Bonding mechanism of binder and low-rank coal during carbonization
2023-01-04WANGMingyiCHANGZhiweiLIUYuehuaSHANGGUANJuDUWenguangMARuiWANGQiLIUJunjieLIUShoujunYANGSong
WANG Ming-yi , CHANG Zhi-wei , LIU Yue-hua , SHANGGUAN Ju , DU Wen-guang , MA Rui ,WANG Qi , LIU Jun-jie , LIU Shou-jun,3,* , YANG Song,*
(1.College of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan 030024, China;2.Shanxi Engineering Center of Civil Clean Fuel, Taiyuan University of Technology, Taiyuan 030024, China;3.State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, China)
Abstract: Low-rank coal is rarely used in the industry of carbonized briquette due to its poor cohesiveness.In order to replace lump coal and utilize low-rank pulverized coal as much as possible in a carbonized briquette process, washing oil residue (WOR) was used as an enhanced binder to enhance the bonding strength of resulted carbonized briquette.The effects of blending ratio and carbonization temperatures on binding strength were investigated, and moreover, a reasonable bonding mechanism was deduced.The results showed that the best crushing strength was obtained when the weight ratio of WOR and low-rank coal is 3∶7 at 800 °C, and its crushing strength of M25 (M25) can reach to 97%, while the thermoplastic properties of WOR is thought to be responsible for the obtained good crushing strength, where WOR can be softened and coated on the surface of coal particles during carbonization, and then a coal-binder interface can be formed, resulting in the loose inert coal particles can be combined and the strength of coke is improved significantly.
Key words: low-rank coal;washing oil residue;carbonization;bonding mechanism
Nowadays, approximately 0.4 billion people is using raw coal as the dominant household combustion fuel, and this data may continue to climb in the next two decades[1].Coal combustion can affect the environment and people’s health, and it is also one of the important reasons for the fact that PM2.5and PAHs levels in most part of China is higher than the standard of the World Health Organization[2-4].Besides the air pollution from industrial boilers, emissions from household stoves is also nonnegligible, because of the device shortage and incomplete combustion[5].Therefore, how to solve the pollution from civil scatter coal is one of the key issues in atmospheric environmental management[6,7].
Civil clean fuels (CCF) have been proven to be a good substitute of raw coal to decrease SO2emission during combustion, but the raw material of CCF must be caking coal[8,9].Low-rank coals are non-caking coals and can’t generate the caking structure, and thus the strength of coke produced by low-rank coals cannot meet the requirements of producing CCF.
Coke, which acts as the solid product of coal pyrolysis, is the key component of CCF.Therefore, in order to using low-rank coal as raw material to produce CCF, the key point is to enhance the cohesiveness of coke produced by a low-rank coal.For the sake of improving coal caking property, some additives were added[10-12].Takanohashi et al.[13,14]investigated the effect of the proportion of non-caking coal in a coke blend on the coke strength, and they found that the Drum Index of the coke decreased with increasing proportion of non-caking coal, however, the coke strength was recovered when 5%-10% of Hyper-coal was added.Moreover, various binders such as coal tar pitch,petroleum asphalt and waste plastics have been reported[15-18].Benk et al.[16]used low volatile anthracite as raw material to produce metallurgical quality formed coke.When phenolic resins and air blown coal tar pitch blended binder was added to the raw material, the tensile strength of coke is improved, and the largest tensile strength was 64.6 MPa.Zhong et al.[18]produced a briquetted fuel from waste coal by adding low density polyethylene as a binder.It is found that the heating value increased from 21.9-24.3 MJ/kg when 10% ofbinder was added, indicating this binder has positive influences.
Washing oil residue (WOR), which consists of many polycyclic aromatic hydrocarbons, is a byproduct of the destructive distillation of coal, and it can soften and melt with increasing temperature[19,20].Therefore,WOR can be a promising binder owing to its thermoplastic properties[21].In this study, the effect of WOR and temperature during carbonization were investigated.Meanwhile, on the basis of morphology changes of coke, a bonding mechanism between binder and low-rank coal was proposed.
1 Experimental
1.1 Materials
In this work, a non-caking coal from Shaanxi province (SXC) was selected as sample, and WOR was used as a binder.The proximate and ultimate analyses of raw coal and the WOR are shown in Table 1, and the caking index (G) are also shown in it.
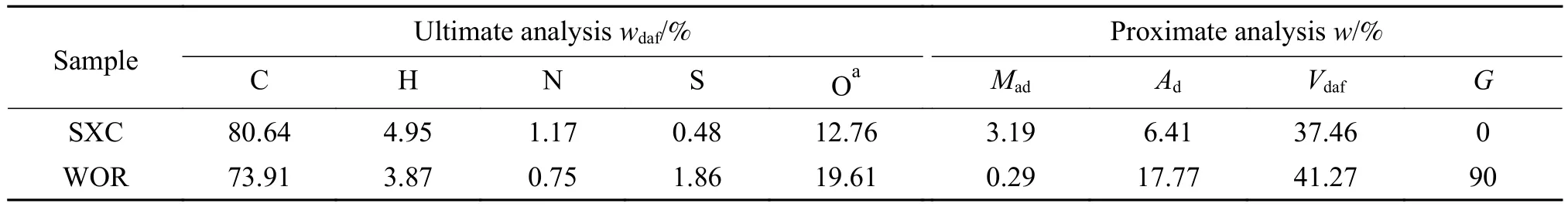
Table 1 Proximate and ultimate analyses of the coal sample and WOR
1.2 Experimental procedure
The preparation process of coke is shown in Figure 1.Coal sample was crushed and sieved to below 3.0 mm, and then dried at 105 °C.The WOR was ground to below 0.2 mm.After mixing, stirring and briquetting, a massive solid mixture of coal with 0-40% of WOR was achieved, and the fixed pressure was 60 kN.
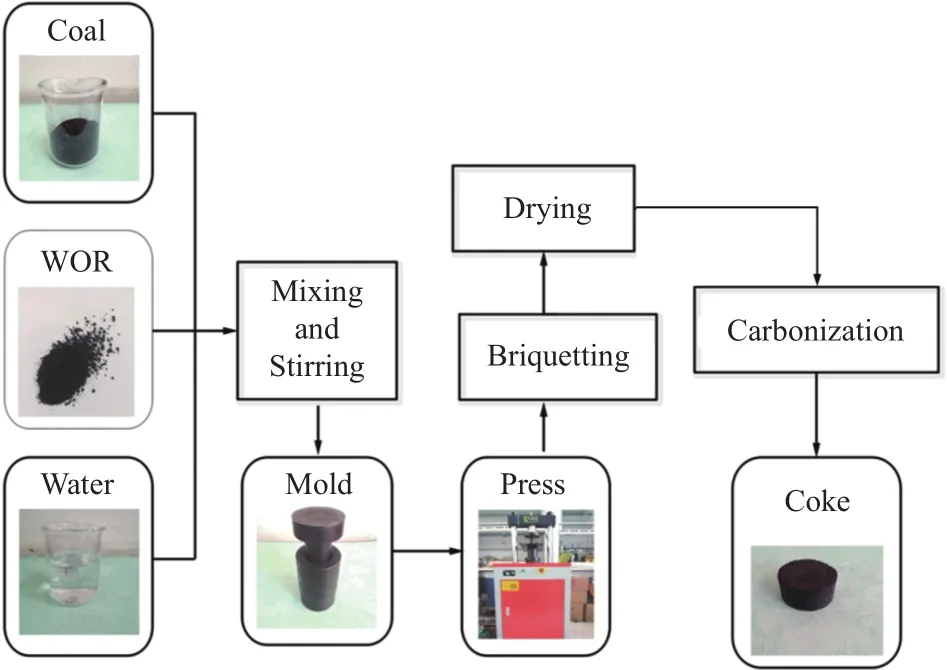
Figure 1 Scheme of coke preparation process
Then, about 12 g of massive solid mixture was filled into a steel reactor, and the flow rate of N2was 50 mL/min.The reactor was heated to the setting temperature with a heating rate of 10 °C/min, and kept for 60 min, as shown in Figure 2.Then, the prepared coke was put into liquid nitrogen quickly.Coketwas used to distinguish the coke samples obtained under different carbonization temperatures, wheretrepresents the carbonization temperature.
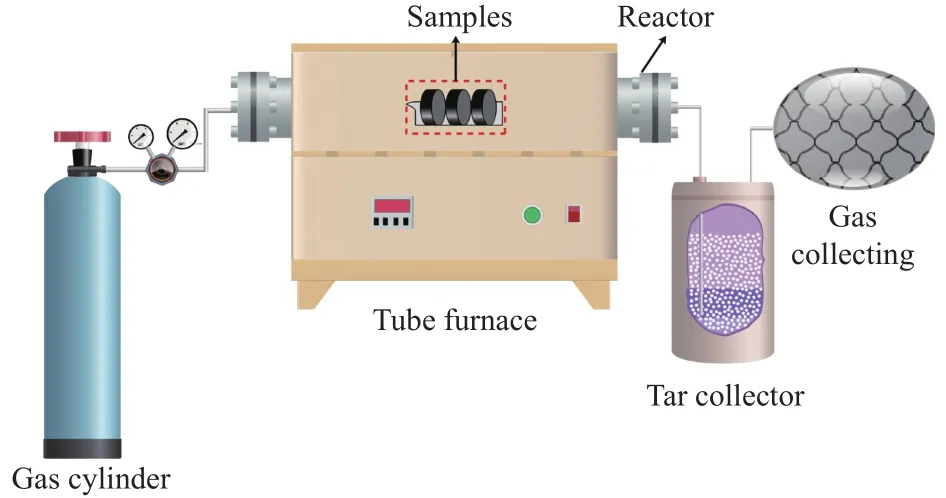
Figure 2 Schematic diagram of the experimental for briquette pyrolysis
1.3 Characterization of coke
1.3.1 Mechanical strength
Mechanical strength of cokes was evaluated by a drum which was often adopted to evaluate the coke strength.The drum strength was divided into crushing strength and wear resistance.A self-made drum machine was used and shown in Figure 3.At first,weighed sample was put into the drum and the speed was set to 25 r/min, then the drum was stopped after 4 min and stand for 1-2 min.After the dust falls, the coke powder was collected and sequentially sieved with 25 and 5 mm round hole sieves.For parts larger than 25 mm, hand piercing must be carried out (when sieving, it was necessary to strive to screen the net, and also prevented the force from being too hard to cause the coke to collide and break).The mass of each grade of coke that of larger than 25, 25-5 mm and less than 5 mm were weighed, and the crushing strength ofM25(M25) and the wear resistanceM10(M10) were calculated as follows:

Figure 3 Homemade coke drum machine

where,mstood for the weight of coke,M25was the weight of samples whose diameter was larger than 25 mm after the drum test, andM10is the weight of the samples whose diameter was less than 10 mm after the drum test.
1.3.2 Thermoplastic properties
The caking properties of coals were characterized by the caking index and they were obtained according to GB 5447-85.Plastic layer index (Y) was obtained on the basis of pyrolysis, the colloid was solidified to form a semi-coke, and the maximum thickness (Y), final shrinkage (X) and volume curve of the colloid layer were used to characterize the coking property of bituminous coal.An automatic colloidal layer tester(HH-JCY/A) was used to test colloidal layer according to GB/T 479—2000.
1.3.3 Structural analyses
The morphology changes of SXC, WOR and coke were measured by a German Zeiss EVO MA15 SEM analyzer.Fourier transform infrared spectroscopy (FTIR) was employed to analyze the changes in the surface functional group of coke samples.The crystal structure of coke samples was analyzed by an X-ray diffraction(XRD, Bruker D8 Focus Powder Diffractometer) with a CuKα radiation (λ= 0.15406 nm) at 40 mA and 40 kV as the X-ray source.The scan range was from 5°-85° and the scanning speed was 4(°)/min.
2 Results and discussion
2.1 Effect of WOR content on coke strength
The effect of WOR content in the mixture is investigated at 800 °C, and the relationship between WOR content and drum strength is shown in Figure 4.When binder is added, the crushing strength increases and the wear resistance decreases until its content reaches 40%.At the same time, the maximum crushing strength is 97% and the strength goes down sharply below 30% with 40% of WOR addition.
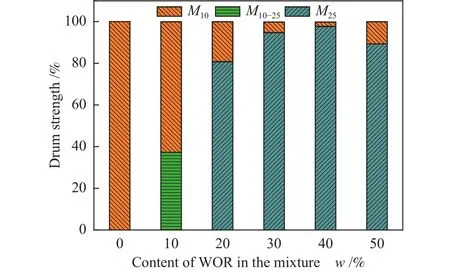
Figure 4 Effect of WOR content on drum strength
To investigate the effect of binder on the caking properties of cokes, the caking index of different fraction of WOR binder is shown in Figure 5.It can be found that the WOR binder has significant effect on the caking properties, the caking index keeps rising when the content of WOR increases from 10% to 50%.What’s more, when the fraction of WOR binder is 10%, the caking index is only 2, while the fraction of WOR binder is 40%, its caking index increases to 41.As the content of the binder increases, more metaplast is produced in the coke, resulting in a higher caking index for the coke[22].
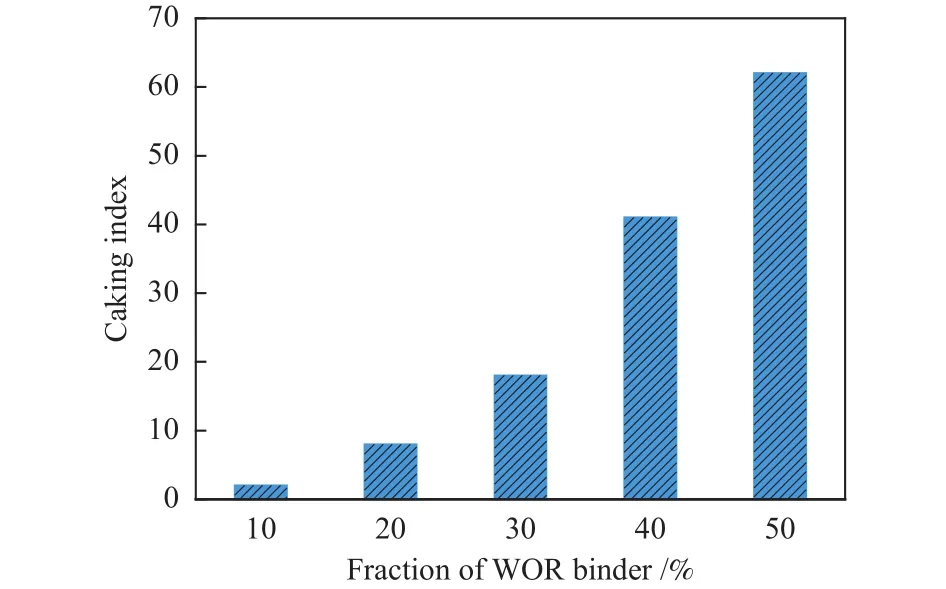
Figure 5 Effect of different fraction of WOR binder on the caking properties of coke
2.2 Bonding effect of WOR
Figure 6 shows the effect of carbonization temperature on the coke (SXC∶WOR=7∶3) drum strength, here the blends ratio of coal and WOR is 7∶3.M25can reach almost 95% when the carbonizationtemperature of coke is 800 °C.Meanwhile, M25decreases when the temperature decreases from 800-200 °C and increases from 800-1050 °C, and the standard coking temperature in the coking industry is 1050 °C.When the carbonization temperature is 200 °C,M10can reach almost 100%, M25is almost 0, indicating that the coke has a very poor drum strength.It is also founded that the molten component produced by the WOR can strengthen the bonding of many inert particles due to the excellent dispersion of the WOR when the carbonization temperature is high, while WOR doesn’t produce the molten component at a lower temperature, leading to a bad drum strength.However, M25decreases from 62%-40% when the carbonization temperature increases from 300-400 °C.
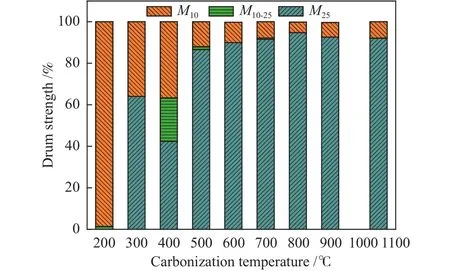
Figure 6 Effect of carbonization temperature on drum strength
The SEM images of coke at different carbonization temperature are shown in Figure 7.The inert coal particles are relatively dispersed and large coal particles become small particles during coal pyrolysis, which can also be found in Figure 7(a) and Figure 7(b)[23].The surface of coke is uneven, indicating that the WOR binder doesn’t soften and melt at this temperature.And in Figure 7(b), the vaporization of a large number of volatiles form a structure with large hollows, which makes the binding of the binder and inert coal particles become lost, and the strength of coke400is significantly reduced.While the temperature rises to 500 °C (shown in Figure 7(c)), part of the large-grained coal particles disappears, and many small particles are formed and agglomerated together,indicating that the binder has begun to soften and melt,and part of inert coal particles is tightly bonded together.In Figure 7(d), the surface of the coke600is very flat, indicating some remarkable agglomerations have occurred.Melting and subsequent re-solidification but no cracks were observed from the surface of coke600, indicating that the binder has completely softened, melted and re-solidified, and almost all of the inert coal particles have been bonded together[24].In addition, pores are again observed in the surface of coke700and coke800(Figure 7(e) and Figure 7(f)) due to the polymerization reaction of the WOR, which leads to a production of hydrogen.The coke expansion pressure increases, this can lead to a tighter bond between the coal particles[25].When the temperature reaches 1050 °C, it can be found that the coke1050undergoes a significant shrinkage and the pore structure of the surface is significantly reduced.It can be explained by the polycondensations of the decomposition residues, and the aromatic carbon network in coke is continuously increasing, which makes the coke arrangement regular and the density increases, resulting in a higher coke strength.Moreover, the structure of coke1050seems more homogeneous and tighter than that of coke300because of the carbonization of the WOR, which leads to an increase in coke drum strength after carbonation process.
The FT-IR spectra of cokes at different pyrolysis temperatures are shown in Figure 8 and four bands are observed.The absorption bands are assigned based on standard patterns.The absorption band at near 3400,1620 and 700-900 cm-1are belonged to -OH,C=C/C=O and aromatic C-H (Car-H), respectively[26].The absorption band at 3000-2800 cm-1are all belonged to aliphatic C-H (Cal-H), and it can be separated into five parts, the bands at 2953 and 2865 cm-1are attributed to -CH3.The bands at 2922 and 2850 cm-1are attributed to -CH2-, and the band at 2895 cm-1is attributed to C-H[27].
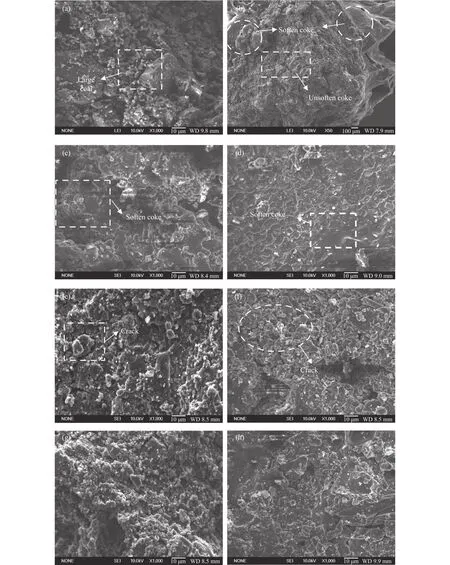
Figure 7 SEM images of coke at different carbonization temperatures
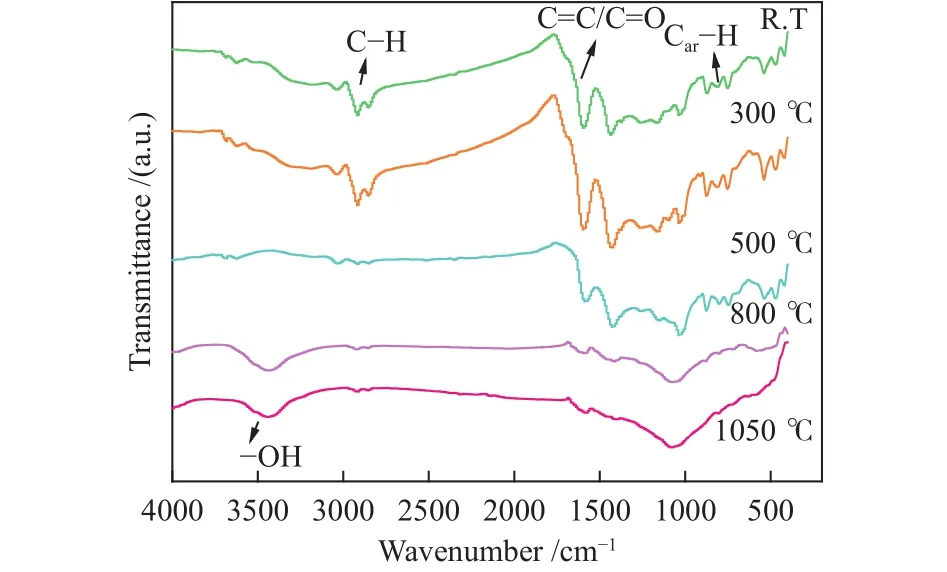
Figure 8 FT-IR spectra of coke produced at different temperatures
The peak intensity at 2850 cm-1decreases with increasing pyrolysis temperature due to the loss of Cal-H.A significant decrease in intensity of aromatic C-H group is observed when the pyrolysis temperature reaches to above 500 °C, and it also can be related to the transform from smaller aromatic rings to the aromatic rings having six or more benzene rings during pyrolysis, which produces hydrogen and more condensed aromatic rings.The strength of the bands caused by C=O and C=C groups in the carbonized briquette decreases significantly at 500 °C, indicating that they are thermally unstable above 500 °C and are removed during pyrolysis[28].Meanwhile, the carbonized briquette produced at a high temperature is composed of polycyclic aromatic hydrocarbons and few branched chains which can be verified by the disappearance of the feature peaks in the FT-IR spectra of coke800and coke1050[29].The peak at near 3400 cm-1shows that the hydroxyl characteristic peak exists in coke800and the coke1050.What’s more, the hydrogen bonds play an important role in maintaining the macromolecular structure of low-rank coals which can affect the application of low-rank coals significantly.
It is generally believed that the microcrystalline structure of carbonized briquettes is a random layer structure, including the graphite flakes and the random layer microcrystals[30].The microcrystalline structure of carbonized briquettes changes with increasing carbonization temperature.The X-ray diffraction is used to analyze the microcrystalline structure of carbonized briquettes which can help further understand the bonding effect of coal and binder[31].
As shown in Figure 9, a broad peak is found in the 2θrange of 15°-35° and another peak in the 2θrange of 40°-50°.The bands of 002 and 100, which are at about 25° and 45°, are related to the degree of aromatic layers stacking and aromatic interlayer spacing,respectively[32].The band of crystalline graphite at 25°is symmetrical and narrow, and the broadening and apparent asymmetry of this peak in coketreveals the existence of bands located around 002 band.As the carbonization temperature increases, the diffraction angle of the 002 diffraction peak gradually increases,indicating that the microcrystalline structure of carbonized coal tends to be ordered, which is related to the fact that the side chains and the aromatic cycloalkyl continue to fall off, the oxygenated functional groups gradually decrease and the aromaticity increases with increasing temperature[33].
The colloidal index (Yd,Xd) can accurately reflect the cohesive and coking properties of the blended coal during pyrolysis.And Figure 10 shows the colloidal layer index of the coke.
When the carbonization temperature increases from 250-730 °C, the final shrinkage (X) increases from 0 to 38.4.Compared with the SXC (Y=0), the maximum thickness of plastic layer is 11.8.Meanwhile, the colloids are formed by the side chains and functional groups which are shed from the macromolecular structure of coal, and the number of side chains directly determines the cohesiveness of the coal[34].As shown in Figure 8, the macromolecular functional group of coke at high temperature disappears, which indicates that the WOR binder produces colloids during the pyrolysis and significantly improves the mechanical strength of the coke[35].
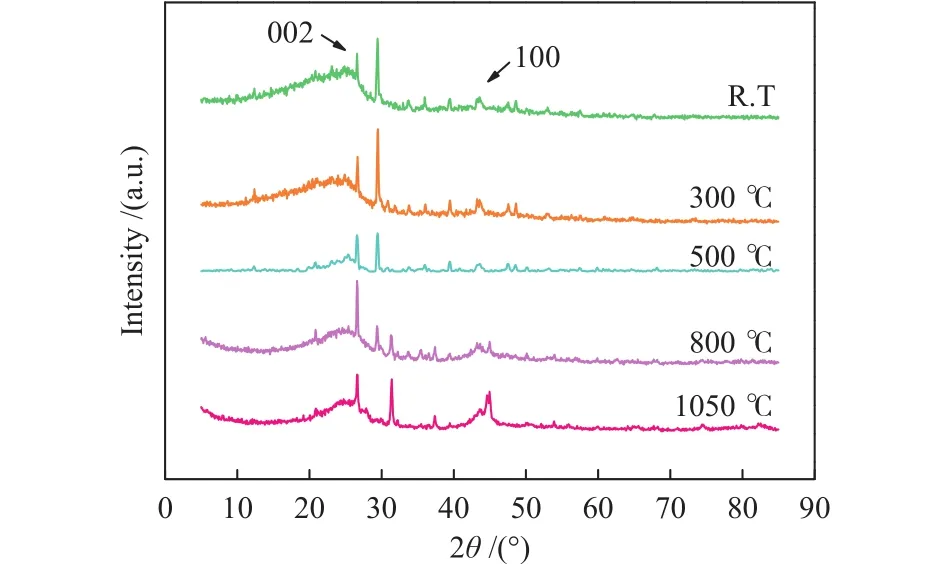
Figure 9 XRD patterns of coke produced at different temperatures
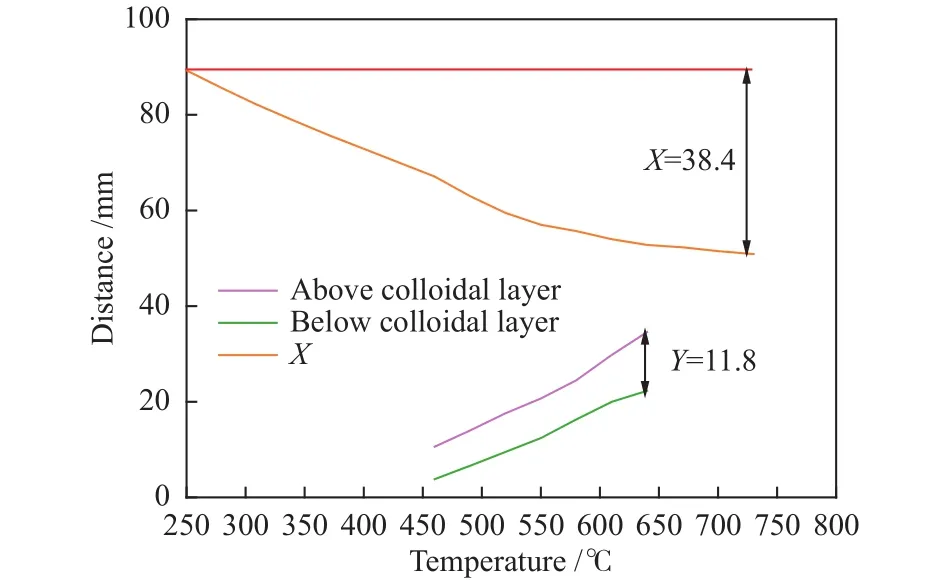
Figure 10 Colloidal layer index of coke (SXC: WOR=7:3)
2.3 Bonding mechanism of WOR and coal
In order to further explore the function of WOR during coal carbonization, the structural changes of coal and WOR are studied.
Figure 11 shows the SEM images of SXC at different carbonization temperatures.C-300, C-400, C-500 and C-800 refer to the coke produced at the carbonization temperature of 300, 400, 500 and 800 °C,respectively.Meanwhile, there is no obvious change is observed in the pure coal particles at different temperatures.As the temperature increases, some cracks appear in the coal particles, which indicates that the coal particles gradually generate more volatiles.
Figure 12 shows the SEM images of WOR at different carbonization temperatures, where W-300, W-400, W-500 and W-800 refer to WOR at carbonization temperatures of 300, 400, 500 and 800 °C, respectively.In Figure 12(a) and Figure 12(b), it can be found that the binder softens and melts at 300 and 400 °C and isfused into one bulk.When the temperature rises to 500 °C (shown in Figure 12(c)), a large number of cracks and holes appear, indicating that a large number of volatiles are escaped.And the expansion pressure increases, making the strength of coke500improve significantly.In Figure 12(d), it can be found that the binder particles are obviously stuck together and a compact structure is formed at 800 °C.

Figure 11 SEM images of SXC at different carbonization temperatures

Figure 12 SEM images of WOR at different carbonization temperatures
Based on the above experimental results, a bonding mechanism of WOR and low-rank coal during carbonization is proposed and shown in Figure 13.A low rank non-caking coal does not show thermoplastic properties, and the structure which is similar to coke can’t be formed.When WOR and coal are carbonized together, WOR can be softened and coated on the surface of coal particles because of its thermoplastic properties during pyrolysis, and a coal-binder interface is formed, polymerization reaction occurs and coal particles are bonded together, resulting in a coke with high strength.
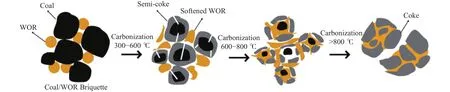
Figure 13 Schematic of the bonding mechanism of WOR and low-rank coal during carbonization
3 Conclusions
In this study, high-strength cokes were produced by adding WOR into non-caking low-rank coals.The effects of binder content and carbonization temperature on the strength of fuel were studied.The results showed that the coke800, a qualified coke, could be obtained by blending with 30% of WOR.SEM was employed to characterize the produced coke.And the results showed that WOR could be softened and coated on the particles surface of coal during pyrolysis because of its thermoplastic properties, and a coalbinder interface can be formed, which significantly improved the strength of coke.Therefore, a possible approach for low-rank coals to produce cohesive coke was proposed, and the bonding theory of WOR and low-rank coals was deduced.
