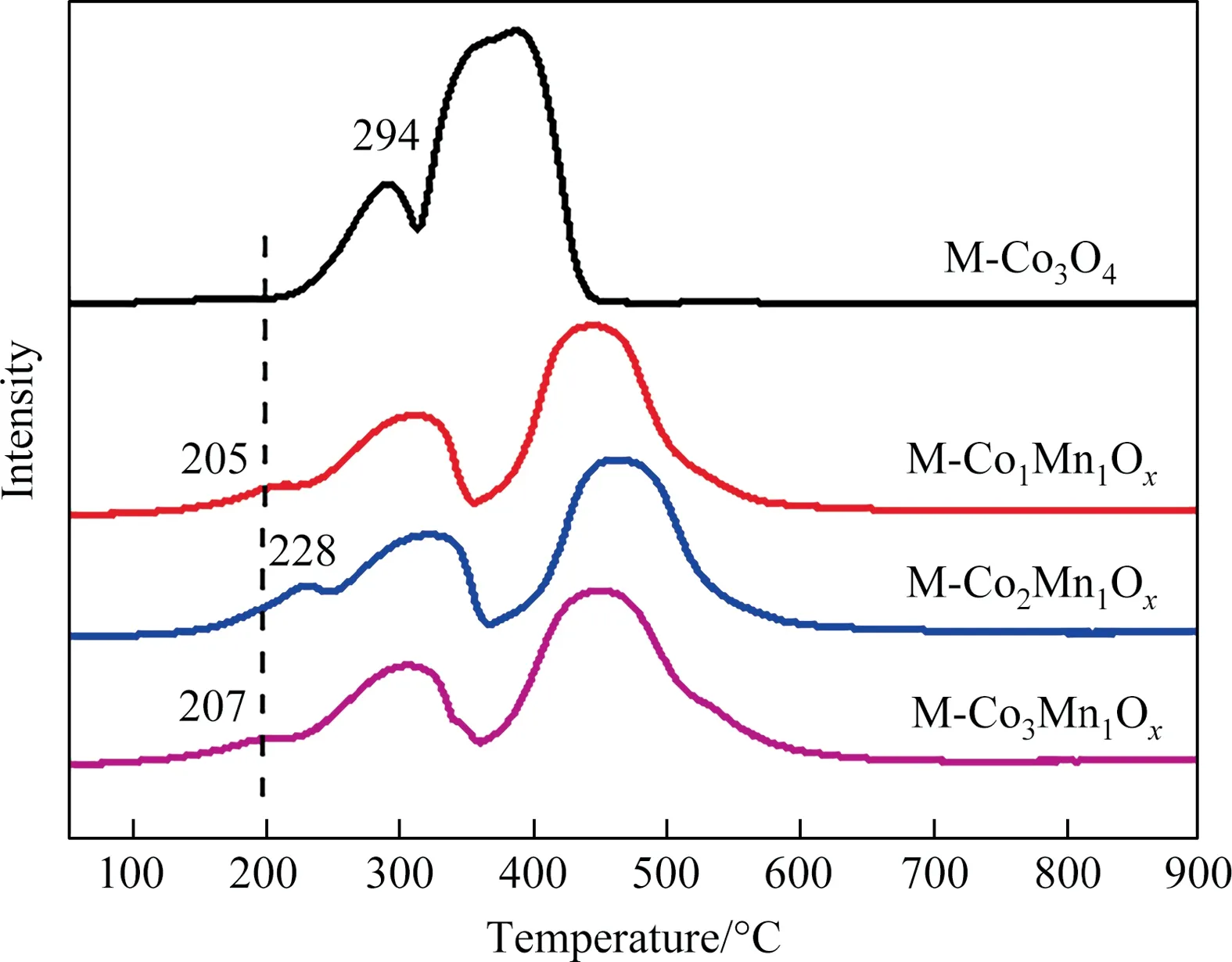
Fig.3. H2-TPR of all samples.
The reducibility of catalysts also plays a vital role in toluene degradation.Aiming to explore their reducibility feature, H2-TPR profiles of M-CoaMnbOxand M-Co3O4are presented in Fig.3 and the quantitative analysis on the peaks in the H2-TPR profiles of the samples have been made and showed in Table S1.Obviously,with the roughly the same total H2consumption,M-CoaMnbOxdisplayed three reduction peaks, one more than that of M-Co3O4.Moreover, M-CoaMnbOxshow the first reduction peak at much lower temperature in comparison to that of M-Co3O4, indicating superior reducibility for M-CoaMnbOx.The first reduction peak of M-Co1Mn1Oxemerges at the lowest temperature, which means the M-Co1Mn1Oxowns the highest oxygen mobility.According to the reported research, MnOxand Co3O4in Co-Mn combined metal oxides interact with each other on the coupled interface,which can improve the reducibility and oxygen mobility of the catalysts[25,40].
Peaks in the range of 200–300 °C are assigned to the change of valence state of Co from + 3 to + 2 (Co3++ e-→Co2+).While the peaks at 300–350 °C may result from the reduction progresses of Co2++ 2e-→Co0and Mn4++ e-→Mn3+[25,41–43].Peaks centered at 440–470°C represent the reaction of Mn3+to Mn2+,corresponding to the reduction of Mn3O4to MnO [39].This is a more favorable evidence for the existence form of Co (Co3O4) and Mn(Mn3O4and MnO2), in line with the results of the XRD, HRTEM and XPS.

Fig.4. Raman spectra of the synthesized M-CoaMnbOx and M-Co3O4.
Fig.4 shows the Raman spectra of the M-CoaMnbOxand MCo3O4catalysts.Apparently, the five typical peaks of Co3O4nanocrystals are detected on all the catalysts.No characteristic peaks for MnOxwere detected for its average distribution, providing a more convincing evidence for XRD analysis [44,45].Taking the peaks of M-Co3O4as a contrast,the Raman peaks of the Co-Mn metal oxides catalysts all shift negatively.The redshift phenomenon is attributed to the generation of more lattice defects[25,46,47],which may benefit from the strong interaction between Co and Mn.The lattice defects can facilitate the mobilization of oxygen to formulate more oxygen vacancies, which is positive to toluene catalytic oxidation [46,47].The most obvious redshift occurred for M-Co1Mn1Ox, indicating more oxygen vacancies as well as smaller grain sizes of the catalyst[48].The results are consistent with that of XRD and XPS.
3.2.Catalytic performance
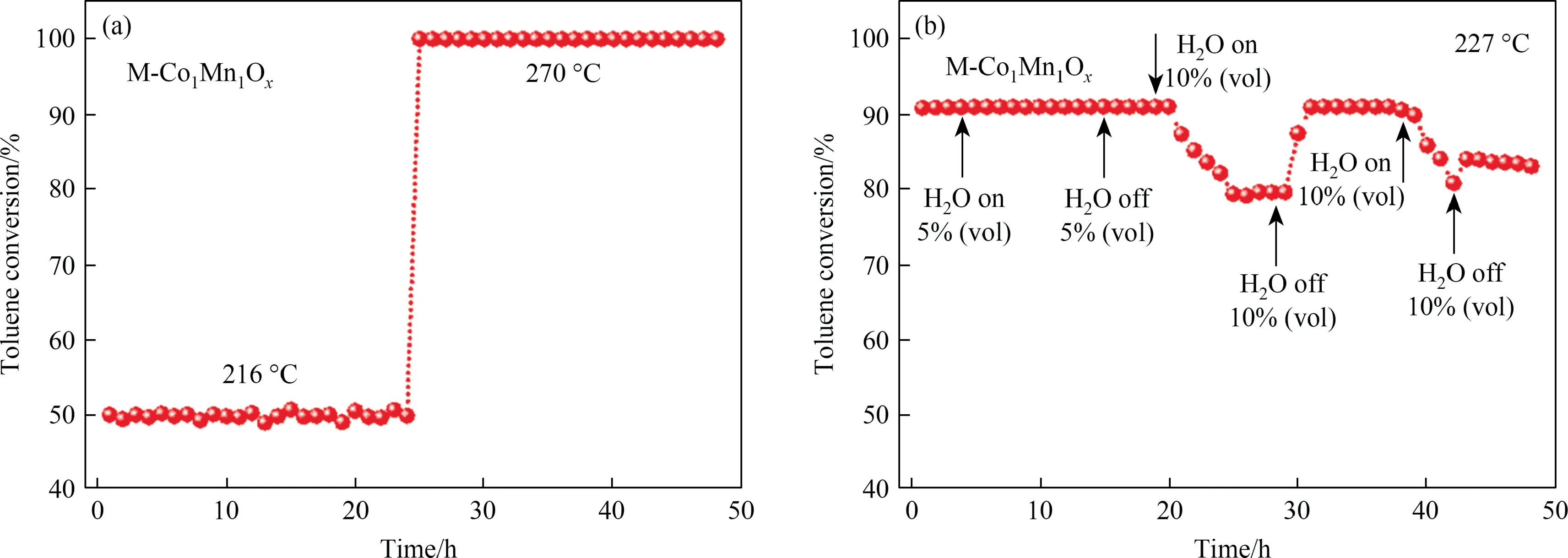
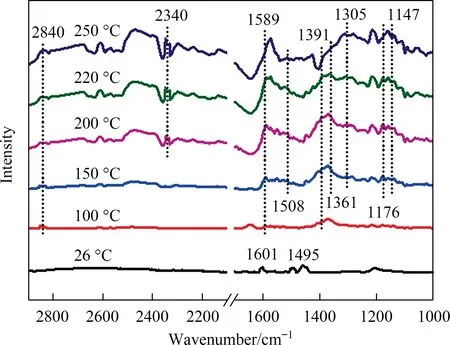
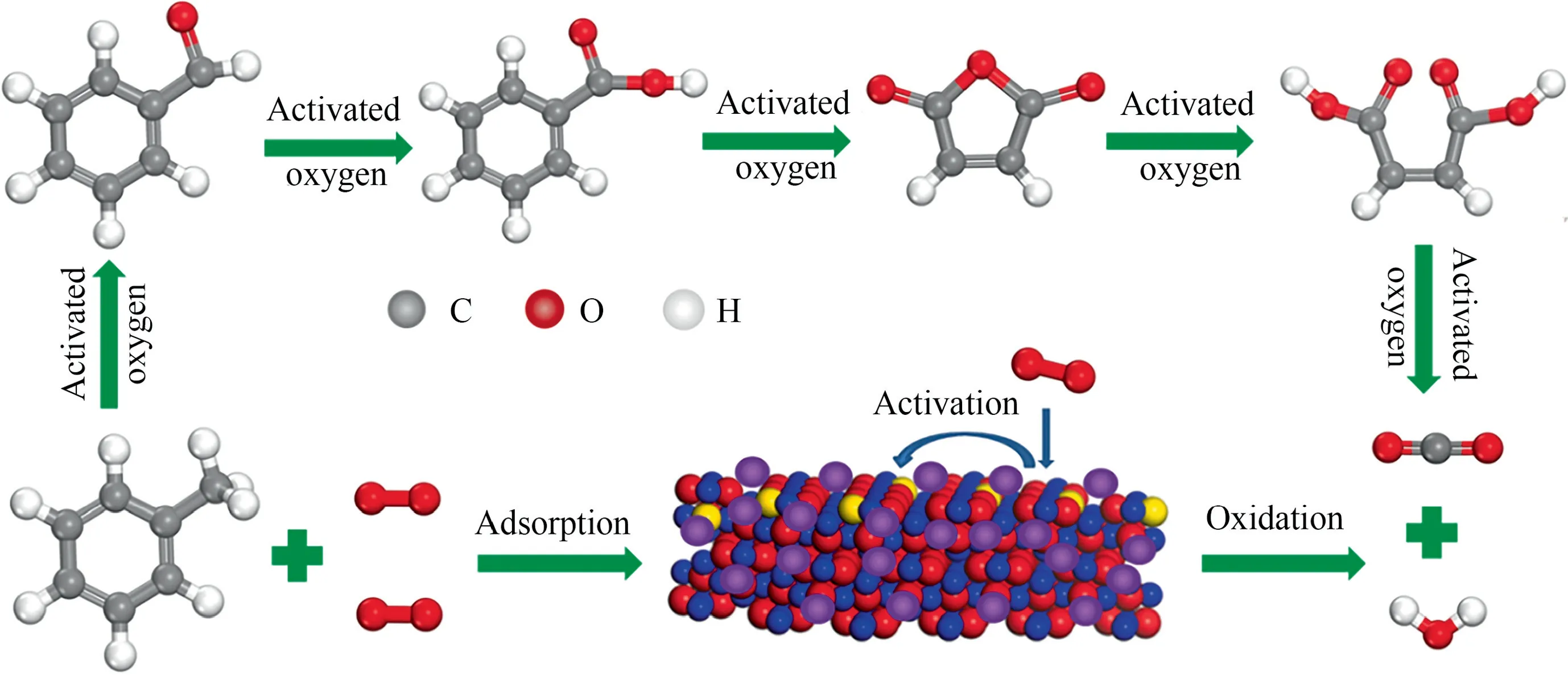
Fig.5(a) shows the degradation rate of toluene over MCoaMnbOxcatalysts and M-Co3O4.Fig.5(b) enlarges the view of toluene degrading efficiency in Fig.5(a) at higher temperatures.Overall,the catalytic activities of M-CoaMnbOxare better than that of M-Co3O4,certifying the promoting effect to toluene oxidation of Mn.The M-Co1Mn1Oxin which the Co:Mn=1:1 showed a more outstanding catalytic performance by comparison.For an intuitive understanding about the catalytic activities of the catalysts, the temperatures at which the removal efficiency of toluene reach 10%,50%,90%and 100%(the GHSV=20000 ml∙g-1∙h-1)are all presented in Table 2.Taking M-Co3O4as a comparison,T10%,T50%andT90%of M-Co1Mn1Oxreduce to 27, 16 and 12 °C, respectively.MCo0.5Mn1Ox(Co:Mn=0.5:1) and M-Co3Mn1Ox(Co:Mn=3:1) performed much closer for toluene degradation, better than MCo2Mn1Ox(Co:Mn=2:1) in performance, even the variation is not obvious.This also indicates that regularity between the doping amount of Mn and the catalytic activity of the Co-Mn catalysts is not uniform.As listed in Table S2, M-Co1Mn1Oxshows a superior catalytic performance among the series of reported Co3O4catalysts under the same test conditions.
The Arrhenius fitting plots is displayed in Fig.5(c), at toluene degradation rate of the M-CoaMnbOxcatalysts ≤20%.Table 2 lists the calculatedEa(apparent activation energy), which are ranked as follows: M-Co1Mn1Ox(55.4 kJ∙mol-1) < M-Co3Mn1Ox(58.4 kJ∙mol-1) < M-Co0.5Mn1Ox(58.6 kJ∙mol-1) < M-Co2Mn1Ox(58.8 kJ∙mol-1) Table 2Catalytic properties and apparent activation energies of the catalysts Fig.5(d) displays the catalytic performance of M-Co1Mn1Oxwith different GHSV.Significantly, the GHSV has an effect on the catalytic property of the samples.With the GHSV increases from 20000 to 80000 ml∙g-1∙h-1, the catalytic activity decrease gradually.TheT50%andT90%are 241 and 257°C at GHSV=80000 ml∙g-1-∙h-1, respectively, which are 12 and 18 °C higher than that of GHSV=40000 ml∙g-1∙h-1, 25 °C and 30 °C higher than that of GHSV=20000 ml∙g-1∙h-1.This further signifies that the extended exposure time between toluene and M-Co1Mn1Oxcan facilitate the catalytic efficiency of the catalyst to some extent. To further confirm the superior activity of M-Co1Mn1Ox, The reaction rate (toluene consumption rate) of M-CoaMnbOxwere evaluated at 180 and 230 °C, other test conditions are consistent with those in activity evaluation.Fig.6 is the bar graphs of the results.Apparently, reaction rate of the Mn-doped Co-Mn metal oxides catalysts are all higher than that of M-Co3O4at both the temperatures.The M-Co1Mn1Oxowns the fastest toluene consumption rate among the Mn-doped catalysts.The differences were not obvious between the M-CoaMnbOxcatalysts at 180 °C,but widened significantly at 230 °C, which is a full proof for the highest catalytic efficiency of M-Co1Mn1Ox. From the above analysis,we know that the doping of Mn effectively promotes the catalytic performance of M-Co3O4for toluene combustion.M-Co1Mn1Ox(Co:Mn=1) performs prominently among the M-CoaMnbOxwith different Co/Mn.The analysis results of the adopted characterization techniques reveal some outstanding physical and chemical properties of M-Co1Mn1Oxcompared with other M-CoaMnbOx.The broader and weaker peaks showed in XRD spectrum imply smaller crystal size of the catalyst, which can improve the utilization efficiency of catalyst [49].Rough and porous surface of M-Co1Mn1Oxexhibited in SEM suggests abundant surface defect sites on the catalyst.The HRTEM information states that {1 1 0} facets exposed over Co-Mn metal oxides catalysts, which are the active crystal plane for toluene degradation[10,33].Higher Co3+/Co2+, Mn3+/Mn4+and Oads/Olattof MCo1Mn1Oxcatalyst are obtained by the XPS analysis results.It has been proved that Co3+and Oads(activated oxygen that is surface adsorbed) are closely associated with high catalytic activity of Co3O4[36].The interaction between Mn3+and Co3+should not be overlooked in toluene degradation.A stronger reduction property in low temperature of M-Co1Mn1Oxin H2-TPR contributes a lot to its high catalytic activity [50].As for Raman part, the abundant oxygen vacancies and the smaller grain sizes of the M-Co1Mn1Oxare both advantageous to catalytic oxidation of toluene [48]. To further investigate the catalytic stability of M-Co1Mn1Ox,216 °C (T50%) and 270 °C (which is higher thanT100%) were picked as the temperatures,at which the on-stream reaction experiments were proceeding for 24 h, respectively.The test results are shown in Fig.7(a), in the first phase of stability testing, toluene degradation rate over M-Co1Mn1Oxfloat up or down around 50% for 24 h.Then the catalytic reaction of toluene was going on for another 24 h under 260 °C.The toluene degrading efficiency was staying at 100%during this process.Clearly,M-Co1Mn1Oxhas an outstanding catalytic activity together with excellent stability.Moreover,the H2O exists in the practical application of toluene oxidation,thus the effects of water vapor should be considered. Fig.7(b)exhibits the toluene degradation rate in the presence of 5%or 10%water vapor at 227°C(T90%).Clearly,the introduction of 5% water vapor has no effect on the catalytic performance of MCo1Mn1Ox, the toluene removal rate was maintained at about 90% for 12 h.However, a significant decline of toluene conversion appeared after the induction of 10% water vapor.The toluene degradation rate decreased to 80% in 7 h and kept at about 80%for 5 h, then the water vapor was shut down, the removal rate of toluene was coming back to 90%in 3 h.Unfortunately,the toluene degradation rate decreased to 80% with 10% water vapor on at about 37 h,but just recovered to 83%with 10%water vapor off.The results show that higher concentration of water vapor can inhibit the catalytic activity of M-Co1Mn1Oxto a certain extent, which may be attributed to the competitive adsorption of H2O and toluene on the catalyst [49]. Fig.5. (a)Toluene degradation rate,(b)enlarged view of(a),(c)Arrhenius plots of the catalysts,with 1000 μl∙L-1 toluene and SV is 20000 ml∙g-1∙h-1;(d)toluene degradation rate over M-Co1Mn1Ox at different GHSVs. The XRD patterns of M-Co1Mn1Oxafter the stability test with the effect of water vapor at 227 °C was detected and presented in Fig.S5.It is clear that some new X-ray diffraction peaks emerged after the stability test, which are agree with those of the standard SiO2(PDF-#-46-1045).The result is that some silica sand(the main component of which is SiO2)were put in the fixed bed reactor to fix the M-Co1Mn1Oxin the certain position during the stability test.Hence, in the process of collecting the M-Co1Mn1Oxsample after stability test, SiO2was mixed in inevitably.Except for the characteristic peaks of SiO2, other peaks of M-Co1Mn1Oxafter stability test show good match for that of M-Co1Mn1Oxbefore stability test.The SEM images of M-Co1Mn1Oxbefore and after combustion reaction are showed in Fig.S6.It is observed that M-Co1Mn1Oxafter the stability test is still with abundant pore structure and partially kept the octahedra morphology of the ZSA-1.Overall, the M-Co1Mn1Oxhas a satisfying stability and a good water resistance to a certain degree.Therefore, Mn-doped ZSA-1 is an optimized precursor for promising Co-Mn mixed metal oxides for toluene combustion. Fig.6. Toluene consumption rate under 230 and 180 °C: (I) M-Co0.5Mn1Ox, (II) MCo1Mn1Ox, (III) M-Co2Mn1Ox, (IV) M-Co3Mn1Ox and (V) M-Co3O4. To detect the products of toluene at different temperatures over M-Co1Mn1Ox, theinsituDRIFTS was utilized.Clearly in Fig.8, the infrared peaks represent benzene ring at 1495 and 1601 cm-1emerged on M-Co1Mn1Oxcatalyst at room temperature, which implied the successful adsorption of toluene on M-Co1Mn1Ox[51].Then,as the temperature goes up to 100°C,the infrared peaks of toluene decreased rapidly and disappeared gradually, implying the adsorption of O2on the catalyst and toluene catalytic oxidation was carried out.Then at 100 °C, the characteristic peaks of benzaldehyde and benzoic acid at 2840 and 1391 cm-1were observed. This suggests that benzaldehyde and benzoic acid are the first two intermediate products, which can be easily formed during the degradation of toluene over M-Co1Mn1Ox.Peaks corresponding to anhydride and maleate species appeared at 150°C,which are at 1305 and 1508 cm-1, respectively [5,30,52].As the temperature rose from 150 to 220 °C, peaks at 1391, 1305 and 1508 cm-1became much stronger, indicating benzoic acid, anhydride and maleic acid substances has accumulated on M-Co1Mn1Ox.At 250 °C, peaks of benzoic acid and maleate species become weak obviously.Peaks at 1361, 1147 and 1176 cm-1respectively represent acetate species, aldehydes and ketones [51,52], which emerged at 150°C and the peak strength became obviously stronger as the temperature raised.This means benzoic acid and maleate species are further broken down to short chains of aldehyde and ketones at higher temperature.Notably, the peak strength of CO2(2340 cm-1)and H2O(1589 cm-1)got stronger gradually during the temperature-rise period(from 150 to 250°C)[51],demonstrating carbon dioxide and water were the end products of toluene degradation.Compared with the in suit infrared characteristic peaks of M-Co3O4at the same temperature, the rate of the benzoic acid and maleate species formation were dramatically accelerated [14].The faster reaction rate of toluene on MCo1Mn1Oxmay benefit from the lower activation energy. Fig.7. (a) Catalytic stability over M-Co1Mn1Ox (at 216 and 270 °C) and (b) the effect of water vapor at 227 °C under the same conditions of toluene degradation rate test. Fig.8. In situ DRIFT spectra of toluene catalytic combustion over M-Co1Mn1Ox. Apparently,benzaldehyde,benzoic acid,anhydride and maleate species are the key intermediate materials of toluene catalytic degradation over M-Co1Mn1Ox.Fig.9 shows the process of toluene combustion, the adsorption of toluene and the oxygen in the air stream take place on M-Co1Mn1Oxcatalyst firstly,and the O2is further activated to be some reactive oxygen species.Toluene is then decomposed into benzaldehyde, further degrade into benzoic acid and anhydride.Subsequent catalytic oxidation break the benzene ring to maleate species and then to short chains of aldehyde and ketones and acetate species,ultimately to CO2and H2O.The whole catalytic oxidation process is analogous to some reported works[5,25,30]. Fig.9. The degradation mechanism of toluene over M-Co1Mn1Ox. In conclusion,with different molar ratio of Co:Mn,M-CoaMnbOxcatalysts were derived from Mn doped ZSA-1.The introduction of MnOxshowed a positive driving effect to toluene oxidation of MCoaMnbOxcompared with M-Co3O4.The MnOxinteracts with Co3O4thus accelerate the generation of lattice defects and the electron transfer of M-CoaMnbOx,enhance the content of Co3+,Oadsand strengthen the reducibility of the catalysts.M-Co1Mn1Oxexhibited the superior toluene catalytic activity withT90%andT100%reaching at 227 and 235 °C, which is much more outstanding than that of no-doped M-Co3O4.In addition, the degradation pathways of toluene was detected by theinsituDRIFTS, the finally CO2and H2O are in turn derived from toluene, benzaldehyde, benzoic acid,anhydride and maleate species.In a word,Co-Mn composite metal oxide derived from Mn-doped Co-MOF is a facile and effective method and the catalysts are promising for toluene combustion. Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgements The authors acknowledge the financial support of National Natural Science Foundation of China(Nos.22078215,21671147),Natural Science Foundation of Shanxi Province(No.201901D211117),Coal Bed Methane Joint Foundation of Shanxi (No.2016012004),Science and Technology Innovation Project of Higher Education Department of Shanxi Province (No.2020L0632).Young Academic Leaders Funding Program of Taiyuan Institute of Technology (No.2020 × S03), the Shanxi Province Natural Science Foundation for Youths(202103021223347),and the Taiyuan Institute of Technology Scientific Research Initial Funding (2022KJ010). Supplementary Material Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2021.11.027.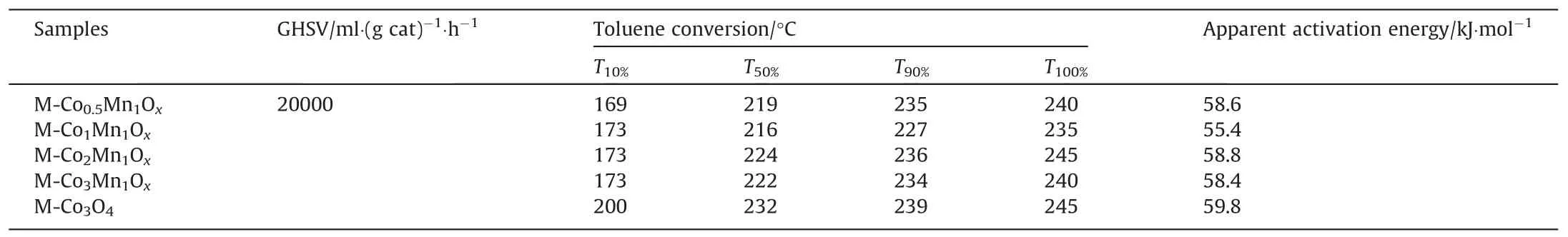
3.3.Stability test


3.4.Mechanism for combustion of toluene over M-Co1Mn1Ox catalyst
4.Conclusions
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Preparation of lithium carbonate by microwave assisted pyrolysis
- Simulation and design of a heat-integrated double-effect reactive distillation process for propylene glycol methyl ether production
- Hyper-parameter optimization of multiple machine learning algorithms for molecular property prediction using hyperopt library
- High-efficiency and safe synthesis of tonalid via two Friedel-Crafts reactions in continuous-flow microreactors
- Improvement of synergistic effect photocatalytic/peroxymonosulfate activation for degradation of amoxicillin using carbon dots anchored on rod-like CoFe2O4
- Boosting the hydrogen storage performance of magnesium hydride with metal organic framework-derived Cobalt@Nickel oxide bimetallic catalyst
