Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents
2023-01-03WeiyiChuChiWeiPhnSengJoeLimAbdulSlmBbji
Weiyi Chu, Chi Wei Phn,b,c,*, Seng Joe Lim, Abdul Slm Bbji
a Department of Pharmaceutical Life Sciences, Faculty of Pharmacy, Universiti Malaya, Kuala Lumpur 50603, Malaysia b Clinical Investigation Centre, University Malaya Medical Centre, Lembah Pantai Kuala Lumpur 59100, Malaysia
c Mushroom Research Centre, Universiti Malaya, Kuala Lumpur 50603, Malaysia
d Department of Food Sciences, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, UKM Bangi, Selangor 43600, Malaysia
e Innovation Centre for Confectionery Technology (MANIS), Faculty of Science and Technology, Universiti Kebangsaan Malaysia, UKM Bangi, Selangor 43600, Malaysia
Keywords:Antioxidant Composition Edible bird nest Neurodegenerative disease Neuroprotection Sialylated mucin glycopeptide
A B S T R A C T Neurodegenerative diseases are often associated with the accumulation of oxidative stress and neuroinf lammation. Edible bird’s nest (EBN) is a glycoprotein (sialylated mucin glycopeptides) found to be benef icial against neurodegenerative diseases. Antioxidative, anti-inf lammatory, and anti-apoptotic properties of EBN in preserving neuronal cells were widely researched using in vitro and in vivo models. Functional effects of EBN are often linked to its great number of antioxidants and anti-inflammatory glycopeptides.Bioactive compounds in EBN, especially sialic acid, add value to neurotrophic potential of EBN and contribute to neuronal repair and protection. Various studies reporting the neuroprotective effects of EBN,their molecular mechanisms, and neuroactive composition were gathered in this review to provide better insights on the neuroprotective effects of EBN.
1. Introduction
Neurodegenerative disease refers to a range of disorders that are caused by damage of neurons in the human brain. Neurodegenerative disease remains the second leading group cause of death in the world.It was shown to contribute to 16.5% of deaths from all causes [1].The World Health Organization (WHO) estimates that there are 10 million new cases of dementia each year [2]. The increase in the prevalence of dementia is related to the “Silver Tsunami”, a term used to describe the expected increase in the senior population of age 65 years and above. Globally, the share of the population aged 65 years or over increased from 6% in 1990 to 9% in 2019. It is expected to rise further to 16% by 2050, so that one in six people in the world will be aged 65 years or over [3].
Aging is a well-known crucial risk factor for neurodegenerative diseases [4]. It is believed that aging leads to the accumulation of various unrepaired cellular damage, weakening cellular repair, and compensatory mechanisms [5]. Oxidative stress and neuroinf lammation are the two prominent pathophysiology processes that damage the nervous system. Both are closely related, where the increase in reactive oxygen species (ROS) or reactive nitrogen species (RNS) will induce an altered intracellular signaling, leading to dysregulation of the inflammatory response [6]. The occurrence of inflammation will in-turn release reactive species and proinf lammatory cytokines, causing the repetition of this vicious cycle [7].Weakened compensatory mechanisms such as the antioxidant defense system and impairment in tissue repair further worsen oxidative stress and neuroinflammation. A series of neurodegenerative cascades is then initiated, leading to apoptosis of neuronal cells. Thus, the brain becomes more susceptible to the progression of neurodegenerative diseases.
Currently, pharmacological treatments are widely used in the management of neurodegenerative diseases. However, these drug therapies are often associated with undesirable side effects, and they only provide short-term delay in the progression of disease.Therefore, alternative methods such as the use of functional food with potent antioxidative and anti-inflammatory activities should be developed to prevent these neurodegenerative diseases. Functional food is natural or processed food that is enriched with a biologically active compound known to provide clinically proven and documented therapeutic benefits, over and above its nutritional properties [8]. They are known to be able to strengthen one’s overall well-being including reducing the risk of chronic diseases.
Edible bird’s nest (EBN) is a functional food popular among the Chinese community. It is a glycoprotein (EBN sialylated mucin glycopeptides) made from the saliva of swiftlet species in the genus ofAerodramusandCollocalia[9]. EBN is believed to have high nutritional and medicinal value and has been consumed since the Tang(618-907 AD) and Sung (960-1279 AD) dynasties [10]. Since then,the medicinal value of EBN has been investigated and documented.It was found that EBN has health supplementing properties including antiviral, antiaging, growth promoting, complex enhancement, bone strengthening, osteoarthritic preventing effect, and improvement in one’s immune system [11,12].
These health benefits lie within EBN’s bioactive component and nutritional composition. EBN was found to be rich in protein,carbohydrate, and other essential trace elements such as iron,manganese, zinc, sodium, calcium, and magnesium [13]. The bioactive component of EBN believed to play an important role in neuroprotection isN-acetylneuraminic acid (more commonly known as sialic acid) [14]. Sialic acid is an acidic sugar that is bound to the terminal position of the glycan in EBN glycoprotein, which is known to contribute to healthy nerve and brain function, and memory enhancement. It was often associated with the enhanced neurological and intellectual capacity of infants. Other components of EBN that possess neuroprotective activity and contribute to healthy brain development are lactoferrin, ovotransferrin,N-acetyl galactosamine,and epidermal growth factor.
To the best of our knowledge, the neuroprotective effects of EBN and its potential bioactive components contributing to neuroprotection have not been previously reviewed. Thus, this study aimed to critically review the potential neuroprotective effects of EBN. Here,we further discuss the antioxidative, anti-inflammatory, and antiapoptotic properties of EBN which contribute to the neuroprotection of EBN. The neuroactive compositions present in EBN, as well as the mechanism underlying the neuroprotective effects, and its potential therapeutic applications are also discussed.
2. Oxidative stress and production of ROS in the brain
Oxidative stress signifies the disturbance in the equilibrium between the production of ROS and level of antioxidants in the brain [15]. The neuronal cells are highly susceptible to oxidative damage because of their high polyunsaturated fatty acid content in membranes, high oxygen consumption, weak antioxidant defense, and the presence of redox-active metals such as iron and copper [16].
The major source of ROS generation is from the mitochondrial respiratory chain [17]. Within the electron transport chain of mitochondria, Complex I and Complex III are responsible for most of the generation of superoxide anion (O2-) [18]. Other than the mitochondria, nicotinamide adenine dinucleotide phosphate oxidase(NOX), xanthine oxidase (XO), monoamine oxidase (MAO), and peroxisomal enzymes generate ROS too [19]. ROS produced by MAO has been linked with neuronal cell death in the substantia nigra which is a pathological hallmark of Parkinson’s disease (PD) [20].The consequences of accumulated ROS in the brain are oxidation of protein, DNA, RNA, and lipid peroxidation (Fig. 1).
Increased lipid peroxidation has been identified in AD and PD brains [16,21]. When polyunsaturated fatty acids undergo lipid peroxidation chain reactions, lipid hydroperoxide is the primary lipid peroxidation product formed [22]. Lipid hydroperoxide leads to the formation of highly reactive electrophilic aldehyde and secondary products, including malondialdehyde (MDA) and 4-hydroxy-2-nonenal (4-HNE) [21]. MDA is the most mutagenic product of lipid peroxidation, while 4-HNE is the most toxic [22]. These products induce accumulation ofβ-amyloid (Aβ) and neurofibrillary tangles in AD, andα-synuclein in PD [23].
2.1 EBN and its metabolites provide antioxidative protection via antioxidative enzymes
In order to control oxidative stress, neuronal cells have a high need for effective protection against ROS. The antioxidants are superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase(CAT), peroxiredoxins, and glutathione [18]. EBN was found to be able to increase the expression of these antioxidants.
Anin vitrostudy showed that pre-treatment with EBN could reduce intracellular ROS [24]. The experimental model used was SH-SY5Y human neuroblastoma cells stimulated with hydrogen peroxide (H2O2). SH-SY5Y cells have been extensively used due to their sensitivity to PD-related toxins and cell death associated with mutant genes [25]. SH-SY5Y cells stimulated with H2O2experience oxidative stress due to the increase in hydroxyl radicals and subsequently undergo a cascade of apoptotic consequences [26]. The possible pathway in reducing ROS proposed by Hou et al. [24] is via free radical scavenging activity of EBN and increased SOD activity through increased expression of SOD genes. However, SOD is responsible for the breakdown of highly reactive O2-into less reactive H2O2and oxygen and has no direct effect on H2O2[18]. Therefore,while SOD reduces superoxide ROS, it is reasonable that EBN attenuates the increase of hydroxyl radical caused by H2O2via its own free radical scavenging properties.
Yew et al. [27] found that a low dose of EBN (20 mg/kg)increased the expression of glutathione peroxidase 1 (GPX1) in 6-hydroxydopamine (6-OHDA)-stimulated mice. 6-OHDA treatments are often used for PD models as it causes the loss of dopaminergic neurons. It can also mimic end-stage PD in whichα-synuclein is found in the cerebral cortex of 6-OHDA-stimulated mice [25].GPX1 is an isoform of GPX which is expressed predominantly in the microglia [28]. It is useful in catalyzing the reduction of H2O2and lipid peroxides by utilizing glutathione as an electron donor [18]. Hence, an increase in GPX1 explains the reduction of lipid hydroperoxides in 6-OHDA-induced SH-SY5Y cells treated with EBN [27].
However, Hou et al. [29] reported different findings on the antioxidant enzyme activities. They reported that EBN treatment decreased SOD and CAT activities that were initially increased in ovariectomized rats. Ovariectomized rats are universally used to model menopause in women, signifying the processes behind aging. Menopause is associated with estrogen-deficiency. Estrogen has a positive effect on ROS production and antioxidant defense, thus providing neuroprotection [30]. Therefore, women with low estrogen were said to have higher risk of neurodegenerative diseases. Hou et al. [29]found that SOD and CAT activities increase in ovariectomized rats,which is inconsistent with Huang et al. [31] where they reported ovariectomy caused a decrease in SOD activities. This is also not consistent with the reduction in SOD concentrations noticed in menopausal women [32]. Thus, the difference in findings by Hou et al. [29] compared to other articles signify the need for further works to clarify the antioxidant status and the effects of EBN in estrogen-deficient models.
2.2 EBN and its metabolites reduce the MDA and advance glycation end-products (AGEs) level
The same study by Hou et al. [29] revealed that EBN improves oxidative stress evidence by reducing MDA level. In fact, EBN was more efficient than estrogen therapy, a medication normally used in managing menopausal symptoms. Since the reduction of MDA does not correlate with the antioxidant findings, it shows that the reduction of MDA is again contributed by EBN’s free radical scavenging activities. A study by Ismaeil et al. [33] reported that EBN treatment in chronic cerebral hypoperfusion induced neurodegeneration in rats decreases hippocampal F2-isoprostane level. F2-isoprostane is a product from lipid peroxidation of arachidonic acid, which makes it a biomarker for oxidative stress [34]. The reduction of F2-isoprostane is associated with an increase in viable neuronal cell count. The mechanism behind the reduction of F2-isoprostane remains unknown.
Another possible pathway isvia EBN activity on reducing AGEs. AGEs are inevitable components of the aging process, which causes inflammation and oxidative stress [35,36]. Another study by Hou et al. [37] indicated that EBN increases serum estrogen of ovariectomized rats. As mentioned earlier, estrogen has neuroprotective effect. Thus, both decrements of AGEs and increment of estrogen allow the attenuation of oxidative stress, making EBN neuroprotective. However, the mechanisms behind the decrease in AGEs and increase in estrogen are yet to be discovered. Future works to identify these mechanisms could be performed to develop EBN as an alternative to prevent aging-related disorders or aid in estrogendeficiency conditions.
Besides neurodegenerative experimental models, desirable neuroprotective effects were also spotted in pups breast-fed by maternal mice consuming EBN. When a high dose of EBN (9 g) was fed to maternal mice throughout pregnancy and lactation, the SOD concentration in the offspring increased remarkably, while MDA level reduced [38]. It was noted that EBN increases free sialic acid content in mice breastmilk. Large amounts of sialic acid in human milk were often associated with the early stages of neurodevelopment in infants [39]. With that, this shows EBN functions in neurodevelopment and protecting neuron from oxidative stress.
3. Anti-(neuro)inflammatory activity of EBN
Neuroinflammation is another prominent feature shared by various neurodegenerative diseases. Out of all neuroglia cells in the central nervous system (CNS), microglia are involved in the first line innate immunity [40]. They are responsible for immune surveillance within the CNS [41]. During an infection or injury,microglia are the first to be activated. Activation of microglia is induced by lipopolysaccharide (LPS) and abnormal protein aggregates such as Aβandα-synuclein that are widely found in AD and PD,respectively [42]. Upon activation, these aggregates lead to the production of pro-inflammatory cytokines, ROS, and RNS [7]. When there is an excessive accumulation of these factors, chronic activation of microglia worsens oxidative stress, resulting in a series of apoptotic cascades (Fig. 1) [43].
Based on a study carried out by Yew et al. [27], both pancreaticdigested crude extract and water extract (freeze dried filtrate of soaked and boiled EBN) of EBN exert neuroprotective effects by inhibiting microglial activation. Pancreatic digestion simulates human gastrointestinal tracts, allowing the proteolytic breakdown of EBN to produce bioactive components. The difference between crude extract and water extract is due to the presence of nutrients, where water extract of EBN is believed to contain mainly water-soluble substances while crude extract contains lower molecular weight glycopeptides and glycans [27]. Despite the differences in nutrients present in both EBN samples, it was found that the microglia marker CD11b was reduced in 6-OHDA-stimulated mice pre-treated with both EBN samples. This study also reported the reduction of nitric oxide (NO)production in 6-OHDA-stimulated SH-SY5Y cells pre-treated with both EBN samples [27]. In a previous study, the expression of CD11b in neurodegenerative diseases is upregulated via NO production was reported [44]. Thus, reducing NO production by EBN signifies its ability to reduce microglial activation and neuroinflammation.
Careena et al. [45] also showed that EBN inhibits the upregulation of pro-inflammatory cytokines, tumor necrosis factor (TNF)-α,interleukin (IL)-1β, and IL-6 in LPS-stimulated rats. LPS is widely used inin vitroandin vivoprotocols to model AD and PD. LPS activates microglia by activating toll-like receptor 4, which then induces pro-inflammatory genes and produces inflammatory mediators [46]. The decrease in pro-inflammatory cytokines by EBN is associated with a decrease in ROS and MDA levels [45]. This finding is consistent with a study where EBN attenuates high fat diet-induced oxidative stress and inflammation in rats [47]. It was found that EBN increases expression of hepatic antioxidant genes and decreases serum pro-inflammatory cytokines, such as C-reactive protein, IL-6, and TNF-α, which was initially increased in rats fed with high fat diet [47]. Therefore, this shows a relationship where decreasing neuroinflammation could reduce oxidative stress too.
Another possible reason behind the reduction of pro-inflammatory cytokines might be due to the increase in anti-inflammatory cytokines in EBN treated cells. In a study investigating the immunomodulatory effect of EBN against influenza A virus infection, it was reported that EBN causes the increase of IL-4 and IL-10, while reducing IL-1β,IL-2, and IL-6 [48]. Both IL-4 and IL-10 are anti-inflammatory cytokines that are shown to down-regulate pro-inflammatory cytokine receptors, hence counter-regulate the production and function of pro-inflammatory cytokines [49]. Since this result was not obtained from a neurodegenerative disease experimental model, it would be interesting to investigate the effect of EBN on anti-inflammatory cytokines in neurodegenerative disease models. Furthermore,estrogen is neuroprotective by inhibiting microglial activation, while the decrease in AGEs reduces its interaction with Aβ, thus reducing inflammation [50,51]. Hence, the effect of EBN on increasing serum estrogen and decreasing AGEs produce anti-inflammatory effects to protect against neurodegenerative diseases. Beneficial effects of EBN in oxidative stress and neuroinflammation are shown in Fig. 1.

Fig. 1 Mechanisms of oxidative stress and neuroinfalmmation and the effects of EBN on each pathway., inhibitory effects of EBN; , upregulated by EBN.
4. Anti-(neuro)apoptotic activity of EBN
Apoptosis occurs when there are high levels of intracellular proteolysis. There are abundant signs of apoptosis in the form of aberrant protein aggregates in brains undergoing neurodegeneration.Accumulation of these protein aggregates is not only a sign but serves as an inducer for apoptosis. Liu et al. [52] demonstrated that overexpression ofα-synuclein induces cell death via an array of mechanisms in the pathogenesis of PD.
Apoptosis can occur via the intrinsic or extrinsic pathway, where it can be triggered by cellular stress and TNF respectively (Fig. 2).Both pathways alter the mitochondrial membrane permeability, but the intrinsic pathway has more influence over it than the extrinsic pathway [53]. This is because activated caspase-8 in the extrinsic pathway causes apoptosis either by increasing mitochondrial membrane permeability or by cleaving effector caspase-3 directly [54].Increased mitochondrial membrane permeability will release proapoptotic factors, such as cytochrome C into the cytoplasm. Under the caspase-dependent pathway, this will activate initiator caspases such as caspase-9 in the presence of apoptosomes, which then cleave caspase-3 [55]. Later, activated caspase-3 cleaves essential protein substrates such as poly (ADP-ribose) polymerase (PARP), triggering DNA fragmentation, hence apoptosis [53].
Apoptotic cells can be easily identified by observing their morphological features. These features include shrinkage, chromosome condensation, DNA fragmentation, and the presence of apoptotic bodies.Both Yew et al. [27] and Hou et al. [24] showed that EBN could inhibit apoptosis. It was found that there were reduced morphological changes when neurotoxic cells were pre-treated with EBN.
4.1 The interaction of EBN and caspases
Detecting apoptosis inin vitroandin vivoexperimental models can also be done via measuring the expression of pro-apoptotic genes,mitochondrial membrane potential (MMP), and quantifying caspases.Both Yew et al. [56] and Hou et al. [29] reported that EBN could inhibit caspase-3 cleavage. Hou et al. [29] realized EBN exerted this effect greater than estrogen, showing its ability to be a better therapy than estrogen. In addition, Yew et al. [56] reported that a water extract of EBN could inhibit this process. In fact, this finding was enhanced with the result where water extract of EBN could ameliorate early apoptotic membrane phosphatidylserine externalization.Phosphatidylserine externalization is one of the characteristics of early apoptosis that occurred as a result of the activation of caspases [54].Thus, inhibition of caspase activation by EBN prevents apoptosis.
4.2 The interaction of EBN and other apoptosis related genes
The effects of EBN on apoptosis-related genes, i.e., poly [ADPribose] polymerase 1 (PARP1), presenilin (PSEN1andPSEN2), and amyloid precursor protein (APP) genes, were also investigated. Cleavage of PARP1 by effector caspases is the hallmark of apoptosis [57].PARP1 is an enzyme essential for initiating various forms of DNA repair. Cleaving PARP1 will cause the inactivation of DNA repair mechanisms [58]. Therefore, the increase in the expression of thePARP1gene by EBN will aid in cell survival, which corresponds with findings where EBN increases cell viability and reduces apoptotic activity [24]. Presenilin 1 is the catalytic component ofγ-secretase, an enzyme responsible for cleaving APP into Aβ, whereas presenilin 2 helps in forming a fully functionalγ-secretase complex [59].Accumulation of Aβis pronounced in AD, which can trigger apoptosis via caspase-3 activation [60].PSEN1,PSEN2, andAPPgenes were found to be downregulated in cells pre-treated with EBN [29]. Thus, these findings further suggested the possible functions of EBN in preventing neuronal apoptosis.
4.3 The interaction of EBN and MMP
Increased mitochondrial membrane permeability (MMP) is central to apoptosis [61]. The effect of EBN on MMP was investigated to determine whether EBN has a beneficial influence on mitochondria in preventing apoptosis [56]. MMP is a reliable measure of cell stress and apoptosis, as it is compromised during early stress response and is a good indicator of ongoing cell death [62]. It was reported that EBN does not affect MMP. Thus, this implies that EBN does not inhibit apoptosis via the mitochondria, but rather viainhibition of the caspase-dependent pathway. The anti-apoptotic effects of EBN were shown in Fig. 2.
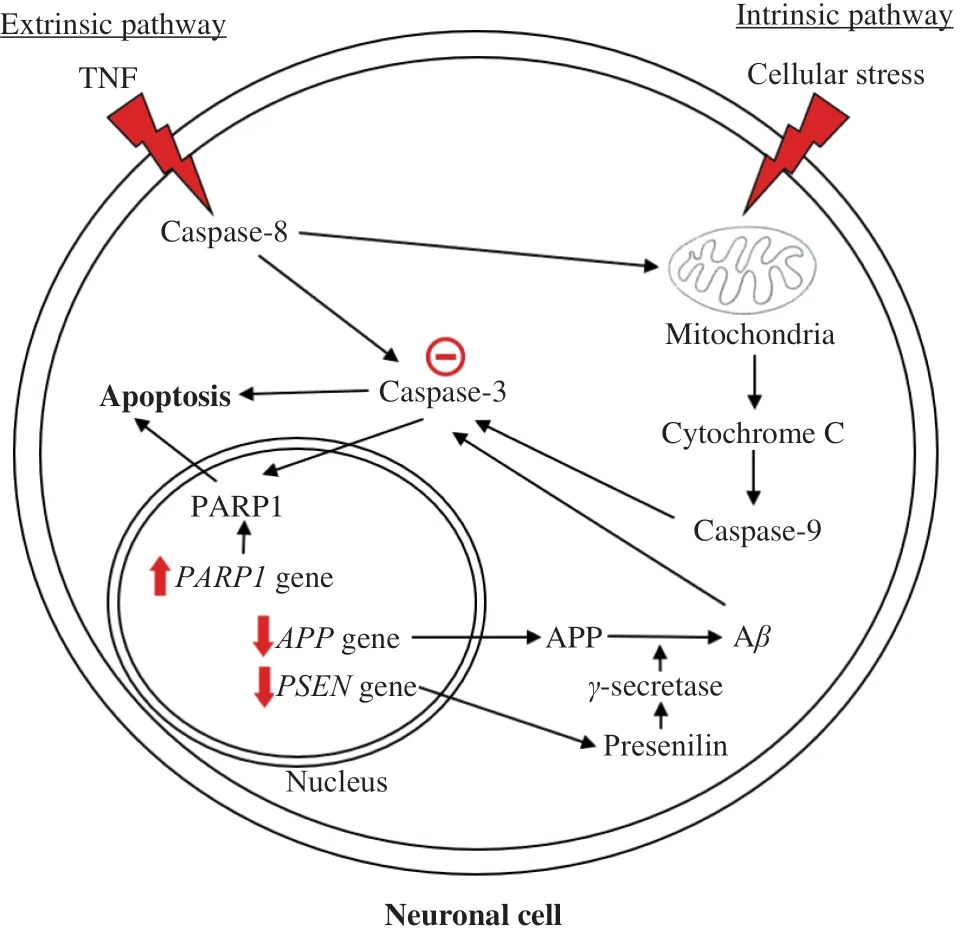
Fig. 2 Schematic diagram on the extrinsic and intrinsic pathways of apoptosis and the anti-apoptotic activities of EBN., inhibitory effects ofEBN; , upregulated by EBN; , downregulated by EBN.
5. Therapeutic potentials of EBN on different neurological disorders
5.1 Improvement of memory and learning and its underlying mechanisms
Dementia is a commonly known symptom of neurodegenerative diseases. The effects of EBN on memory and learning behavior of mice were investigated by Hou et al. [37], Careena et al. [45], and Xie et al. [38]. These studies used the Morris water maze (MWM)test. The MWM test is a test of spatial memory and learning where rodents navigate from start locations around the perimeter of an open swimming arena to locate a submerged escape platform [63]. This test choice is appropriate because compared to appetitive mazes, water presents an equal motivator that balances motivation to escape over a wide range of body weight differences, which are often found in experimental mice models [63]. The MWM test strongly correlates with hippocampal synaptic plasticity, which plays a major role in learning, memory, and spatial navigation [64]. A deficit in synaptic plasticity is often the reason behind AD [65]. EBN treatment could enhance the learning and memory capacity of mice as they showed lower escape latency and longer times spent in a target quadrant [37,45].In fact, the effect was dose-dependent and comparable toGinkgo biloba, a well-known supplement for dementia [45,66].
A high dose of EBN (9 g) administered to maternal mice throughout pregnancy and lactation showed recurring crossing and longer time spent in target quadrant by their pups, thus indicating improved memory functions [38]. A study by Mahaq et al. [67] also showed that EBN improved learning and memory functions in two consecutive generations of offspring due to the EBN supplementation of mothers, possibly through placenta and maternal milk. This is correlated with the increase in synaptic vesicles densities in both groups, hence altering synaptic plasticity required for memory consolidation [67].
These findings are strengthened with the realization that EBN could preserve hippocampal sirtuin-1 (SIRT1) expression [37]. SIRT1 is a member of the sirtuin family, critical for maintaining synaptic plasticity for normal acquisition and consolidation of short-term and long-term hippocampus-dependent memories [68], hence, a possible role in regulating memory and learning. Besides, acetylcholine and brain-derived neurotrophic factor (BDNF) significantly improve memory, learning, and cognitive function [69]. Cholinergic neuronal loss and decrease in BDNF occur in AD patients. Acetylcholine can bind to synaptic receptors to stimulate downstream neuronal actions, leading to the release of BDNF. BDNF will then modulate memory formationby enhancing synaptic plasticity [70]. EBN was found to increase choline acetyltransferase (ChAT), decrease acetylcholinesterase (AChE), and increase BDNF in pups of maternal mice consuming EBN [38,67]. Increased ChAT will promote the synthesis of acetylcholine while decreased AChE will reduce the breakdown of acetylcholine, thus increasing acetylcholine concentration. Therefore, with the preservation of hippocampal SIRT1 expression and increasing acetylcholine and BDNF, EBN could boost memory and learning functions.
5.2 Improvement of locomotor activity and its underlying mechanisms
Movement disorders are also one of the signature symptoms of neurodegenerative diseases. Yew et al. [27] investigated the effect of EBN on PD models using open field and beam tests. The open field test is commonly used to observe general ambulatory ability and exploratory behavior of the animal, while the beam test is normally used to assess fine motor coordination and balance [71,72]. It was shown that EBN-treated mice have more coordinated movements and can travel a longer distance. They had better balancing ability and used a shorter time to cross the beam. However, PD patients are often associated with non-motor symptoms such as olfactory deficits and mood fluctuations. Thus, more comprehensive behavioral tests such as an elevated plus maze and buried pellet test could be utilized to develop a better understanding of EBN effects in PD models [73]. In the same study, EBN was found to prevent the loss of dopaminergic neurons through attenuation of oxidative stress and neuroinflammation. Hence,EBN could be a potential therapy for PD following on from evidence of improving locomotor activity and preserving dopaminergic neurons in mice PD models.
Moreover, EBN was found to stimulate proliferation, migration,and differentiation of neural stem cells (NSC) [74]. Transplantation of NSC is a new therapeutic approach for neurodegenerative diseases.As neuronal loss is evident in neurodegenerative disease, NSC transplantation aims to replace these degenerating neurons [75].Nutrition was found to be able to support the growth and differentiation of newly generated cells [76]. Thus, the neurogenesis effect of EBN presents a window of opportunity for EBN in nutrition associated NSC therapy for neurodegenerative disease patients. All neuroprotective effects and mechanisms of EBN are summarized in Fig. 3, while the results from studies included in this review are summarized in Table 1 [24,27,29,33,37,38,45,56,67].
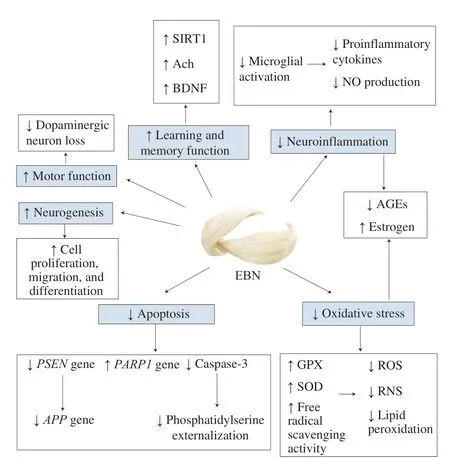
Fig. 3 Summary on the neuroprotective mechanisms of EBN. ↑, increase; ↓, decrease.

Table 1 (Continued)
6. Chemical compositions of EBN and its associated neuroprotective activities
EBN has an LD50of more than 5 000 mg/kg, classifying it as category five or an unclassified category of the globally harmonized classification system [48,77]. Thus, it is considered safe for human consumption. However, there are reports of allergic symptoms and food-induced anaphylaxis following ingestion of EBN [78]. It was demonstrated that these allergic reactions are IgE mediated and are most probably due to the presence of mites, fungal and bacteria on the surface of EBN [79]. Contaminations may occur due to improper cleaning, storage and transportation of EBN. According to the Standard and Industrial Research Institute of Malaysia (SIRIM) on Edible Bird Nest (EBN) Specification [80], the tolerance level for bacteria, yeast and molds are documented and the final product should be within specified limits. However, concerns about the permissible level of mites and mite allergens in EBNs remain unresolved. Such guidelines should be in placed to ensure the safety of consumption of EBN in view of increasing reports of anaphylactic reactions.
Essentially, EBN is a sialylated mucin glycopeptide, and the protein has been reported to contain all the 20 amino acids [81,82].Most analyses reported a high amount of protein, followed by carbohydrate and ash, while the fat content is low (Table 2). However,Huda et al. [83] reported a different finding with a wide range of protein and carbohydrate content. This is due to the differences in harvesting locations. Areas with high rainfall provide good environment for insects to proliferate, which increases the abundance of feed for swiftlets, leading to higher protein and lower carbohydrate composition of EBN.
As shown in Table 2, moisture content was reported to have a wide range of 5.58% to 24.30%. Moisture content is a parameter indetermining the quality of EBN, where the acceptable limit is less than 15% [80]. High moisture content is undesirable as it will disrupt the compositions of other nutritional compounds. Moreover, low moisture content reduces the probability of fungal growth on the EBN [84].This also ensures the stability of sialic acid, whose concentration should be constant throughout 42 days of storage [38].EBN in Saengkrajang et al. [85] was found to contain higher moisture content due to the fact that the EBN samples were harvested near the seashore.
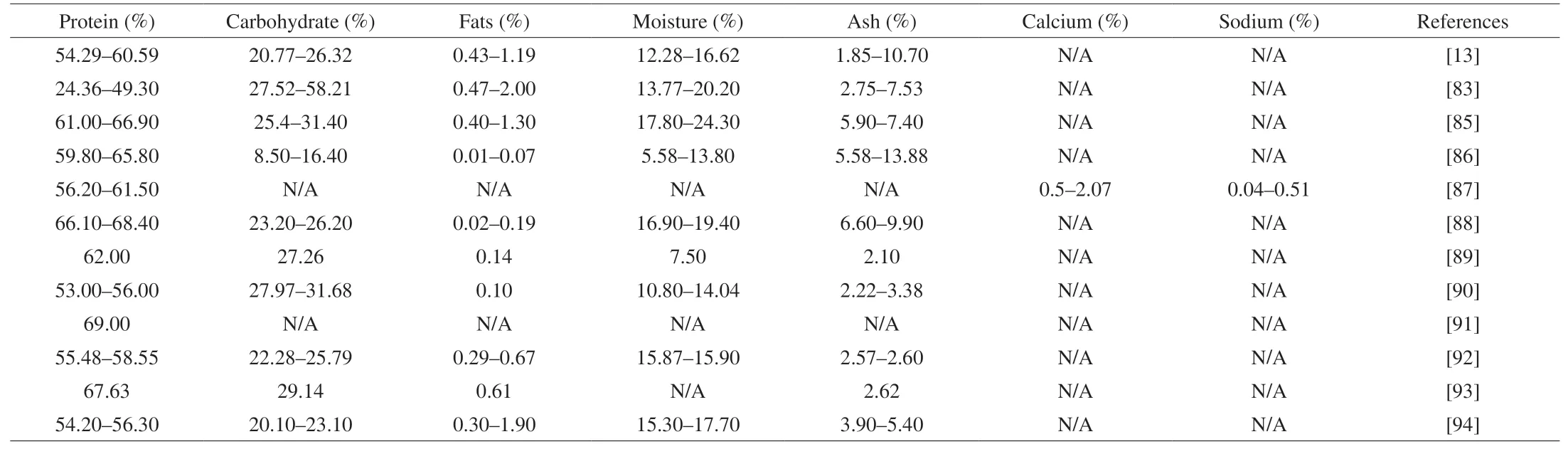
Table 2Summary on the proximate analysis of EBN composition.
Ash content is another parameter that showed a wide range between 1.85% and 13.88%. Generally, the ash content is a measure of total amount of minerals present within a food. However, it is highly influenced by the presence of impurities. A high percentage of ash was reported by Hamzah et al. [86] and Noor et al. [13] as both were analyzing unclean EBN, which signifies the presence of impurities such as feathers and foreign matters. Typically, clean EBN has lower ash content, which means a lower mineral content present in EBN. Nevertheless, elemental analyses identified high sodium and calcium content among other trace elements [87]. Calcium is important for neuroprotection as it is involved in nerve transmission.
In addition, amino acid analyses were also carried out, which discovered the presence of all essential amino acids [13,82]. Free radical scavenging activity of EBN mentioned previously is possibly linked to the presence of hydrophobic and aromatic amino acid in EBN. Amin et al. [93] reported 34.04% of hydrophobic amino acids in EBN. Hydrophobic amino acids exert antioxidative effect by interacting with hydrophobic targets to enhance bioavailability of antioxidant properties, while aromatic amino acids stabilize radicals through resonance or delocalization [84]. Table 2 summarizes the composition of EBN obtained from various studies [13,83,85-94].
Ghassem et al. [95] identified two novel antioxidant pentapeptides from EBN: Pro-Phe-His-Pro-Tyr and Leu-Leu-Gly-Asp-Pro.Both are mainly composed of hydrophobic and aromatic amino acids. Furthermore, they are resistant toin vitrodegradation by gastrointestinal proteases, suggesting their stability in the gastrointestinal tract to be absorbed in order to exert their free radical scavenging activity [96].
Although EBN fat content is low, fatty acids of EBN were analysed to be 48.43% of polyunsaturated fatty acid, 25.35% of saturated fatty acids, and 24.74% of monounsaturated fats [97].Significant free radical scavenging activity was found to originate from EBN unsaturated fatty acids. The presence of oleic acid and linoleic acid provides antioxidative properties. EBN triglycerides exhibit high paraoxonase activity. Paraoxonase 1 is an enzyme capable of protecting against lipid peroxidation and it is highly implicated in AD [98]. Thus, exhibiting high paraoxonase activity signifies neuroprotection, although further works should be carried out onin vivomodels to determine its effect.
Another two glycoproteins believed to contribute to EBN’s antioxidative effects are lactoferrin and ovotransferrin [24]. Housefarmed white EBN contains higher lactoferrin and ovotransferrin concentration. It was found that both lactoferrin and ovotransferrin have free radical scavenging activity [24]. However, their effect does not entirely reflect the same activity as EBN, where EBN has higher scavenging activity. Therefore, it is highly possible that synergistic effect between lactoferrin, ovotransferrin, and other antioxidative compounds mentioned contributed to the free radical scavenging activity of EBN, consequently enhancing neuroprotective effects.
The increase in serum estrogen reported by Hou et al. [37] is possibly due to the presence of several hormones identified in EBN.Ma et al. [99] detected six hormones in EBN, which are testosterone,estradiol, progesterone, luteinizing hormone, follicle-stimulating hormone, and prolactin. Another study also identified several progestogenic hormones and estrogenic hormones in EBN [100].The progestogenic hormones detected were 19-norethindrone,21α-hydroxyprogesterone, 17α-hydroxyprogesterone,D-norgestrel,medroxyprogesterone, chlormadinone acetate, progesterone, and medroxyprogesterone-17-acetate. On the other hand, the estrogenic hormones detected were estradiol, estriol, ethinylestradiol, estrone,diethylstilbestrol, dienestrol, and hexestrol. Therefore, it can be postulated that these hormones contributed to the increase of serum estrogen, thus providing antioxidative and anti-inflammatory effects of EBN.
Sialic acid remains the main bioactive component as it has a critical function in neuronal outgrowth, synaptic connectivity,and memory formation [39]. It is the precursor for the synthesis of polysialic acid glycan, which may reinforce endogenous mechanisms of brain repair [101]. As mentioned, Xie et al. [38] showed the capability of EBN sialic acid in aiding the neurodevelopment of pups.The ability to protect the hippocampus from inflammation was also postulated to be due to the anti-inflammatory effects of sialic acid [45].Norhayati et al. [87] reported 0.7%-1.5% of sialic acid present in EBN,while Tung et al. [102] reported 10.2% to 12.1%. Ling et al. [103]reported sialic acid contents of 5.9% to 20.0% in raw and enzymatically hydrolyzed EBNs. Clearly, the variation in sialic acid content is contributed by the extraction and preparation methods, their origins, as well the difference in harvesting locations [14]. According to Ling et al. [14,103], different extraction methods like acid,aqueous, and enzymatic extraction would directly impact the recovery yield of the sialic acid level in EBN. Origin and harvesting location wise, Quek et al. [88] found that EBN samples collected from houses in Peninsular Malaysia have higher sialic acid content than those from the caves and East Malaysia. Thus, an optimum environment in farming, as well as extraction methods, should be standardized to ensure consistent production of EBN in an industrial scale.
N-acetylgalactosamine, galactose, and fucose are the other major monosaccharides found in the glycans of EBN glycoproteins.N-acetylgalactosamine is important in synaptic function, while galactose and fucose are vital for brain development [12]. Recently,more proteomic analyses have been carried out to identify the bioactive components of EBN. Kong et al. [104] and Tan et al. [90]identified epidermal growth factor, which is a protein known to modulate neurogenesis patternsin vivo[105]. Yew et al. [74] and Zukefli et al. [106] identified a few neuroprotective compounds such as RGM domain family member B and heat shock 70 kDa/78 kDa glucose-regulated protein.
Therefore, in view of the wide variety of compounds present in EBN, their synergistic effect may have potentiated EBN neuroprotective activity. The systematic review by Ismail et al. [107]concluded that EBN could improve the cognitive functions of experimental animals and the efficacy of thein vitroneuroprotective effects of EBN was dose-dependent, which could be attributed to the ability of EBN to attenuate the neuroinflammation and neurooxidative stress. Table 3 summarizes the neuroprotective compounds discovered from various studies and their functions [24,45,48,74,87-90,95,97,102,104,106,108-110].

Table 3Summary on the neuroprotective compound of EBN.
7. Conclusions and perspective
In this review, we have summarized the beneficial effects of EBN on neuronal health, with emphasis on its antioxidative, antiinflammatory, and anti-apoptotic effects. The effects of EBN on enhancing memory, learning, and motor functions while stimulating neurogenesis proved that it may have therapeutic values in preventing neurodegenerative diseases. Analyses of the nutritional and chemical compositions identified many compounds that contribute to the antioxidative, anti-inflammatory and neuroprotective effects,supporting EBN usage as a functional food. However, as the studies discussed here are mainlyin vitroandin vivoexperiments, usage in human models must be investigated with greater care to have better understandings on the pharmacological and adverse effects of EBN.Any possible interactions between drugs and EBN should also be identified. More exhaustive analyses should be carried out to search for bioactive components of EBN as the composition varies among EBN of different geographical locations. Seeing that different EBN preparation methods produce different chemical compositions; an optimum extraction method should be determined to produce EBN with best nutritional values and functional properties. To conclude,this review provides valuable insights into the neuroprotective effects and compositions of EBN while promoting interest in developing EBN as an alternative to prevent neurodegenerative diseases.
Conflict of interest
All authors declare no conflicts of interest.
Acknowledgment
This review was supported by the Research Excellence Consortium (KKP/2020/UKM-UKM/5/1) provided by Ministry of Higher Education, Malaysia. The work is partly supported by the Fundamental Research Grant Scheme (FRGS), Project No. FP016-2019A, Reference Code: FRGS/1/2019/SKK09/UM/02/2. We would like to thank Professor Ian Macreadie from RMIT University for proofreading the article.
杂志排行
食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Flowers: precious food and medicine resources
