Ameliorative effect of Lacticaseibacillus rhamnosus Fmb14 from Chinese yogurt on hyperuricemia
2023-01-03HongyunZhoXioyuChenFnqingMengLingZhouXinyiPngZhoxinLuYingjinLu
Hongyun Zho, Xioyu Chen, Fnqing Meng, Ling Zhou,Xinyi Png, Zhoxin Lu,*, Yingjin Lu,*
a College of Food Science and Technology, Nanjing Agricultural University, Nanjing 210095, China
b College of Food Science and Engineering, Nanjing University of Finance and Economics, Nanjing 210023, China
Keywords:Lacticaseibacillus rhamnosus Fmb14 Hyperuricemia Inosine degradation Uric acid metabolism
A B S T R A C T Hyperuricemia is a critical threat to human health, and a high inosine diet can increase the prevalence of it.Lacticaseibacillus rhamnosus Fmb14 was isolated from traditional fermented Chinese yogurt, and its inosine degradation rate reached 36.3% at 109 CFU/mL for 24 h. LC-MS analysis revealed that high concentrations of inosine could activate compensatory metabolic pathways of L. rhamnosus Fmb14 to catalyse inosine as an energy source and produce intracellular folic acid and ribof lavin. The contents of folic acid and ribof lavin were 6.0 and 4.3 fold increased after inosine treatment in the cell-free extracts (CFE). L. rhamnosus Fmb14 CFE treatment ameliorates hyperuricemia through xanthine oxidase (XOD) inhibition and ATP-binding cassette subfamily G member 2 (ABCG2) promotion, both of which are responsible for uric acid (UA)synthesis and secretion in HepG2 and Caco2 cells, respectively. The in vivo results showed that the serum UA level decreased from 236.28 to 149.28 µmol/L after 8 weeks of oral administration of L. rhamnosus Fmb14 in inosine-induced hyperuricemia model mice. Our results revealed that L. rhamnosus Fmb14 has a potential as a biological therapeutic agent in hyperuricemia prevention.
1. Introduction
The past two decades have witnessed dramatic changes in people’s lifestyles worldwide, and some metabolic syndrome diseases have occurred due to changes in lifestyle conditions [1]. Hyperuricemia is the most important risk factor for gout and some other metabolic diseases, such as hypertension, acute and chronic kidney disease,obesity, fatty liver and diabetes mellitus [2] and it has been reported that more than 7.44 million cases of gout exist worldwide [3].Hyperuricemia is def ined as high levels of uric acid (UA) in plasma or serum, and the normal thresholds of serum or plasma UA are 420 and 360 µmol/L in men and women, respectively [4]. In addition to causing acute arthritis, asymptomatic hyperuricemia also stimulates human cells in chronic low-grade inflammation conditions [5] and results in autophagy disruption or pyroptosis [2], which has a high risk for human health.
Hyperuricemia patients are often advised to avoid high purine diets, such as seafood, red meat and animal protein, which is quite disruptive to their daily life [6]. Inosine is a purine that is metabolized from adenosine monophosphate (AMP), inosine monophosphate (IMP)and guanosine monophosphate (GMP) in food and it can further catalyzed hypoxanthine, xanthine and UA [7]. Purines are mainly catalyzed to UA strictly restricted by the content and activity of XOD in the liver, and the commonly used hyperuricemia drugs such as
allopurinol could signif icantly decrease the activity of XOD [8]. Serum
UA is secreted through the intestine (30%) and kidney (70%) and is regulated by transporters such as ATP-binding cassette subfamily G member 2 (ABCG2) and glucose transporter 9 (GLUT9) [7].Benzbromarone [9] has been used as uricosuric to increase the amount of UA secreted in renal tissue to ameliorate hyperuricemia, but current treatment approaches are limited due to serious drug resistance and adverse effects [10].
Probiotic intervention therapy has become one of the conventional methods for the treatment of metabolic diseases due to its regulated
effect on host metabolism disorders and intestinal flora dysbiosis [11].Although probiotics have a long-term safety history, the efficacy of
the applications of probiotics in hyperuricemia has been seriously neglected. Probiotics could produce intracellular uricase in the gastrointestinal system and degrade UA to allantoin [12,13].Lactobacilluscould colonize the gut, regulate the dysbiosis caused by hyperuricemia and facilitate purine and UA metabolism [14].Moreover, high serum UA could destroy the homeostasis of the intestinal flora, which damages the gut barrier and decreases UA transporter biosynthesis, such as ABCG2 and GLUT9 [15,16].Multiple studies have demonstrated the indirect effect of probiotics on hyperuricemia, but understanding of the interactions of specificLactobacilluswith purine metabolism and UA secretion systems in depth will be crucial for the prevention of hyperuricemia.
Accordingly to the urgent need to develop new biological resource as an adjuvant with high inosine dietary to prevent hyperuricemia. We isolated an array ofLactobacillusand found thatL. rhamnosusFmb14 has the ability to degrade inosine. Therefore, the aims of this study were to determine the mechanism by whichL. rhamnosusFmb14 alleviates hyperuricemia bothin vitroandin vivo.
2. Materials and methods
2.1 Materials and reagents
Seven traditional fermented materials were obtained from 4 regions in Northwest China as shown in Table 1. Dulbecco’s modified eagle’s medium (DMEM) and fetal bovine serum (FBS)were purchased from Gibco Co., Ltd. (Carlsbad, USA). The UA,inosine and hypoxanthine were purchased from Aladdin Co., Ltd.(Shanghai, China). The primary antibodies against NACHT, LRR,and PYD domain component protein 3 (NLRP3), caspase1, ABCG2 and GLUT9 were purchased from Boster Biological Technology Co., Ltd. (Wuhan, China). The secondary antibody goat anti-rabbit immunoglobulin G (IgG) (peroxidase conjugate) were obtained from Boster Biological Technology Co., Ltd. (Wuhan, China). The enzyme linked immunosorbent assay (ELISA) kits for UA, XOD,hypoxanthine phosphoribosyltransferase1 (HPRT1), ABCG2,interleukin-1β (IL-1β) and Interleukin-18 (IL-18) were purchased from Meimian Institute of Biotechnology Co., Ltd. (Nanjing, China).Blood urea nitrogen (BUN) and creatinine (CRE) kits were purchased from Jiancheng Institute of Biotechnology Co., Ltd. (Nanjing,China). All other analytical reagents were purchased from Sinopharm ChemicalReagent Co., Ltd. (Shanghai, China) and Solarbio Science & Technology Co., Ltd. (Beijing, China).

Table 1Specific information of the samples from Northwest China.
2.2 Isolation and identification of inosine degradation strains
Microorganisms were isolated from traditional fermented materials by Man-Rogosa-Sharpe (MRS) culture and lactic acid bacteria were identified by biochemical test including H2O2catalase test and Grams examinations. Single isolates were incubated in MRS broth mediums for 48 h and then strains were obtained by centrifuge at 10 000 ×gfor 10 min. Single inosine growth experiments were using as primary screen and then 1 000 µmol/L inosine PBS were prepared to test inosine degradation ability of each of isolates by high performance liquid chromatography (HPLC). The screened strains were deeply identified by 16S rRNA gene sequence similarity.
2.3 Preparation of different cell-free extracts (CFEs)
L. rhamnosusFmb14was incubated in MRS broth culture for 24 h. Then,L. rhamnosusFmb14 (109CFU/mL) was centrifuged at 8 000 ×gfor 10 min to collect live microorganisms and fermentation broth. Then microorganisms were washed with PBS solution (pH 7.2)three times and finally re-suspend the cell pellets in different suspension solutions. Afterwards, cell suspension was placed in ice bath and treated with ultrasonic disruption for 20 min to break bacterial cells to obtain CFE. After centrifugation (8 000 ×g) at 4 °C for 10 min, the resulting supernatant was collected for further experiments. The CFE-1 was extracts from.L. rhamnosusFmb14 cultivated in MRS broth medium and the CFE-2 was extracts fromL. rhamnosusFmb14 cultivated in inosine-PBS for 24 h, respectively.
2.4 Measurement of purines and UA by HPLC
Degradation test of inosine and hypoxanthine in the filtrate samples were analyzed by HPLC (Agilent 1100, Santa Clara, USA).UA, inosine and hypoxanthine potassium phosphate (pH 7.2) with gradient concentration was prepared to establish a calibration curve(Fig. S1). In specific, 900 µL prepared standard solution and 100 µL perchloric acid was added to tubes, and 20 µL of the mixture was injected to HPLC. The purine and UA in the mixture were analyzed on Agilent separation module system (Agilent 1100, Santa Clara, USA) equipped with an ultraviolet detector and ZORBAX SB-Aq column (4.6 mm × 250 mm, 5 µm, Agilent Corporation, Santa Clara, USA) at a flow rate of 1.0 mL/min at 25 °C. Chromatographic separations were carried out using the following gradient of HPLC grade methanol (acetonitrile A), and water (eluent B): 0-15 min, 0% A,15-20 min, 0-10% A, 20-25 min, 10%-0 A. Chromatograms were obtained at 290 and 250 nm for UA and inosine (hypoxanthine),respectively.
2.5 Liquid chromatographic-triple quadrupole tandem mass spectrometry (LC-QqQ-MS/MS) analysis
Chromatographic separation was achieved using kine tex LC column (100 mm × 2.1 mm, 1.7 µm, Phenomenex, Santa Clara,USA) in a gradient mode. Mobile phase A was 100% 10 mmol/L ammonium acetate. Mobile phase B was methanol. The flow rate was 0.3 mL/min. The elution program was as follows: 0-5 min, 1% B,5-7 min, 70% B, 7-9 min, 95% B, 9-10 min, 1% B. Analysis time was 10 min and the injection volume was 2 µL.
Qualitative and quantitative analyses were performed using liquid chromatograph coupled with triple quadrupoleTM5500 (Sciex,Framingham, USA) operating in multiple reaction monitoring (MRM)mode with electrospray ionization (ESI). Liquid chromatograph(Agilent, Santa Clara, USA) consisted binary pump, column oven(Agilent, Santa Clara, USA) and CTC auto sampler (CTC Analytics,Zwingen, Switzerland). Samples were analyzed using ESI in a negative ionization mode and the specific parent ions of UA and purine were shown in Table S2. (Data was obtained from The Human Metabolome Database), the specific parent ions were usually used to identify the materials.
2.6 Cell cultures and cell treatment
HepG2 and Caco2 cell lines were obtained from the Cell Bank of Chinese Academy of Science (Shanghai, China). Cells were cultured in DMEM supplemented with 10% (V/V) of FBS, streptomycin(100 μg/mL), and penicillin (100 units/mL), as well the culture condition was 5% CO2and 95% air at 37 °C. After activation, all cells used in experiments were within 20 generations. The UA and inosine were added into DMEM medium to obtain UA and inosine stock solution. HepG2 and Caco2 (106cells/mL) were cultured in the medium for 24 h and then the cells were treated with different concentrations of FBS-free UA or inosine solution to obtain simulating hyperuricemia models.
2.7 Cell viability assay
Effects of CFEs treatment on the viability of HepG2 and Caco2 were measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [17]. HepG2 and Caco2 cell lines were adjusted to a concentration at 106cells/mL and plated in 96-well plates. The cells were divided into 3 groups: control group,UA-treated group, and inosine-treated group to test the effect of UA or inosine treated to cell viability. Then the cells in the models divided into 3 groups: control group (treat with PBS), CFE-1 treated group and CFE-2 treated group. MTT solution was added into each well and incubated for 4 h. Then, DMSO was added to each well followed by discarding the MTT solution. The absorbance at 570 nm was recorded. The cell viability was calculated using the following equation:

2.8 Reverse transcription and quantitative real-time PCR(qRT-PCR) assay
Total RNA were isolated from cultured HepG2 and Caco2 cells by homogenization in TRIzol reagent (TransGen Biotech., Beijing,China), used for RNA extraction. Then, RNA was reverse-transcribed into cDNA using a HiScript II Q RT SuperMix kit (Vazyme Biotech Co., Ltd., Nanjing, China) and the quality of cDNA was measured using a Nano Drop 2000 Spectrophotometer (Thermo Fisher Scientific Inc., Waltham, USA). Quantitative PCR was operated with HieffTMSYBR Green Master Mix (Yeasen Biotech Co., Ltd., Shanghai,China) and a RT-PCR detection (Applied Biosystem, Carlsbad, USA)at the following cycling conditions: 95 °C for 2 min, followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s and 72 °C for 30 s. The melting curve was also done from 65 °C to 95 °C with 0.5 °C/s increments to exclude unspecific amplification after the last reaction. The relative expression levels in terms of fold changes of target genes were calculated by 2-ΔΔCTmethod. The primers of Abcg2, Glut9, Hrpt1,Xod and β-actin were shown in Table S1. The melting curve was used to check the specificity of the primers.
2.9 Determination of proteins
The expression of XOD, HPRT1 and ABCG2 were measured by ELISA kit (Meimian Technology, Yancheng, China) according to manufactures’ protocols. The absorbance was detected using a microplate reader at 450 nm. 100 µL RIPK was added to every plate to lyse cells and intracellular proteins were harvest from supernatant after centrifugation at 4 000 ×g.
2.10 Determination of ABCG2 and GLUT9 by immunocytochemistry
Pre-set a 50 mm × 50 mm coverslip in every well in the 6-well plates and the cell were then cultured in the plates for 24 h at 37 °C.The groups were divided as described in section 2.9 and cell on the coverslip was fixed in the 4% paraformaldehyde for 10 min. Cells were treated in 3% H2O2for 10 min to scavenging peroxide and blocked non-specific site by normal goat serum for 1 h at 37 °C in dark. Slides were incubated with primary antibody overnight at 4 °C. This was then detected by incubation with horseradish peroxidase-tagged antibody followed by color development using DAB as chromogen. Stained slides were photographed in bright-field microscope (NIKON ECLIPSE 80i, Nikon Co., Tokyo, Japan) and evaluated by mean optical density (MOD) by imagej as IHC scores.
2.11 Determination of ABCG2 by fluorescence immunocytochemistry
Pre-set a 50 mm × 50 mm coverslip in every well in the 6-well plates and the cell were then cultured in the plates for 24 h at 37 °C.The groups were divided as section 2.9 described and cell on the coverslip was fixed in the 4% paraformaldehyde for 10 min. Cells were blocked non-specific site by normal goat serum for 1 h at 37 °C in dark. Slides were incubated with primary antibody overnight at 4 °C. This was then detected by incubation with Fitc-tagged antibody,DAPI and Tubulin-Tracker Red as chromogen for 10 min and photographed in fluorescence microscope (NIKON ECLIPSE 80i,Nikon Co., Tokyo, Japan).
2.12 Animal experiments designed
The animal experiments were carried out according to regulations controlled by Laboratory Animal’s Research Centre of Nanjing Agriculture University (Nanjing, China). All experimental protocols were approved by Ethics Committee of Nanjing Agriculture University (Nanjing, China, Approval number: NJAU.No. 20210311012).
Four-week old male Kunming mice were purchased from Yangzhou University (Jiangsu, China) and all animals were kept under specific pathogen-free conditions. Mice were housed normally for 7 days to allow environmental adaptation, illuminated with artificial light for 12 h every day (06:00-18:00 light cycle,18:00-06:00 dark cycle), fed with standard laboratory food, and allowed to drink water freely. The environment temperature was 25 °C and relative humidity was 60%. After the acclimation period(7 days), 30 mice were distributed into 3 groups of 10 mice each:control group (C), inosine-induced hyperuricemia model group (M),L. rhamnosusFmb14 administration group (Fmb14). Sample size was based on availability of animals at the facility and every 5 mice were fed in one cage.
L. rhamnosusFmb14 were grown in MRS liquid medium (20 g glucose, 10 g beef extract, 10 g peptone, 5 g yeast powder, 5 g sodium acetate, 0.25 g manganese sulphate, 0.58 g magnesiumsulphate,2 g ammonium citrate dibasic, 2 g dipotassium phosphate, 1 mL of Tween 80 and water to 1 000 mL) at 37 °C for 16 h under anaerobic conditions. Next, the medium was washed with saline and the number of viable bacteria was adjusted to 109CFU/mL to obtain suspensions of the probiotic strains. To elicit hyperuricemia, the mice of control and Fmb14 groups were orally administrated inosine(0.25 mg/(day·100 g body weight) and potassium oxonate(0.35 mg/(day·100 g body weight) dissolved in carboxymethylcellulose sodium (CMS). Mice in Fmb14 groups were administrated 100 µLL. rhamnosusFmb14 dissolved in saline with concentrations at 109CFU/mL while control and model group received saline solution only. All mice were received CMS and saline alone.
2.13 Evaluation of serum and urine index
To verify the effect ofL. rhamnosusin hyperuricemia mice,experiments were carried out for 8 weeks. Blood and urine samples were collected after 12 h fasted on week 8 for UA, CRE and BUN analyses. Specifically, 50 mL of blood was collected from the tail vein of each animal and the serum was obtained by centrifugation at 1 350 ×gfor 5 min. Urine were collected in beakers and then transferred to a tube for centrifugation and then urine supernatant were obtained.
2.14 Determination of colonized L. rhamnosus Fmb14 in faeces
Feces were collected on week 8 forL. rhamnosusFmb14 colonization detected. The determination ofL. rhamnosusFmb14 was performed using qPCR method modified as report in literatures.The primers sequences for qPCR are listed:L. rhamnosus-F:GACGCAGCCGGTTGACCCA,L. rhamnosus-R: GGCGGCAGTT GCCCCAGAAT, Bacteria-27F: AGAGTTTGATCCTGGCTCA,Bacteria-1492R: GGTTACCTTGTTACGACTT. Mouse fecal DNA was extracted according to the manufacturer’s instructions using Stool DNA Kit (Solarbio Science & Technology Co., Ltd., Beijing, China).The DNA of FMB14 cultured in MRS for 24 h was used as reference.qPCR was operated with HieffTMSYBR Green Master Mix (Yeasen Biotech Co., Ltd., Shanghai, China) and a RT-PCR detection (Applied Biosystem, Carlsbad, USA) at the following cycling conditions: 95 °C for 2 min, followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s and 72 °C for 30 s. The melting curve was also done from 65 °C to 95 °C with 0.5 °C/s increments to exclude unspecific amplification after the last reaction. The relative expression levels in terms of fold changes of target genes were calculated by 2-ΔΔCTmethod. All measurements were performed in sextuplicate.
2.15 Statistical analysis
Statistical analysis was performed using SPSS software (Version 16.0, SPSS Inc., Chicago, USA). Results were expressed as mean ± SD and significance was controlled by one-way analysis of variance(ANOVA) and multiple comparisons of graphs. The graphs were calculated by GraphPad Prism 8.0.1.244 (GraphPad software Inc.,CA, USA) andP< 0.05 indicated statistical significance.
3. Results
3.1 Isolation and identification of inosine degradation strains
To obtain more highly efficient probiotics to ameliorate hyperuricemia, we first isolated 75 lactic acid bacteria from different Chinese traditionally fermented foods through MRS medium. The inosine degradation ability of the isolates was screened through growth experiments on single inosine plates and HPLC tests (the calibration curves are shown in Fig. S1)in vitro. The superior strains for inosine degradation were identified asL. rhamnosusthrough physiological biochemistry and 16S rRNA sequence similarity, and we named itL. rhamnosusFmb14 (accession number MZ707550).L. rhamnosusFmb14 were preserved in the China Center for Type Culture Collection (CCTCC, Wuhan, China; preservation number:M 20211270).
3.2 Characterization of the inosine degradation of L. rhamnosus Fmb14 in vitro.
Live bacteria, CFE and fermentation supernatant were added to a sterile PBS (pH 7.2) solution containing inosine (500 µmol/L) for cultivationin vitroto analyse the inosine degradation characterization ofL. rhamnosusFmb14. We found that both CFE and live bacteria could degrade inosine, while the fermentation supernatant did not have this degradation ability (Fig. 1A). The results showed that the live bacteria (107CFU/mL) could degrade 100% inosine to hypoxanthine (88.4%) and some other compounds (11.6%) within 3 h. The greatest degradation rate of inosine was observed after 48 h of cultivation (Fig. 1B). As presented in Figs. 1C and D, the inosine degradation rate increased based on the different concentrations ofL. rhamnosusFmb14, while the inosine concentrations showed no significant decreases. According to these results, the final degradation ability ofL. rhamnosusFmb14in vitrowas tested, and Figs. 1E and F show thatlive bacteria can degrade inosine from 506.9 µmol/L to 322.0 µmol/L hypoxanthine at 109CFU/mL for 6 h. The 36.3%degradation rate indicates the potential ofL. rhamnosusFmb14 for hyperuricemia prevention.
In addition, the effect of CFE-1 (CFE ofL. rhamnosusFmb14 cultured in MRS broth medium) on inosine degradation was observed through a 72 h time course determination, and we found that an unsteady process occurred (Fig. 1G). Then, CFE-2 ofL. rhamnosusFmb14was obtained after inosine treatment for 24 h by ultrasonication, and the results showed that the degradation rate of CFE-2 could reach 21.7% after 3 h under steady conditions (Fig. 1H).
3.3 Mechanisms of L. rhamnosus Fmb14 on inosine degradation
Intracellular compounds ofL. rhamnosusFmb14 were first determined by HPLC, and we found no significant inosine and hypoxanthine accumulation in cells, and the number of intracellular peaks increased from 7 to 15 after inosine treatment (Fig. S2).Then, LC-QQQ-MS was used to detect purine-related intermediate metabolites (Table S2), and the ion chromatograms of CFE-1 and CFE-2 on MRMm/z440 (folic acid) andm/z375 (riboflavin) were shown in Fig. S3. Folic acid and riboflavin were confirmed through characteristic ions by secondary mass spectrometry (Fig. 2), and both vitamins were found to be increased 6.0-fold (folic acid) and 4.3-fold(riboflavin) after inosine treatment (Table 2).
3.4 Effects of CFE, inosine and UA on cell viability
HepG2 and Caco2 cells were chosen as UA synthesis and secretion models induced by inosine and UA, respectively. The effects of different concentrations of inosine and UA on cell viability were tested by MTT assays, and the results showed that the addition of inosine and UA did not affect the cell viability of Caco2 cells after 24 and 48 h (Figs. 3G and H). UA treatment had an effect on HepG2 cells for 24 and 48 h (Figs. 3E and F), but inosine showed no cytotoxicity against HepG2 cells at 24 and 48 h (Figs. 3E and F).Then, inosine induction was chosen as the UA synthesis model for HepG2 cells, and UA induction of Caco2 cells was chosen as the UA secretion model. For the apoptosis of HepG2 cells induced by UA, the effect of CFE on apoptosis was deeply researched in another article.
The effects of CFE-1, CFE-2 and the supernatant ofL. rhamnosusFmb14on cell viability were evaluated in HepG2 and Caco2 cells.The results showed both CFEs and supernatant had no cytotoxicity on HepG2 (Fig. 3A) or Caco2 (Fig. 3C) for 24 h, and for the consumption of nutrients, the cell viability of the treated group showed a significant decrease (P< 0.05) relative to the control but no significant difference(P> 0.05) from the PBS treated group (Fig. 3) for both HepG2(Fig. 3B) and Caco2 (Fig. 3D) for 48 h. To ensure the safety ofthe CFE treatment, the effect of CFE on the hyperuricemia models induced by inosine and UA was evaluated.

Fig. 1 The effect of L. rhamnosus Fmb14 on purine degradation in vitro. (A) Different inosine degradation efficiencies of live bacteria, CFE and supernatant.(B) Effect of L. rhamnosus Fmb14 on inosine degradation for 72 h. (C) Effect of different concentrations of substrates on the inosine degradation rate after 48 h. (D) Effect of different concentrations of L. rhamnosus Fmb14 on the inosine degradation rate after 48 h. (E) Time course of L. rhamnosus Fmb14 on inosine degradation at 109 CFU/mL in 500 µmol/L inosine. (F) The degradation rate of inosine by L. rhamnosus Fmb14. (G) Effect of CFE-1 on inosine degradation for 72 h. (H) Effect of CFE-2 on inosine degradation for 24 h. The mean ± SD (n > 3 per group) was represented by bars, and different lowercase letters represent significant differences at P < 0.05.

Fig. 2 Secondary mass spectrometry chromatogram and structure of the characteristic ion peak on MRM m/z 440 and m/z 375. (A) MRM on m/z 440(folic acid). (B) MRM on m/z 375 (riboflavin).
3.5 Effects of CFE-2 on UA biosynthesis in vitro
Inosine induction led to an increase in XOD, which is the most dangerous signal of hyperuricemia, but the addition of CFE-2 reduced XOD expression at both the mRNA and protein levels(Figs. 4A and C) in the HepG2 and Caco2 models. CFE-2 treatment also enhanced the expression of HPRT1 at both the mRNA and protein levels (Figs. 4B and D), which means thatL. rhamnosusFmb14 effectively regulates the host energy system in terms of purine metabolism dysbiosis.
3.6 Effects of CFE-2 on the UA secretion in vitro
The most important transporter of UA secretion was ABCG2,
which was upregulated at the mRNA level but it showed no significant
difference at the protein level, as detected by ELISA kit (Fig. 4E).The inconsistent expression levels of ABCG2 at the mRNA and protein levels may be because ABCG2 is mainly located in the cell membrane, while the RIPK lysate and a centrifuge cannot separate the protein from the cell membrane. Therefore, immunocytochemistry and fluorescent immunocytochemistry of ABCG2 were performed,as shown in Fig. 5A. Our hypothesis was proven since ABCG2 was expressed on the cell membrane of Caco2 cells and the addition of CFE-2 increased its biosynthesis (Fig. 5C). The mRNA expression ofGLUT9was not significantly changed in the models, but the immunocytochemistry results showed that UA induction enhanced the reabsorption of UA in Caco2 cells and that CFE-2 treatment
significantly reduced adverse transport (Fig. 5B).
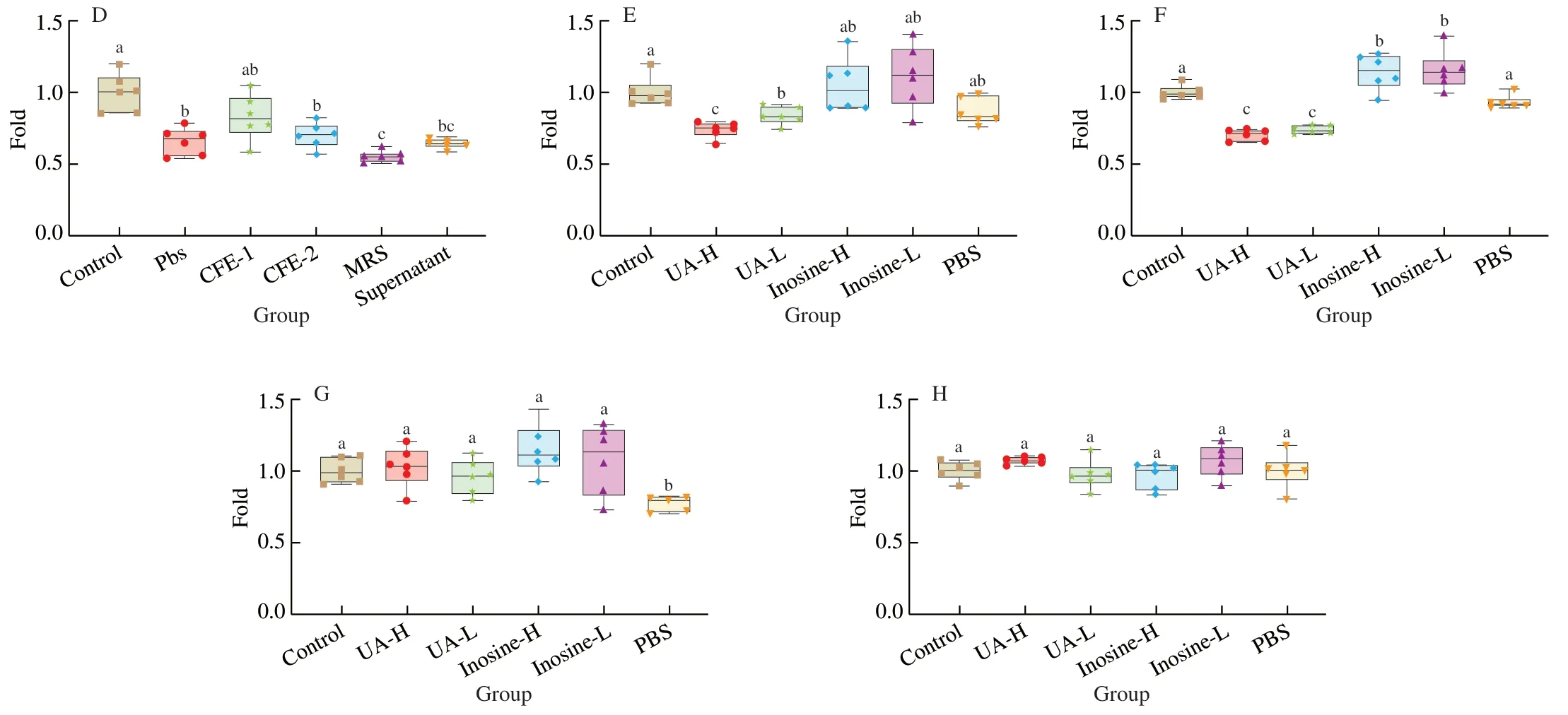
Fig. 3 (Continued)
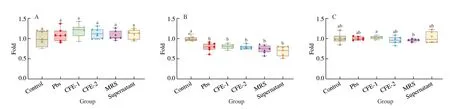
Fig. 3 Effects of CFE of L. rhamnosus Fmb14 treatment on cell viability and the safety of hyperuricaemia models. (A, B) The effect of CFEs and supernatant on the viability of HepG2 cells after 24 (A) and 48 h (B). (C, D) The effect of CFEs and supernatant on the viability of Caco2 cells after 24 (C) and 48 h (D).(E, F) The effect of different concentrations of UA and inosine stimulation on the viability of HepG2 cells after 24 (E) and 48 h (F). (G, H) The effect of different concentrations of UA and inosine stimulation on the viability of Caco2 cells after 24 (G) and 48 h (H). The mean ± SD (n > 6 per group) was represented by bars,and different lowercase letters represent significant differences at P < 0.05.
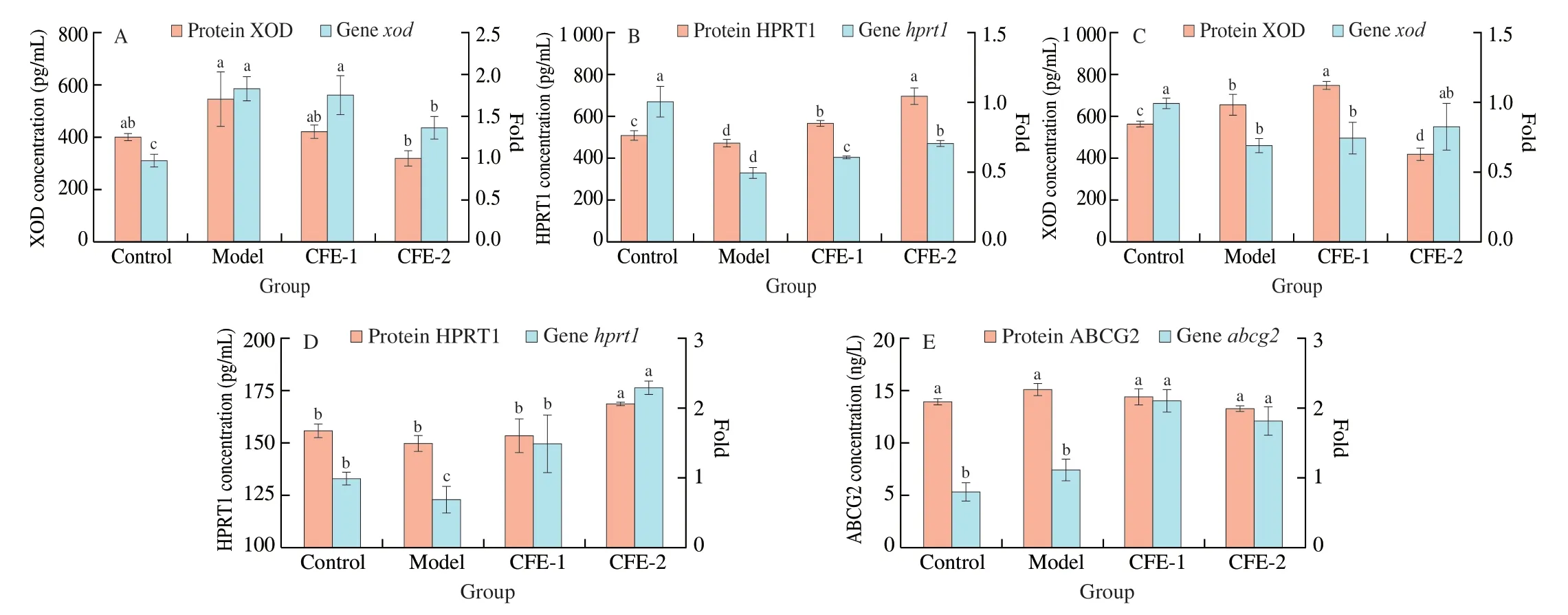
Fig. 4 Effects of CFE treatment on biomarkers of hyperuricaemia on gene and protein expression. (A, B) The effect of CFE treatment on XOD (A) and HPRT1(B) expression in HepG2 cells after 24 h. (C, D) The effect of CFE treatment on XOD (C) and HPRT1 (D) expression in Caco2 cells after 24 h. (E) The effect of CFE treatment on ABCG2 expression in Caco2 cells after 24 h. The mean ± SD (n > 3 per group) was represented by bars, and different lowercase letters represent significant differences at P < 0.05.
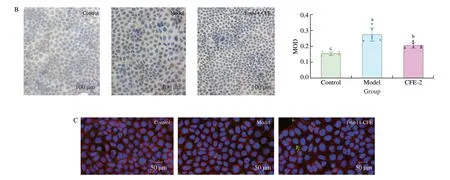
Fig. 5 (Continued)
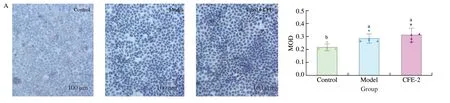
Fig. 5 Effect of CFE treatment on ABCG2 and GLUT9 expression in Caco2. The immunocytochemistry image of (A) ABCG2, (B) GLUT9, (C) ABCG2. Blue represents nuclei in A, B and C, brown represent ABCG2 and GLUT9 in A and B, respectively, green represent ABCG2 and red represents microtubules in C. The mean ± SD (n > 3 per group) was represented by bars, and different lowercase letters represent significant differences at P < 0.05.
3.7 Ameliorative effects of L. rhamnosus Fmb14 on chronic inosine-induced hyperuricemia model mice
To verify the ameliorative effect ofL. rhamnosusFmb14 on inosine-induced hyperuricemia mice, experiments were carried out, as shown in Fig. 6A. Instead of injection of potassium oxonate and UA into mice to establish acute hyperuricemia, the proper doses of inosine and potassium oxonate were orally gavaged to the mice for 8 weeks.The results showed that the serum UA levels of the model mice were dramatically elevated from 139.52 µmol/L to 236.28 µmol/L compared to the control group, and the administration of Fmb14 significantly alleviated the UA concentrations to 149.28 µmol/L (Fig. 6C).High-dose inosine oral gavage decreased the body weight of the mice by almost 30% compared to that of the control group, while Fmb14 treatment showed no effect (Fig. 6B). Simultaneously, qPCR results of different groups showed that FMB14 is colonized in the gut of mice (Fig. 6B).

Fig. 6 (Continued)

Fig. 6 Ameliorative effect of L. rhamnosus Fmb14 on hyperuricaemic mice. (A) Experimental chart of Fmb14 in the treatment of hyperuricaemic mice.(B) Relative expression of L. rhamnosus in faeces of each group at week 8. (C-E) The serum levels of uric acid, CRE and BUN in each group. (F-H) The urine levels of uric acid, CRE and BUN in each group. The mean ± SD (n > 6 per group) was represented by bars, and different lowercase letters represent significant differences at P < 0.05.
To analyse the effect of Fmb14 on the hyperuricemia-related metabolic disorders of the mice, the levels of CRE and BUN were tested, and CRE showed a decrease, while BUN showed an increasing trend in the model group. The serum levels of two biomarkers in the Fmb14 group were partially restored from the model to the control levels by 18.5% and 38.4%, respectively (Figs. 6D-E). Meanwhile,the urine concentrations of UA, CRE and BUN were also tested, and the results indicated that all indices were strongly increased in the model group and were reduced in the Fmb14 group (Figs. 6F-H).All of these results indicated that Fmb14 has a positive effect on dietary-induced hyperuricemia amelioration.
4. Discussion
In this study, we evaluated a probioticL. rhamnosusFmb14 isolated from handmade yogurt in Zhangye, Gansu Province,Northwest China. An investigation showed that the incidence of gout and hyperuricemia in eastern areas was higher than that in Northwest China due to high purine dietary habitats [14].In this article, we collected fermented foods from Northwest China to improve the screening efficiency of excellent strains for hyperuricemia amelioration. Many traditional fermented foods,such as milk or liquor, have been proven to be beneficial to health through the intestinal colonization of probiotics or direct fermentation effects [18,19]. Another advantage of fermented food was the safety of the products and probiotics, which have been reported in many literatures [20]. Therefore, lactic acid bacteria isolated from natural traditional fermented food in low-risk hyperuricemia regions are promising for the prevention of hyperuricemia, and our results support this hypothesis.
Hyperuricemia causing gout, which is the most common form of inflammatory arthritis, primarily affects adult men [21].Hyperuricemia means high levels of serum UA, which involves two factors, both exogenous and endogenous [5]. Animal and fish meats have a high content of inosine [6], and excessive intake of these foods may cause hyperuricemia. Therefore, we chose inosine as an inducer to establish a model. The addition of inosine enhanced the process of hyperuricemia by improving XOD expression in HepG2 and Caco2 cells (Figs. 4A and C). Purine-degrading organisms have been reported, but the normal metabolic pathways in probiotics only degrade purine into hypoxanthine under equivalent conditions, which makes its effect on ameliorating hyperuricemia unclear [22]. Our findings regardingL. rhamnosusFmb14 showed that the metabolic flux tended to produce folic acid and riboflavin more than hypoxanthine, which decreased the true inosine content by 36.3% (Fig. 7A). Research on folic acid-producingLactobacillus[23]is consistent with our findings, but it has not been previously considered in hyperuricemia prevention or treatment.
Inosine is a nucleic acid metabolite that may be transformed into hypoxanthine and xanthine as regular metabolic intermediates and finally excreted from the body as UA [24]. The addition ofL. rhamnosusFmb14 as a supplementary foodstuff could prevent high serum UA from occurring due to its high degradation of inosine(Fig. 7A). ATP and GTP possess energy regulation effects in the biometabolic system [25], and diverse microbial communities play a crucial role in these aspects by vitamin synthesis [26]. These previous viewpoints strongly verified our research onL.rhamnosusFmb14 for its protective effect against hyperuricemia (Fig. 7B).
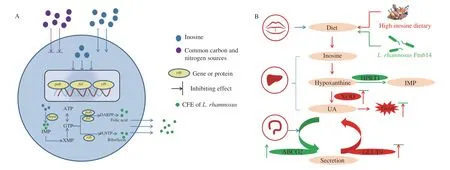
Fig. 7 Possible prevention mechanisms of L. rhamnosus Fmb14 and its CFE treatment against hyperuricaemia. (A) The metabolic changes of L. rhamnosus Fmb14 in inosine-enriched environments. L. rhamnosus Fmb14 cells preferentially use common carbon and nitrogen sources, such as glucose and yeast extracts,and reactions of hypoxanthine to IMP are inhibited. When resource exhaust and excessive inosine enter the intracellular space, inosine is immediately catalysed to hypoxanthine by punA, and substrate induction is activated. The levels of hprt1 were transcribed to produce IMP, which is a precursor of alkaloids (folic acid and riboflavin) and ATP. (B) Once a high purine diet was presented, the balance of purine metabolism was broken down by the high expression of XOD and low HRPT1 in the liver. The addition of L. rhamnosus Fmb14 could decrease the XOD in the liver by competitive absorption of inosine by enterocytes. The highly regulated expression of HPRT1 could benefit the host energy metabolism system by promoting the utilization rate of purines. Moreover, the colonization of L. rhamnosus Fmb14 induced the promotion of ABCG2 and inhibition of GLUT9, both of which increased the secretion of UA. Red arrows represent the effects of a high inosine diet induction. Green arrows represent the ameliorative effects of L. rhamnosus Fmb14 in hyperuricemias.
Lactobacillusis a common beneficial strain colonizing the human gut, andL.reuteriTSR332 has been reported to assimilate 90% inosine [27]. Purine degradation efficiency is strain-specific,but catalysing inosine to hypoxanthine or xanthine cannot prevent hyperuricemia [28].L.rhamnosusFmb14 could use inosine as a carbon and nitrogen source to maintain the biological activity against purines, which makes it superior in relieving hyperuricemia(Fig. 7A). Probiotics have been reported to produce folic acid, such asL.plantarum,L.caseiandL.acidophilus[23,29], but the mechanisms of these microorganisms in hyperuricemia prevention have not been fully researched.L.rhamnosusFmb14 survived in high inosine concentrations by activating ATP-GTP biosynthesis, which has a regulatory effect on host energy metabolism (Fig. 7A). Our results showed that the compensating metabolic pathway ofL.rhamnosusFmb14 activated by inosine is probably beneficial for hyperuricemia prevention and energy regulation when individuals are consuming a high inosine diet (Fig. 7B).
The biological metabolism of folate and riboflavin is complex but is very close to purine metabolism [30], and this biosynthesis process is mainly regulated byfolandribfamilies [31]. As the compensated metabolic pathways activated by inosine, the CFE ofL.rhamnosusFmb14 may contain not only the vitamins we found but also all related enzymes (Fig. 7A), which would be the main functional ingredient to prevent hyperuricemia. CFEs of probiotics are increasingly being found to play an important role in the prevention and treatment of some metabolic diseases [17,32]. We observed that CFE-2 treatment did not have an adverse effect on cells (Fig. 3) and it ameliorated hyperuricemia by XOD, GLUT9 inhibition and HPRT1 and ABCG2 promotion at the protein level (Figs. 4 and 5). Purine metabolism is commonly considered to be conducted in the liver, but our results indicate that UA synthesis was started immediately once inosine digestion was completed in the intestine (Fig. 4). ABCG2 is responsible for the transmembrane transport of UA from the serum to the intestinal cavity and then excretion in the faeces [33], and our findings support that CFE-2 treatment promotes this process (Fig. 5).
In addition to ABCG2, GLUT9 is an important UA reabsorption transporter in the intestine [15,34], and CFE-2 inhibits its biosynthesis(Fig. 5B). Moreover, CFE-2 treatment modulated the tight junction damage by increasingzo,oclnandcgngene expression in high UA model (Fig. S4). As we described above, the CFE ofL.rhamnosusFmb14 induced by inosine may contain enzymes and growth factors that regulate metabolic dysbiosis mediated by UA (Fig. 7B). This conclusion is consistent with some previous studies onL.ingluviei[35].
Mechanism of probiotics ameliorate hyperuricemia partly including 3 pathway including competitive assimilate purine with host, reduce UA biosynthesis and increase UA excretion. In our study,the findings indicated thatL.rhamnosusFmb14 could decrease UA biosynthesis through inosine assimilation (Fig. 1), XOD inhibition(Fig. 4A) and HPRT promotion (Fig. 4B), as well increase UA excretion through ABCG2 promotion (Figs. 5A and C) and GLUT9 inhibition (Fig. 5B). Probiotics take responsibility to optimize the metabolism of host after colonization and proliferation in the gut. LiveL.rhamnosusFmb14 contributes to reduce UA concentrations by assimilate purine in the diet. On the other hand,L.rhamnosusFmb14 could release CFE to enteric cavity to inhibit XOD and increase ABCG2, which decrease UA levels as synergistic effect with liveL.rhamnosusFmb14 and that explained why the effect ofL.rhamnosusFmb14 on purine induced mice higher thaninvitro.
The establishment of an animal hyperuricemia model is challenging because commonly used laboratory animals, such as mice and rabbits, can express urate oxidase to degrade UA [36]. Our work used potassium oxonate to block the activity of uricase and a high inosine diet to increase UA biosynthesis, and the results confirmed that the method was effective (Fig. 6C). Previously, research on hyperuricemia focused on the acute model, which was mostly developed by the direct injection of potassium oxonate and UA [14].Some approaches to modelling hyperuricemia by high fructose or yeast addition have also been discussed, and the therapeutic effect in previous work was also explored in an acute model [24,37]. Probiotics could only come into effect by colonized in the gut (Fig. 6B) and our results indicated that FMB14 showed good ability in gut colonization and proliferation. In addition to the role of inosine assimilation, high efficient colonization of probiotics also modulate gut microbiota dysbiosis and enhance gut barrier damage, both of which induced by hyperuricemia [24].
Our aims in the chronic hyperuricemia model induced by a high inosine diet and the reduced effect ofL.rhamnosusFmb14 on serum levels of UA in model mice proved our hypothesis (Fig. 6C). The ameliorated effect of FMB14 on serum UA levels reduced 36.8%(from 236.28 µmol/L to 149.28 µmol/L) compared to model group(Fig. 6C). Even the efficient of FMB14invitroandinvivois similar,but considerate the synergistic effect of FMB14 in gut, the actual efficient of FMB14 on purine assimilationinvivomay lower than the theory. This conclusion of FMB14 is consistent to the research ofL.reuteriTSR332 (61.5%),L.fermentumTSF331 (28.4%) andL.amylovorusLa322 (7.0%) on UA degradation for 7 days, but the ability ofL.reuteriTSR332 on UA reducing decreased from 61.5% to 34.6% after 14 days, which means probiotic effects would be affected in the chronic hyperuricemia model [27]. Our results indicated that FMB14 intervention keeps a high degradation rate for 8 weeks model and the longer term effects of FMB14 are also in proceeding. Moreover,L.gasseriPA-3 was found reduce almost 15% absorption of inosine and hypoxanthine in rats which could ameliorate hyperuricemia [22].In a UA oral experiments,LimosilactobacillusfermentumJL-3 was found to decrease 31.3% serum UA levels in mice after 14 days feed [14]. Besides the probiotic direct treatment, the effect of microbial fermented extracts on hyperuricemia has also been determined and decrease rate is below 30% [38]. The disordered serum levels of BUN and CRE indicated that chronic hyperuricemia might damage the metabolism under asymptomatic conditions, which has been reported in many studies [14,15,27,37]. The consistent trends of UA,BUN and CRE contents in urine indicated that kidney dysfunction did not appear until after 8 weeks of hyperuricemia (Figs. 6F-H)so it is worth to extend the model to further explore the risks of long-term hyperuricemia. Compared to the previous researches,FMB14 is prominent on chronic hyperuricemia induced by purine diet and more specific mechanism of Fmb14 on amelioration would explore in the future.
Based on the above experimental results, we found that the mechanism ofL.rhamnosusFmb14 against UA accumulation was its special purine-degrading ability against folate and riboflavin. Second,CFE of inosine-inducedL.rhamnosusFmb14 could also promote the metabolism in both hepatocytes and enterocytes to enhance the decrease in UA. Finally,L.rhamnosusFmb14 intervention in an inosine diet-induced chronic hyperuricemia model was proven to be effective and confirmed our hypothesisinvitro.L.rhamnosusFmb14 is a promising safe bioresource to provide us with a food method due to its special compensation of vitamin production pathways to prevent and ameliorate hyperuricemia.
5. Conclusions
In conclusion,L.rhamnosusFmb14 was isolated from handmade yogurt as a potential probiotic to ameliorate hyperuricemia. The effect ofL.rhamnosusFmb14 treatment on the inosine degradation rate reached 36.3% after 24 hinvitro. The compensatory metabolic pathway ofL.rhamnosusFmb14 to produce folic acid and riboflavin was activated with high concentrations of inosine. We demonstrated that CFE-2 treatment could reduce UA biosynthesis by decreasing XOD and increasing HPRT1 in inosine-induced HepG2 cells. In addition, the effects of CFE-2 treatment on the UA transporters ABCG2 and GLUT9 were both found to be promoted in UA-induced Caco2 cells. Based on our results,L.rhamnosusFmb14 is a promising dietary method to ameliorate hyperuricemia by decreasing the digestive absorption of inosine, reducing the biosynthesis of XOD in both hepatocytes and enterocytes, and enhancing the secretion of UA by promoting ABCG2 and inhibiting GLUT9. The biological therapeutic effectinvivodemonstrated that the level of serum UA was decreased 36.8% after oral administration ofL.rhamnosusfor 8 weeks and that metabolic dysbiosis caused by hyperuricemia internal circulation was restored. The specific and in-depth mechanisms ofL.rhamnosusFmb14 in hyperuricemiainvivowere investigated.
Conflictofinterest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work.
Acknowledgements
Grant support was received from the National Natural Science Foundation of China (32072182).
AppendixA.Supplementarydata
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.10.031.
杂志排行
食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents
