Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
2023-01-03LiTinRuixingZhoXinyiXuZhiwiZhouXiofngXuDongmiLuoZhiqingZhouYuLiuArilKushmroRobrtMrksAnrDinnyQunSun
Li Tin, Ruixing Zho, Xinyi Xu, Zhiwi Zhou, Xiofng Xu, Dongmi Luo,Zhiqing Zhou, Yu Liu, Aril Kushmro, Robrt S. Mrks, Anrás Dinnyés,f,g,h, Qun Sun,,*
a
Key Laboratory of Bio-Resource and Eco-Environment of the Ministry of Education, College of Life Sciences, Sichuan University, Chengdu 610064, China
b Institute for Marine and Antarctic Studies, University of Tasmania, Newnham 7248, Australia
c College of Biomass Science and Engineering, Sichuan University, Chengdu 610064, China
d Department of Urology, Institute of Urology (Laboratory of Reconstructive Urology), West China Hospital, Sichuan University, Chengdu 610041, China
e Avram and Stella Goldstein-Goren, Department of Biotechnology Engineering, Faculty of Engineering Sciences, Ben Gurion University of the Negev, Beer-Sheva 84105, Israel
f BioTalentum Ltd., Gödöllő 2100, Hungary
g Department of Cell Biology and Molecular Medicine, University of Szeged, Szeged H-6720, Hungary
h Hungarian University of Agriculture and Life Sciences, Institute of Physiology and Animal Nutrition, Department of Physiology and Animal Health, Gödöllő 2100, Hungary
Keywords:Lactiplantibacillus plantarum Diabetes Obesity Non-alcoholic fatty liver disease Kidney stone disease Cardiovascular diseases
A B S T R A C T The increased global incidence of chronic metabolic diseases, a vital threat to human health and a burden on our healthcare systems, includes a series of clinical metabolic syndromes such as obesity, diabetes,hypertension, and dyslipidemia. One of the well-known probiotic microorganisms, Lactiplantibacillus plantarum plays an important role in promoting human health, including inhibiting the occurrence and development of a variety of chronic metabolic diseases. The present study provides an overview of the preventive and therapeutic effects of L. plantarum on diabetes, obesity, non-alcoholic fatty liver disease,kidney stone disease, and cardiovascular diseases in animal models and human clinical trials. Ingesting L. plantarum demonstrated its ability to reduce inflammatory and oxidative stress levels by regulating the production of cytokines and short-chain fatty acids (SCFAs), the activity of antioxidant enzymes, and the balance of intestinal microbial communities to alleviate the symptoms of chronic metabolic diseases.Furthermore, updated applications and technologies of L. plantarum in food and biopharmaceutical industries are also discussed. Understanding the characteristics and functions of L. plantarum will guide the development of related probiotic products and explore the modulatory benefit of L. plantarum supplementations on the prevention and treatment of multiple chronic metabolic diseases.
1. Introduction
Lactic acid bacteria (LAB) are non-spore-forming, Grampositive bacteria, which exist widely in the environment, especially in various fermented foods. As one of the important probiotics, LAB has a plethora of benefits, including regulating intestinal m icrobial community balance, enhancing biological barriers, participating in immune system regulation, lowering cholesterol, and lowering blood pressure [1]. Within the LAB community,Lactobacillusplantarumis a facultative heterofermentative member that is generally regarded as safe (GRAS) and widely used as a vital element of fermenters in food production [2].L.plantarumalso has been shown to prevent and treat upper respiratory tract infections by inducing immunomodulatory and anti-inf lammatory effects while promoting mucosal barrier integrity and actions of NK cells [3]. A fter the outbreak of the Corona Virus Disease 2019 (COVID-2019),L.plantarumwas found to have a potential role in fighting against it [4,5]. Recent studies showed that the recombinant spike (S) protein was stably and effectively expressed inL.plantarumsystem by constructing genetically engineered bacteria [4]. Moreover, the combination ofL.plantarumGUANKE (LPG) and SARS-CoV-2 vaccine may enhance the signal transduction of interferon through the gut-lung and gut-spleen axes as well as inhibit cell apoptosis and inflammatory pathway, as the specific neutralization antibody was increased by more than 8-fold in bronchoalveolar lavage (BAL) and 2-fold in serum after SARSCoV-2 vaccine inoculation [5].
Several studies showed thatL.plantarumplayed an important role in regulating human blood pressure, blood lipid, blood sugar balance, and functions of the intestinal tract, nervous systems and immunity [6-10]. Therefore, it has been widely applied in the treatment of inflammatory, psychiatric, and chronic metabolic diseases. The number of patients with chronic metabolic diseases is huge and growing rapidly, posing a greater threat to public health. Overall, hyperlipidemia, hypertension, hyperglycemia and hypercholesterolemia contributed to a 20% global health loss in 2019,nearly double what it was 20 years beforehand and now representing a leading cause of death worldwide [11]. However, due to the complex pathogenesis and limited effectiveness of treatments, a large number of patients are challenging to cure, thus escalating the burden of medical care. Therefore, it is important to find effective methods to prevent and treat chronic metabolic diseases.
The present study reviews the discoveries made in the last five years regarding the role ofL.plantarumin the regulation of chronic metabolic diseases, with special emphasis on diabetes, obesity, kidney stones, non-alcoholic fatty liver, and cardiovascular diseases as the primary outcome, and this is expected to lay the foundation for future in-depth studies.
2. Modulation of chronic metabolic diseases by L. plantarum
2.1 Diabetes
Diabetes is a chronic metabolic disease associated with insulin abnormalities due to inadequate insulin secretion or ineffective use of insulin. According to the data of the International Diabetes Federation(https://www.diabetesatlas.org), the number of adults with diabetes worldwide reaches 537 000 000 (10.5%) in 2021. The number of people with diabetes increased by 740 000 (16%) compared to 2019.Type 2 diabetes mellitus (T2DM) accounts for over 90% of diabetes and is mainly characterized by insulin resistance and relative insulin deficiency [12]. T2DM is currently being treated primarily with lifestyle changes, insulin injections and medication to alleviate insulin resistance [13]. However, current treatment approaches do not always achieve adequate metabolic control, and many patients do not get a satisfactory response. Probiotic therapy is now gaining more attention as a potential supplementary strategy to drug therapy.
Conventionally,in vivostudies emphasized the efficacy ofL.plantarumstrains on diabetic parameters. Most studies focused on the effect ofL.plantarumon the inhibition of enzymes related to glucose metabolism (α-glucosidase orα-amylase), which alleviates the pathological indicators associated with diabetes (Table 1).L.plantarumSS18-5, which was isolated fromL.plantarumGS18 after mutagenesis in space, had high anti-α-glucosidase activities [14].In the high-fat and streptozotocin-induced T2DM rat model,oral administration ofL.plantarumSS18-5 was found to control body weight, reduce fasting blood glucose, glycated hemoglobin and insulin levels, and increase liver glycogen levels [14].L.plantarumCCFM0236 fermentation supernatant could significantly inhibitα-glucosidase activityin vitro[15]. Oral administration ofL.plantarumCCFM0236 could not only reduce the food intake and the levels of blood glucose, glycosylated hemoglobin and leptin level,but also regulate the insulin level, AUCglucoseand HOMA-IR index in diabetic mice [15].L.plantarumProbio-093 significantly inhibited bothα-glucosidase andα-amylase enzymatic activities in anin vitroassay (P< 0.05) [16]. In mice fed with a 45% kcal high-fat diet (HFD),treatment usingL.plantarumProbio-093 significantly reduced body weight (P< 0.05) and improved blood glucose levels (P< 0.05) [16].
Diabetic patients usually exhibit low-grade chronic inflammation associated with elevated levels of circulating pro-inflammatory cytokines.L.plantarumSCS2 exhibited good hypoglycemic and insulinregulating effects by decreasing the levels of MDA and ROS and increasing the levels of antioxidant enzymes in mice with T2DM [17].Regarding diabetes, mice treated withL.plantarumLRCC5310 andL.plantarumLRCC5314 showed that levels of pro-inflammatory cytokines (TNF-α, IL-6) and mRNAs of pro-inflammatory genes(TNF-α,IL-6,Ccl2,leptin) were decreased [18]. These results suggested thatL.plantarumcould inhibit inflammatory responses and suppress stress in diabetic mice.
Alterations in the gut microbiota have recently been recognized as a key factor associated with chronic metabolic diseases (e.g., T2DM) [19].A recent meta-analysis confirmed that alterations in gut microbial composition contributed to the development of insulin resistance in humans [20]. In contrast to healthy individuals, the relative abundance of Firmicutes was reduced and the relative abundance of Bacteroidetes was relatively higher in patients with T2DM [21].Additionally,L.plantarumpMG36e-glucagon-like peptide 1 (GLP-1)caused a substantial reduction in the intestinal pathogenPrevotellaand significant enhancement of butyrate-producingAlistipesgenera [22]. Therefore, ameliorating the gut microbial composition through probiotics (e.g.,L.plantarum) is an important target for the regulation of diabetes. It is worth noting that short-chain fatty acids(SCFAs) produced by intestinal microorganisms play an important role in the regulation of blood glucose in diabetic patients. SCFAs,especially butyric acid, reduce inflammation in adipocytes and improve insulin sensitivity by stimulating the secretion of GLP-1 [23].A previous study showed thatL.plantarumSHY130 ameliorated hyperglycemia in HFD/STZ-induced diabetic mice by regulating the enteroinsular axis [24].L.plantarumFIH treatment readjusted the intestinal flora structure, enhanced the abundance of SCFAproducing bacteria, such asFaecalibaculum,Odoribacter,Alistipes,and increased the levels of SCFAs in diabetic mice (Fig. 1) [24].Oral administration ofL.plantarumHAC01 was found to lower blood glucose, which might be related to its ability to increase the Akkermansiaceae family and increase SCFA in serum. However,fasting plasma glucose, insulin, HOMA-IR, microbial composition,and fecal SCFAs were not significantly altered byL.plantarumHAC01 treatment in a human trial [26]. TheL.plantarumHAC01 treatment clinical trial was the first to examine the effect of a singlestrain probiotic supplement administered in prediabetic subjects. The unsatisfactory results shown that the beneficial regulatory effect ofL.plantarumin animals might not be fully transferable to human.Overall, most studies have shown thatL.plantarumcan alter the composition of the intestinal microflora and increase the content of intestinal metabolites of SCFAs, which promotes pancreatic function and reduces the blood glucose level. The effective dose ofL.plantarumranged 109-1010CFU/mL. It is clear from the hereinabove that although multiple independent and separate studies converge to indicate a positive role in a murine model onL.plantarumeffect in a chronic disease, clearly we are far from understanding an overall mechanism beyond mere observation, thus, explaining the lack of progress in the human adaptation of the said results.
?
?

Fig. 1 Mechanism of the L. plantarum in regulating diabetes [24].
2.2 Obesity
Obesity is the abnormal accumulation of lipids in adipose tissue and can lead to the development of other chronic metabolic diseases such as T2DM, cardiovascular disease, and non-alcoholic fatty acid liver. With the improvement of living standard, worldwide obesity has nearly tripled since 1975, with 39 000 000 children found to be overweight or obese in 2020. Accumulating evidence has revealed thatL.plantarumcould be one of the anti-obesity agents for improving lipid metabolism [27].
An important factor in the regulation of energy expenditure is peroxisome proliferator-activated receptors (PPARs). Probiotics,which activate PPARs expression to accelerate free fatty acid (FA)oxidation, have attracted significant scientific and clinical interest due to their reproducible effects on human obesity (Table 1) [28].L.plantarumFRT10 achieved lipid regulation in obese mice by upregulating hepaticPPARαandCPT1αmRNA expression levels and downregulatingSREBP-1andtDGAT1mRNA expression levels(Fig. 2) [29].L.plantarumKC28 significantly up-regulatedPGC1-αandCPT1-α(P< 0.05) and significantly down-regulatedACOX-1,PPAR-γ(P< 0.001) in a diet-induced obese C57BL/6 mice model [30].Similarly,L.plantarumCQPC01 down-regulatedC/EBP-αandPPARγmRNA expression in obese mice [31].L.plantarumLMT1-48 extract inhibited adipocyte differentiation and lipid accumulation by down-regulating the adipogenic genesPPARγ,C/EBPα,FASandFABP4in 3T3-L1 cells in anin vitrocellular assay [32]. Furthermore,this strain could also downregulate the lipogenic genesPPARγ,HSL,SCD-1, andFAT/CD36in the liver, resulting in the reduction of body weight and fat volume in HFD fed obese mice. Thus, PPARs may be an important target for evaluating the regulatory effect ofL.plantarumon obese patients.

Fig. 2 Mechanism of the L. plantarum in regulating obesity [29].
In 2004, a study using germ-free mice revealed that the gut microbiota played an important role in the pathogenesis of obesity [33].Like in other chronic metabolic diseases, the gut microbiota of obese individuals is characterized by increasing pathogenic species and reducing diversity. The impact of gut microbes on obesity acts via two main avenues. On the one hand, specific gut microbes may help the host digest certain food components [34], whereas on the other hand, there may be a significant change in mucosal protective microbes. Such an effect can be found in individuals with a prolonged high-fat diet that leads to an increase in the ratio of Firmicutes/Bacteroidetes, an increase in lipopolysaccharide-producing bacteria and a decrease in mucosal-protective microbes [35]. It is well documented thatL.plantarumcan inhibit the growth of opportunistic pathogens and promote the proliferation of symbiotic species that can regulate intestinal microbiota composition in obese individuals.Metagenomic analysis of intestinal microbiota in obese mice showed thatL.plantarumHAC01 significantly reduced the abundance of Lachnospiraceae and Ruminococcaceae [36]. The anti-obesity effect ofL.plantarumLB818 was reported to be associated with increased abundance ofAkkermansia,Bifidobacterium,Lactobacillus, and increased the Bacteroidetes/Firmicutes ratio compared to the control [37].L.plantarumFRT10 significantly ameliorated the HFD-induced gut dysbiosis by increasing the abundance ofButyricicoccus,Butyricimonas,Intestinimonas,Odoribacter, andAlistipes, and decreasing the abundance of Desulfovibrionaceae,Roseburia,Lachnoclostridium,Lactobacillus, andBifidobacterium[18].
L.plantarumLC27 treatment reduced HFD-induced Firmicutes and Proteobacteria populations in gut microbiota and fecal lipopolysaccharide production in obese mice [38]. In general, the regulatory effect ofL.plantarumon obesity mainly includes two aspects. On the one hand,L.plantarumcould increase the abundance of probiotics such asAckermania,Bifidobacteriumto improve body metabolism. On the other, it regulated body metabolism by mediating PPARs metabolic pathway. The effective dose ofL.plantarumranged 108-1010CFU/mL. However, the exact molecular mechanisms by which gut microbes regulate obesity are not clear. Further experiments are needed to investigate which metabolites of gut microbes influence host metabolic pathways.
2.3 Kidney stone disease
Kidney stone disease is one of the most common diseases of the urinary system. Calcium oxalate (CaOx) stones are the most common,accounting for about 75%-90% of the cases [39]. The incidence and prevalence of kidney stone disease have increased globally over the past 40 years [40]. Incidences are currently reported to occur in between 5% to 10% in European countries and 1% to 19% in Asian countries [41], with a higher incidence in men than that in women [42].Many people even have reported multiple episodes [43] and eventually leading to kidney failure. The causes of kidney stones are multifactorial, including environment, diet, gender, age, and genetics [44].The mechanisms involved in the formation of kidney stones have not been fully elucidated, as they involve complex physical and biochemical processes. The supersaturation of lithogenic material in urine, the imbalance of renal inhibitors and promoters, the degradation and absorption of oxalic acid in the intestine are all contributing factors [45]. Several chronic metabolic diseases, including metabolic syndrome, hypertension, diabetes, obesity, and arteriosclerosis, are also risk factors for kidney stones [46]. At present, extracorporeal shock-wave lithotripsy, ureteroscopy and percutaneous or burst wave lithotripsy are the main methods to eliminate the stones [47].However, there is no satisfactory drug treatment to cure or prevent the recurrence of stones.
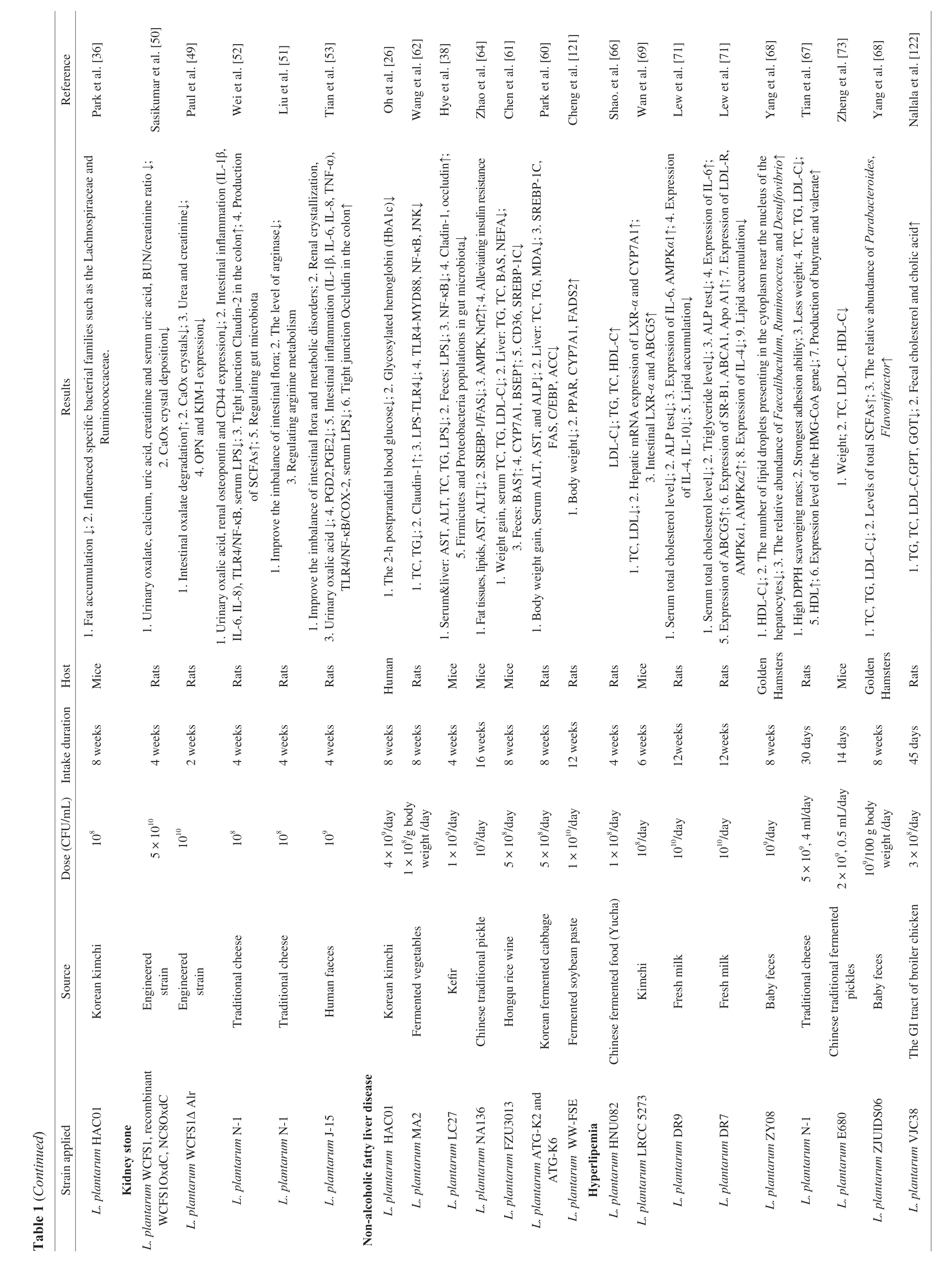
In recent studies, it was shown that LAB not only degraded oxalic acidin vitro, but also reduced uric acid and kidney stonesin vivo[48] (Table 1).L.plantarumWCFS I is one of the most studied LAB strains as its genome sequence is available. Recombinant strains expressed oxalate-degrading activity and secreted allogenic oxalate decarboxylase to degrade oxalate in the intestinal tract of high oxalate-consuming rats and inhibit calcium oxalate stone deposition in the kidney of rats [49,50]. In addition, recent studies showed thatL.plantarumhad the potential to reduce kidney stones [51,52].L.plantarumN-1 was reported to increase the abundance of beneficial SCFAs-producing bacteria and the production of SCFAs,specifically butyric acid. Moreover, its presence in the gut also ameliorated intestinal microbiota disorders, enhanced the intestinal barrier function, and reduced local inflammation by upregulating intestinal tight junction protein, decreasing the expression of cytokines in serum LPS and TLR4/NF-κB signaling pathway [52].L.plantarumN-1 not only promoted SCFAs production in the gut,but also regulated arginine metabolism to reduce urinary oxalate levels, renal osteopontin and CD44 expression, and ultimately renal crystallization [51,52]. In our recent preliminary study,L.plantarumJ-15 [53] was found to reduce the levels of LPS in blood and inflammatory factors in intestine and blood through the bloodgut barrier, and further activate the kidney-gut axis through blood circulation to reduce the level of inflammation in the kidney (Fig. 3) [53].The levels of prostaglandins also decreased in the colon and kidney.Many bile acid metabolism-related substances have changed in the kidney stone group compared to the healthy controls group, which may be one of the future concerns. It can be seen that regulating intestinal flora and metabolic, reducing urinary oxalate is the main role ofL.plantarumin reducing the formation of kidney stones.At present, several studies showed that 108-109CFU/mL was the appropriate dose for non-engineered bacterial supplement, but since there are only a few reports about the optimal dosage for regulating kidney stones, this is still to be evaluated by more investigations.
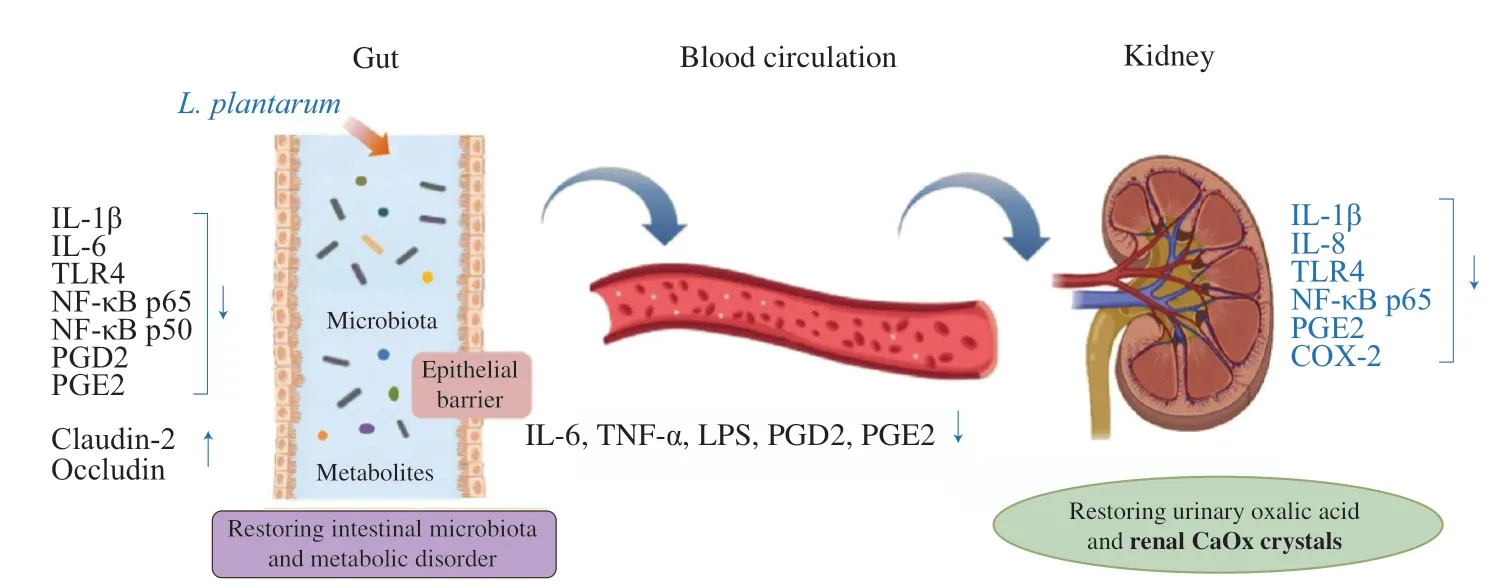
Fig. 3 Mechanism of the L. plantarum in regulating kidney stone [53].
The interactions and metabolic relationships between the host and the gut microbiota are key research areas to be studied further using larger study groups, including clinical studies and using the “omics”technology to explore the mechanism. It is essential to identify the bioactive components and major metabolites ofL.plantarum.Eventually, convertingL.plantaruminto probiotic products for the prevention and treatment of kidney stones is the greatest expectation and challenge.
2.4 Non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) is a common worldwide chronic liver disease that occurs in people of all ages,genders, and races. From 1991 to 2019, the global prevalence of NAFLD increased from 21.9% to 37.3% and has become a widespread concern in recent years [54]. Studies confirmed that NAFLD was closely related to body mass index (BMI), insulin resistance,oxidative stress, dyslipidemia, and genetic susceptibility [55,56].NAFLD presents a range of symptoms, and it can progress from simple fat accumulation to non-alcoholic steatohepatitis (NASH) with hepatic bullae steatosis, associated with inflammation and fibrosis [57].Approximately 2%-7% of NAFLD patients suffer from NASH [56]characterized by hepatitis and liver injury, which may lead to fibrosis [58],cirrhosis, and even hepatocellular carcinoma (HCC) with high morbidity and mortality (Fig. 4) [59]. Long-term exercise, weight reduction, reasonable diet, smoking cessation and alcohol withdrawal can improve or even reverse simple NAFLD to a certain extent.However, there is no quick and effective treatment for NAFLD.L.plantarumisolated from natural or fermented foods has the potential to be developed into an assistant therapy for fatty liver [60]. It has been found that it can improve liver lipid metabolism [60,61], reduce liver injury and inflammation [62], as well as improve the imbalance of intestinal flora [63].L.plantarumwas reported to reduce the content of TC, TG, LDL-C, BAS and NEFA in serum and liver [61].It activates the AMPK/Nrf2 pathway to reduce fat production and increase fatty acid oxidation [64], inhibits lipid synthesis pathway SREBP-1/FAS [60] and the key enzyme of cholesterol synthesis HMGCR [61].L.plantarumalso increases the expression levels of cholesterol 7A-hydroxylase (CYP7A1) and bile salt export pump(BSEP) related to bile acid synthesis and excretion [61].L.plantarumreduces the content of aspartate aminotransferase and alanine aminotransferase in blood and had a certain protective effect on the liver [60]. Meanwhile,L.plantarumreduced the levels of LPS and inflammatory factors in serum, increased the expression of antiinflammatory factors, and reduced liver inflammation by inhibiting TLR4-MYD88 and its downstream NF-κB and JNK pathways [62].Intake ofL.plantarumcould increase the proportion of beneficial intestinal bacteria, reduce the proportion of harmful bacteria thus maintaining intestinal health [62]. Moreover,L. plantalumincreased the expression of intestinal tight junction proteins Claudin-1 and Occludin, enhancing the intestinal barrier function [65].
However, the detailed pathogenesis of NAFLD is not clear.According to previous studies, most of the relevant experiments were carried out using high-fat diet animal models, but this is not the only cause of NAFLD [65]. Wang et al. [62] found thatL.plantarumMA2 significantly improved lipid metabolism, but failed to restore weight in NAFLD rats, which was in contrast to the results of NAFLD animal models on a high-fat diet. Furthermore, it has not been determined whether various positive effects on NAFLD are common to allL.plantarumstrains. Overall, several strains ofL.plantarumfrom different sources have been shown to improve NAFLD by reducing body weight, serum ALT and AST, as well as increasing intestinal SCFAs. Accordingly, it is reasonable to take 108-109CFU/mL doses daily for at least 4 consecutive weeks. In addition, the genome and specific metabolites ofL.plantarumneed to be further studied.
2.5 Hyperlipidemia
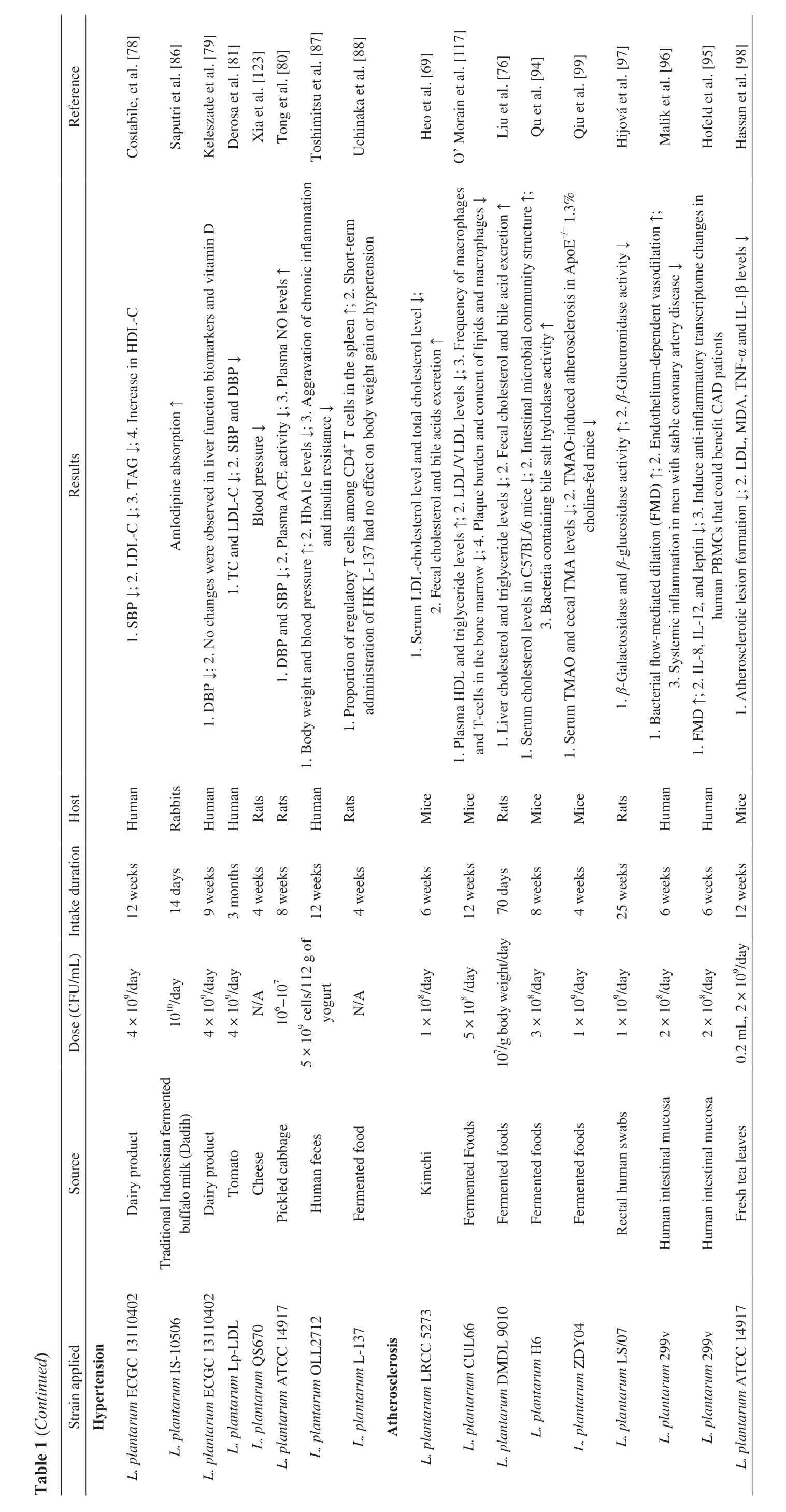
Hyperlipidemia, also known as dyslipidemia, is a chronic metabolic disease characterized by increasing triglyceride (TG) and total cholesterol (TC), increasing low-density lipoprotein cholesterol(LDL-C) and decreasing high-density lipoprotein cholesterol(HDL-C) in plasma [66]. Recent studies found thatL .plantarumcould effectively alleviate hyperlipidemia by reducing the contents of TC, TG, LDL-C and LPS in blood [67], increasing the contents of intestinal SCFAs [67,68], and promoting the excretion of bile acids,organic acids and cholesterol in feces [69] (Table 1). Some studies found thatL.plantarumcould significantly increase the expression levels of LXR-αand CYP7A1, and of hepatic sterol excretion genes of ATP-binding cassette subfamily G members 5 and 8 (ABCG5andABCG8) (Fig. 5) [70].L.plantarumcan up-regulate hepatic energy metabolisms AMPK pathway, while down-regulating the expression of hepatic lipid synthesis andβ-oxidation gene stearoyl-CoA desaturase 1 (SCD1) to alleviate hyperlipidemia [69,71].Additionally, the intake ofL.plantarumcould promote the growth ofBifidobacteria,Lactobacillus,Akkermansia[72] andFaecalibacteriumin the intestinal tract [66]. According to the metabolic pathway analysis of intestinal microorganisms, the synthesis pathways of secondary bile acids and lipopolysaccharide were abundant in the intestinal microflora of hyperlipidemic mice, while those of pyruvate,triglyceride, propionic acid, fatty acids and carotene were abundant in the normalL.plantarum-supplemented mice [66].

Fig. 4 Mechanism of the L. plantarum in regulating non-alcoholic fatty liver disease [59].
Acid resistance, trypsin resistance, bile salt resistance, adhesion to Caco-2 cells, BSH activity and other functional properties can be used as the basis for screening superior strains ofL.plantarum[73].Song et al. [72] compared free and microencapsulatedL.plantarumLIP-1 and found that microencapsulation enhanced colon colonization of LIP-1 cells and improved intestinal microbiota composition and lipid reduction in hyperlipidemic rats. However, the embedding technology for LAB is not mature enough. One of the key issues that needs to be solved in the future, is how to enhance its colonization in the intestinal tract. Current studies have shown thatL.plantarumassists in the treatment of hyperlipidemia by reducing the levels of TC and TG in the blood, improving the structure of intestinal flora and increasing the level of SCFAs. The dosage of 108-1010CFU/(mL·day)seems to be appropriate, and it is better to take continuously for at least 4 weeks to exert its function noticeable. Furthermore, although hyperlipidemia has a good prognosis after comprehensive treatments, it may lead to atherosclerosis or other cardiovascular and cerebrovascular diseases, possible side effects that should not be ignored.
2.6 Hypertension
Hypertension is a typical symptom of cardiovascular disease and is a crucial risk factor for the development of chronic metabolic diseases [74]. Without proper precaution and treatment, chronic hypertension may cause kidney failure, cognitive decline, and dementia [75]. According to the data from the world health organization (WHO), around 1.28 billion adults aged 30-79 years worldwide have hypertension in 2021.L.plantarumhas been shown that this genus has many positive effects on metabolic disorders and cardiovascular diseases [76,77]. In this review, we summarized the research relevant to the application ofL.plantarumon hypertension(Table 1).L.plantarumECGC 13110402, a strain of high bile salt hydrolase activity, was shown to significantly (P< 0.003) reduce the systolic blood pressure (SBP) of mildly hypercholesterolaemic adults [78]. More recently, a study onL.plantarumECGC 13110402 demonstrated that this probiotic strain could also reduce the diastolic blood pressure (DBP) in hypercholesterolaemic adults [79].Furthermore, in randomized placebo-controlled trials, the blood pressure-lowering effect ofL.plantarumhas also been observed using fermented food products [80] and nutraceuticals [81]. Administration ofL.plantarumfermentedAllium hookeriroot (AHR) to spontaneously hypertensive rats (SHRs) decreased DBP and SBP [80].Significant reductions of both DBP and SBP were observed in patients with hypertension after oral administrating nutraceutical containingL.plantarumLp-LDL, arginine, coenzyme Q10 and vitamin B1for 3 months [81]. Besides the positive effects of theL.plantarum,L.plantarumsupplement can also act as an adjuvant agent indirectly inhibiting hypertension. Recent studies revealed thatL.plantarumsupplement improved angiotensin-I-converting enzyme (ACE)inhibitory activities [82-84], enhancedγ-aminobutyric acid (GABA)(Fig. 6) [85], and increased amlodipine absorption [86]. However, the results of relevant studies were not always consistent. In one study,the use ofL.plantarumOLL2712 had significantly increased the blood pressure of active subjects compared to the placebo group [87].An animal study reported that short-term administration of heat-killedL.plantarumL-137 (HK L-137) did not affect body weight gain or hypertension [88]. Due to the strain-specific effect, a particular strain may exert different impacts on hypertension, thus it is recommended to examine each strain separately. It is important to elucidate howL.plantarumalters the structure of gut microbial communities and how this is relevant to hypertension. A better understanding of the host-microbiota-metabolite interactions can contribute to a shift towards the advancement of customized nutritional plans and probiotics that are specifically designed according to bacterial deficiencies. Additionally, the mechanism of the potential antihypertensive effect ofL.plantarumand its protective effect on endothelial function has not been fully understood. Some studies have indicated that hypertension might be associated with an imbalance in the gut microbiota, which indicates that dietary interventions that correct gut microbiota, including probiotics, could be applied as a new therapeutic strategy for hypertension [89,90]. Future studies investigating the functional properties of differentL.plantarumstrains, optimal dosage, appropriate intake, and the underlying molecular mechanisms are recommended to further evaluate the application ofL.plantarumand its relevant products in hypertensive patients.
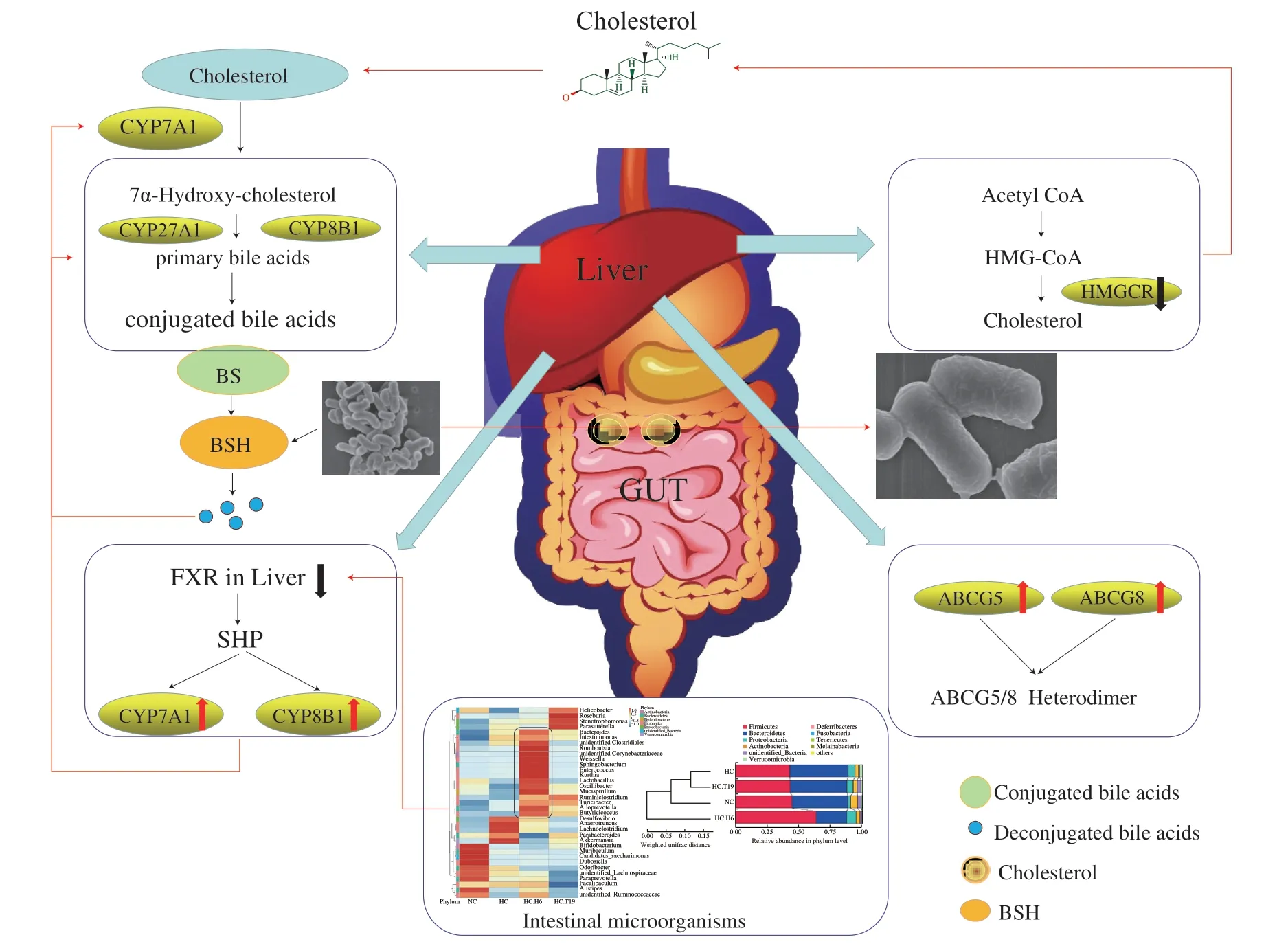
Fig. 5 Mechanism of the L. plantarum in regulating hyperlipidemia [70].
2.7 Atherosclerosis
Atherosclerosis, the potential risk factor of cardiovascular diseases, is leading the global mortality of chronic metabolic diseases. Currently, treatments against atherosclerosis mostly target dyslipidemia associated with the disease, which have a considerable residual risk for cardiovascular disease together with many side effects. One typical outcome of atherosclerosis is inflammation, and its pathological mechanism is complicated. Upgraded levels of lowdensity lipoprotein cholesterol that modulate cell penetrability and affect the blood vessel are directly related to atherosclerosis [91].The inflammatory response is stimulated by a substrate that leads the circulating white blood cells to stick to the endothelial cell surface.This may cause the further attachment of molecules and determinants,promoting monocyte migration to the subendothelial area. Hence,mature monocytes are converted to form macrophages containing engulfed lipid particles, cholesterol, and free fatty acids that are rich in esters, which may then saturate the arterial walls and subsequently lead to the thickening of the arterial wall and increase the possibility to form the necrotic lesion (Fig. 7) [92]. Hypercholesteremia or high cholesterol is one of the key reasons causing atherosclerosis.Due to the multiple benefits of probiotic therapies, interest has increased regarding the use of probiotics as a novel strategy to regulate cholesterol levels and to treat coronary artery disease [93].The bio-therapeutical ability ofL.plantarumon atherosclerosis has been investigated in recent years (Table 1).L.plantarumDMDL 9010 isolated from naturally fermented mustard showed potential reductive effects on total serum cholesterol and LDL-cholesterol levels in Sprague Dawley rats [76].L.plantarumLRCC 5273 andL.plantarumLRCC 5279 isolated from Kimchi were reported to have bile salt hydrolyzing ability on ameliorating serum cholesterol metabolism in hypercholesterolemic C57Bl/6 mice [69]. Likewise, a more recent study conducted on diet-induced hypercholesterolemic C57Bl/6 mice showed thatL.plantarumH6 not only reduced serum cholesterol concentrations, but also optimized the intestinal microbial community by increasing the abundance of high bile salt hydrolase microbes [94]. Furthermore, twoin vivoassessments evaluated the cholesterol-lowering efficacy ofL.plantarumECGC 13110402 in hypercholesterolemic adult patients [78,79]. Besides attenuating the total cholesterol levels,L.plantarumadministration can also inhibit atherosclerosis by reducing systemic inflammation [95,96], regulating enzyme activity [97], and decreasing the progression of atherosclerotic lesion formation [98]. Additionally, microbiota-derived metabolites,including atherogenic molecules such as choline and trimethylamine N-oxide (TMAO) link the gut microbiome to disease. Studies showed thatL.plantarumstrains reduced plasma TMAO, and it was suggested that this effect was achieved via remodeling of the gut microbiota [99].More specifically,L.plantarumZDY04 was proved to significantly reduce the development of TMAO-induced atherosclerosis in ApoE-/-choline-fed mice [99]. However, the mechanism underpinning the cholesterol-lowering effect ofL.plantarumand the optimal dose are still not entirely understood. For instance,L.plantarum299v supplementation was found to enhance vascular endothelial function and attenuate systemic inflammation in men with stable coronary artery disease. The mechanisms of the effect seem to be independent of general cardiovascular risk factors and not associated with modification in TMAO levels [96]. Thus, further studies are needed to enhance the understanding of howL.plantarumand its substrains affect cholesterol metabolism, which would promote further appropriate utilization ofL.plantarum.

Fig. 6 Mechanism of the L. plantarum in regulating hypertension [105].
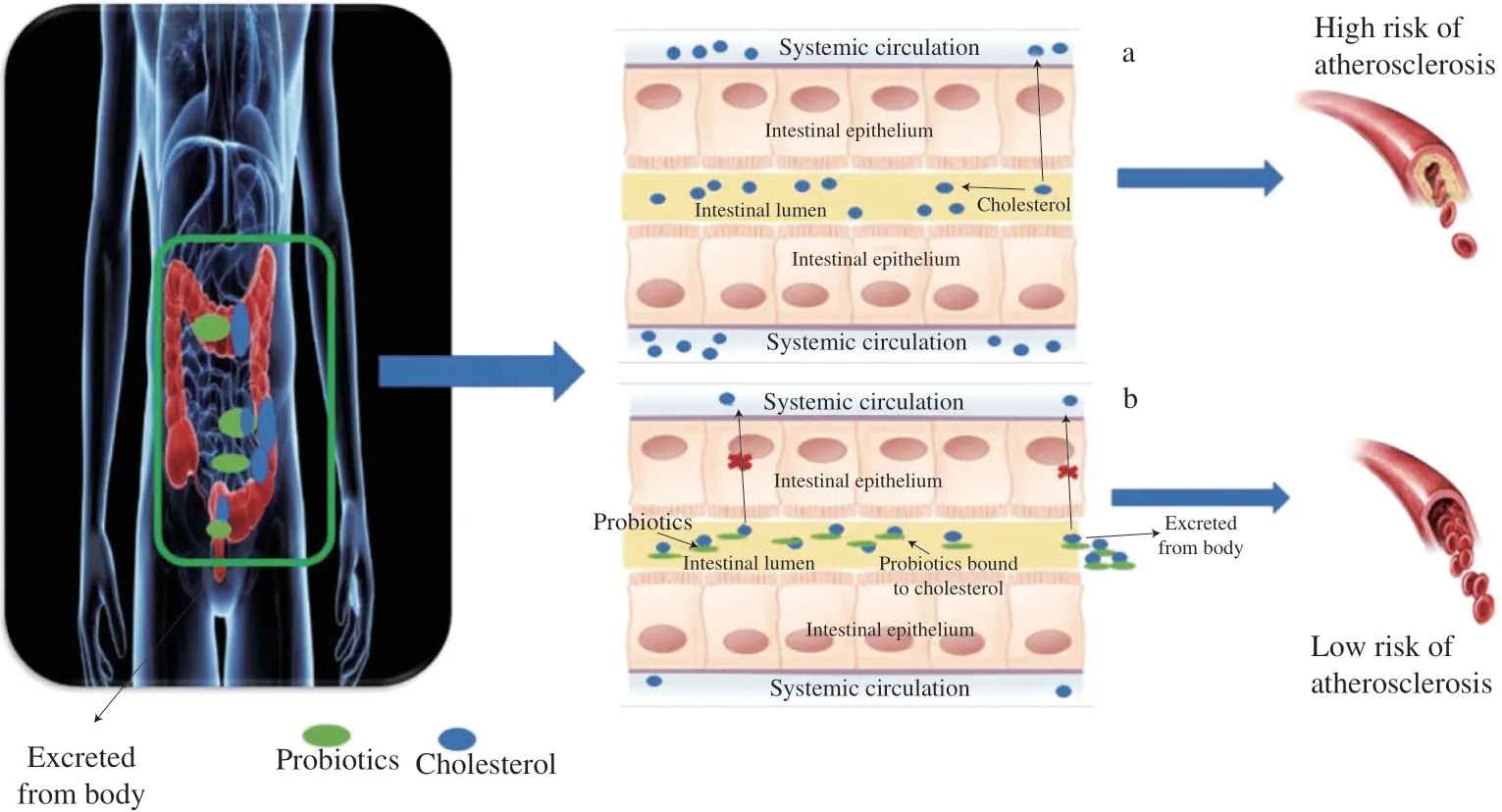
Fig. 7 Mechanism of the L. plantarum in regulating atherosclerosis [92].
3. Applications and technologies of L. plantarum
L.plantarumis an adaptable and culturable LAB that has been widely used in food and biopharmaceutical industries (Table 2).Regarding food products,L.plantarummainly contributes to enhancing the texture and flavor by ameliorating the balance between acetate (volatile) and lactate (nonvolatile) organic acids [100]. Studies have shown that several strains ofL.plantarumcan increase the production of phenols, improve the activity of radical scavenging, and reduce the content of carbohydrates [101,102]. As a vital element of fermenters,L.plantarumhas been widely applied in dairy, vegetable,and meat fermentations [103]. Dairy products are the most common carriers for probiotics because milk has all the factors required for their growth. Hütt et al. [104] found thatL.plantarumTENSIA as an adjunct starter of cheese decreased cardiovascular disease risk, while soy milk fermented byL.plantarumTWK10 was of antihypertensive [105], pressure-lowering and neuroprotective effects [77].Additionally, compared with otherLactobacillusspecies,L.plantarumhas more notable potential as a probiotic bio-preservative to increase safety and shelf-life of fermented foods [106,107].
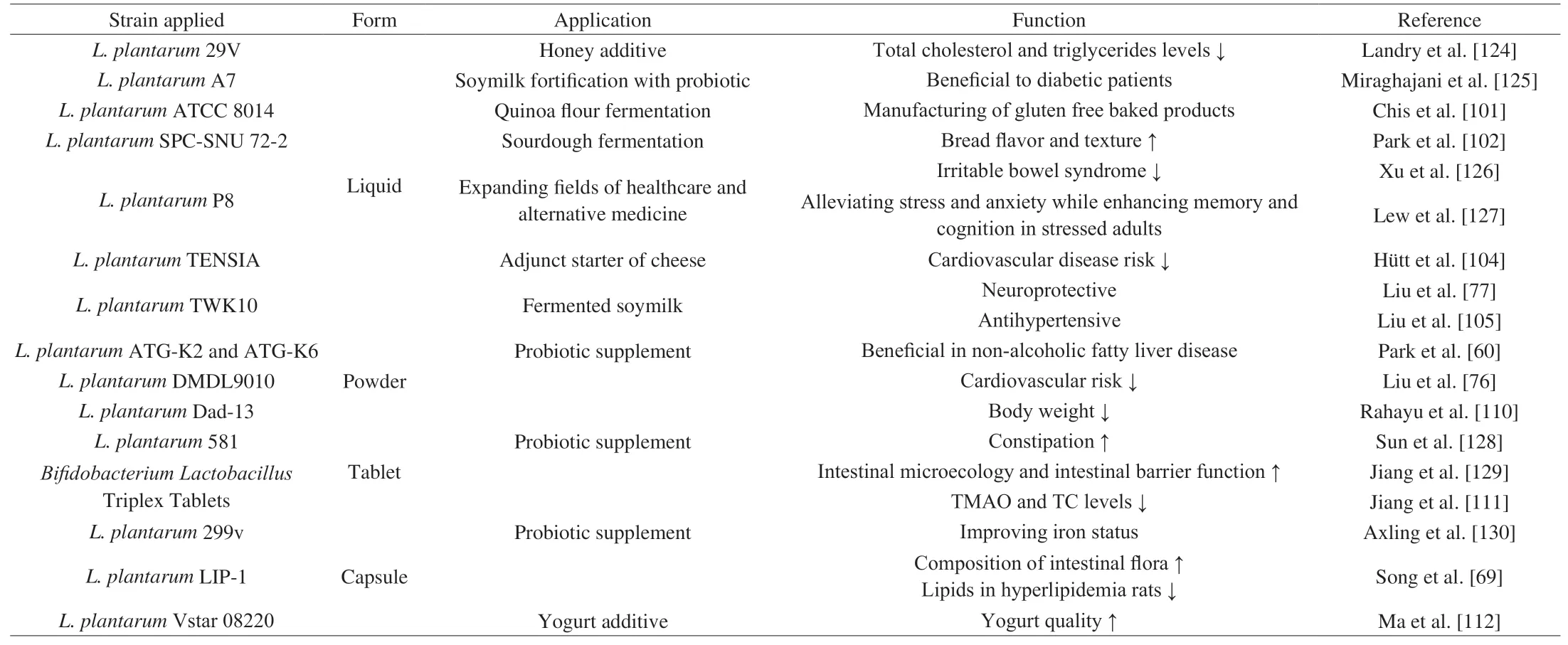
Table 2Summary of the applications and technologies of L. plantarum preparation of varied strains.
A large number ofin vitroandin vivostudies have shown thatL.plantarumhas various health effects, including regulating immune function, modulating chronic metabolic diseases, antagonizing pathogenic infections, regulating intestinal and nerve functions [103].So far,L.plantarum299v, trademarked as LP299V®, is the “star”among the strains since it is safe for human consumption and does not confer antibiotic resistance [108]. With the increasing prevalence of metabolic diseases [11], the application potential ofL.plantarumhas been further explored especially due to its widespread presence in food and strong tolerance to the human digestive tract.
Importantly the effects ofL.plantarumare directly affected by its ability to survive in food products and in the consuming hosts [109].Various technologies have also been advanced along with the applications ofL.plantarumfrom different sources. Relevant technologies, mainly include live bacteria powder, live bacteria tablets,and embedding technology or encapsulation as shown in Table 2.Regarding the application of live bacteria powder, studies showed thatL.plantarumATG-K2 and ATG-K6 [60] were beneficial to nonalcoholic fatty liver disease, whileL.plantarumDMDL9010 [76]reduced cardiovascular risk, andL.plantarumDad-13 [110]decreased body weight. Regarding the application exploration of live bacteria tablets in chronic metabolic diseases, Jiang et al. [111]found thatBifidobacteriumandLactobacillustriplex tablets reduced serum TMAO and TC levels in atherosclerosis (AS) patients. For the application of encapsulation, studies showed thatL.plantarumECGC 13110402 capsule [78] reduced cardiovascular risk and LIP-1 microcapsule [72] reduced lipids in hyperlipidemia rats.Therefore, advanced technologies are needed to protect the viability ofL.plantarumunder various conditions. It is important to note that the efficacy ofL.plantarumis also enhanced by adding prebiotics [109]. Compared to live powder and tablet technologies,embedding technology allows probiotics to be protected in the digestive tract and improves the survival rate of probiotics [112]. Importantly, the drying technology and embedding material during the preparation of microcapsules determine the vitality of probiotics [112-116].
Food fermentation is the most direct and traditional application of probiotics. A variety of application technologies have broadened the application scope ofL.plantarum, and its application value has also been enhanced. Probiotics have entered the healthcare application area but rarely the clinical trial stages. Powder and tablet formats directly expose the probiotics to the digestive tract, which may inhibit their survival and growth. Embedding, acid-resistant coatings, and microencapsulation enhances probiotic efficacy and may become an important trend for their applications.
4. Conclusion and future perspectives
The role and mechanism ofL.plantarumin the prevention and treatment of a variety of chronic metabolic diseases has been summarized in Fig. 8.L.plantarumhas demonstrated its multiple benefits by reducing TMAO, blood glucose and triglycerides,total cholesterol, urinary and intestinal oxalate, and blood pressure during the development of chronic metabolic diseases. The updated knowledge ofL.plantarumand its beneficial effects on chronic metabolic diseases have promoted its application in food and biopharmaceutical industries and built its role in food science and human wellness.
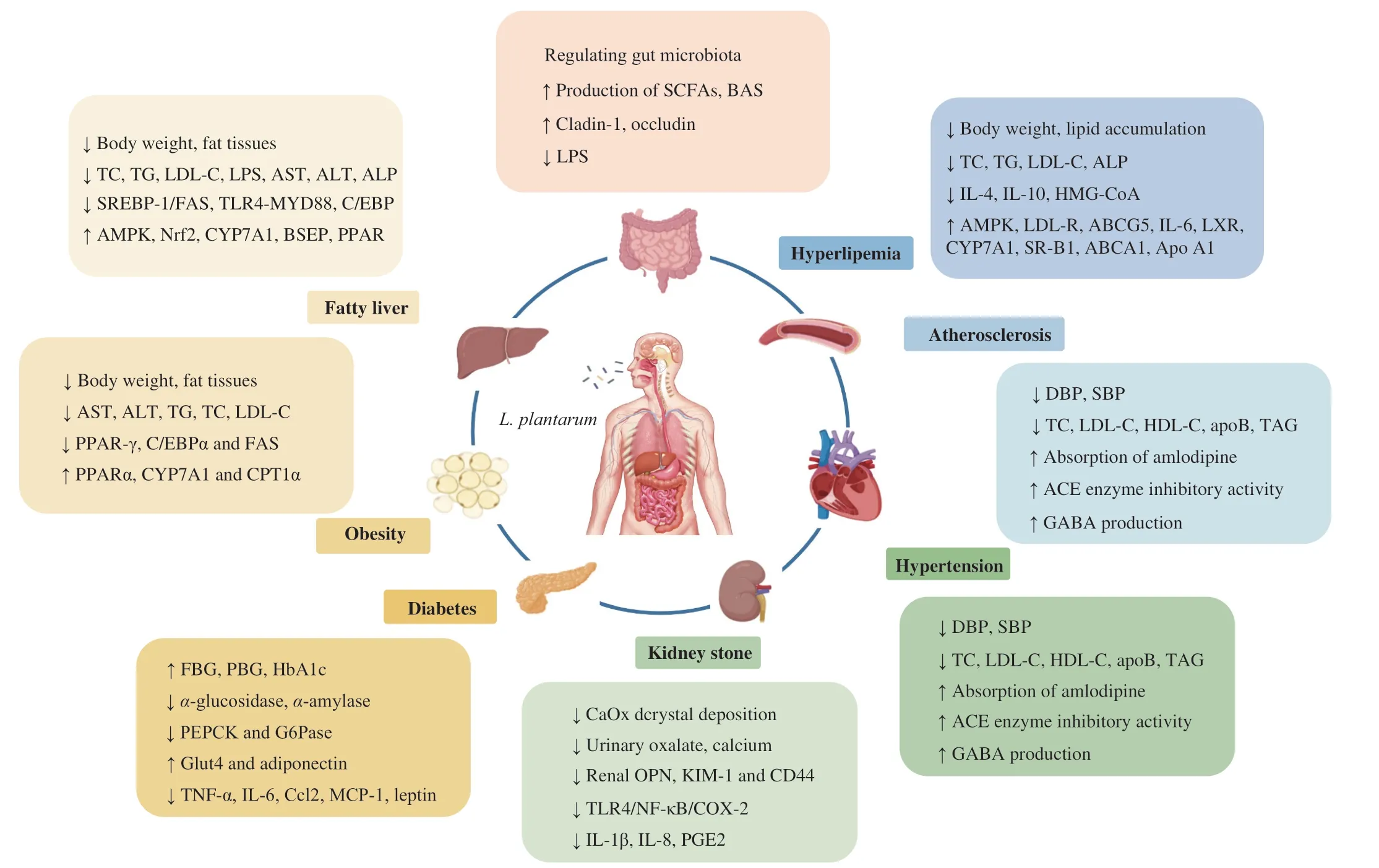
Fig. 8 Mechanism of the L. plantarum in regulating chronic metabolic disease.
So far, numerousL.plantarumprobiotic strains and their fermented products have been tested for animal and human health promotion, the exact regulatory mechanisms of its probiotic effects,however, have not been fully deciphered. More focused and welldesigned meta-analyses are recommended to verify the functions reported and to further investigate the potential mechanisms ofL.plantarumin humans at different health conditions. A wide range ofL.plantarumsub-strains has been included in the current summary,whereas different sub-strains may exert varied functions that need special attention. Besides the diversity in research models and analytical technics, the syndromes of chronic metabolic diseases and the efficacy ofL.plantarummight also be influenced by ethnicity,the living environment, and cultural backgrounds. Despite the accumulated evidence from experimental and clinical studies have demonstrated thatL.plantarumprobiotic consumption is clinically advantageous, there is still no consensus on the optimal dosage,treatment duration and mechanism pathways. As our knowledge on probiotic effect on human wellness grows, a better understanding of the side effects is also worth exploring. Future studies should pay more attention to the standardization of the usage ofL.plantarumproducts as novel therapeutics for the prevention and treatment of multiple chronic metabolic diseases in humans.
Conflict of interest
The authors declare that there were no conflicts of interest regarding either the publication of this article or the funding that they received to complete this study.
Acknowledgements
This work was supported by the National Key Research and Development Projects (2019YFE0103800), Sichuan Science and Technology Program (2021ZHFP0045, 2021YFN0092), International Research and Development Program of Sichuan (2019YFH0113,2021YFH0060, 2021YFH0072), Chinese Hungarian Bilateral Project(2018-2.1.14-TÉT-CN-2018-00011, Chinese No. 8-4) and Food Fermentation Technology Research Team of Luzhou Vocational and Technical College (2021YJTD02).
杂志排行
食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents
- Flowers: precious food and medicine resources
