Study on the interaction between β-carotene and gut microf lora using an in vitro fermentation model
2023-01-03ZhixinLiZhuqingDiEnjunShiPengWnGuijieChenZhongyunZhngYyunXuRuihngGoXioxiongZengDjingLi
Zhixin Li, Zhuqing Di,*, Enjun Shi, Peng Wn, Guijie Chen, Zhongyun Zhng,Yyun Xu, Ruihng Go, Xioxiong Zeng, Djing Li,*
a School of Food and Biological Engineering, Jiangsu University, Zhenjiang 212013, China
b Institute of Agro-product Processing, Jiangsu Academy of Agricultural Sciences, Nanjing 210014, China
c College of Food Science and Technology, Nanjing Agricultural University, Nanjing 210095, China
Keywords:β-Carotene Gut microf lora Retinol Short-chain fatty acids
A B S T R A C T β-Carotene, a typical non-oxygenated carotenoid, is the most efficient source of retinol (VA). The low bio-availability of β-carotene lead to large accumulation in colon; however, the relationship between β-carotene and gut microflora remains unclear. This study intends to explore the interaction between β-carotene and gut microf lora using an in vitro fermentation model. After 24 h fermentation, the degradation rate of β-carotene was (64.28 ± 6.23)%, which was 1.46 times that of the group without gut microflora.Meanwhile, the production of VA was nearly 2 times that of the group without gut microflora, indicating that the gut microflora can metabolize β-carotene into VA. β-Carotene also influences the production of short-chain fatty acids (SCFAs), the production of total SCFAs in 0.5 mg/mL β-carotene (BCM) group was(44.00 ± 1.16) mmol/L, which was 2.26 times that of the blank control (BLK) group. Among them, the production of acetic acid in BCM group was (19.06 ± 0.82) mmol/L, which was 2.64 time that of the BLK group. Furthermore, β-carotene significantly affected the structure and composition of gut microflora,increasing the abundance of Roseburia, Parasutterella and Lachnospiraceae, and decreasing the abundance of Dialister, Collinsella and Enterobacter (P < 0.05). This study provides a new way to understand how β-carotene
1. Introduction
Carotenoids are natural pigments with a C30or C40backbone [1].They are widely distributed in green vegetables and yellow-orange fruits. Carotenoids play important roles in anti-oxidation, immune regulation, prevention of cardiovascular diseases, eye protection,etc., and are the main source of VA for human being [2,3]. Among them,β-carotene can be converted into two-molecule VA by bio-transformation at the central cleavage of C15-C15’double bond, which has attracted more attention as the most efficient source of VA [4].Human cannot synthesize carotenoids by themselves, they must be absorbed by diet [5]. Moreover, the effectively absorptivity of carotenoids is less than 5%, with massive carotenoids accumulated in colon [6].
The human intestinal tract, especially the colon, is inhabited with a large number of microorganisms. They can interact with nutrients and play important roles in nutrition metabolism, energy metabolism and immune regulation [7,8]. Many studies on gut microflora and nutrients focus on carbohydrates and polyphenols,while carotenoids pay less attention. However, existing research about the effects of gut microf lora and nutrients are mutual. For example,the polysaccharides from tea flowers can modulate gut health and ameliorate cyclophosphamide-induced immunosuppression through deglycosylation, hydrolysis, demethylation, and cyclolysis, and its metabolites are more effective than the original compound [9,10]. In addition, nutrients affect human health by selectively proliferating or inhibiting certain bacteria and regulating the structure of gut microflora. For example, catechin and its metabolites can selectively proliferateLactobacillus, inhibit pathogenic bacteria and amine carcinogens, thereby reducing liver fat content and alleviating obesity symptoms in obese mice [11].
The interaction betweenβ-carotene and gut microflora has limited information. However, some studies indicated that the gut microflora may be a new target forβ-carotene’s efficacy. A result showed that germ-free or antibiotic-treated rats accumulated moreβ-carotene in their livers than conventional rats, which proved that the microflora contributed toβ-carotene metabolism [12]. Another study indicated that excessive growth of harmful bacteria, such as Proteobacteria, would damage mucosal epithelial cells and increase permeability in the intestine, which decrease the absorption of carotenoids [13]. The absorption of carotenoids is different between different individuals [3,14]. These studies indicated that different composition of microflora may be the key factor. Furthermore, anin vitroanaerobic fermentation study using colonic fecal samples showed that the retention rate of carotenoids was only 10%-50% after 24 h fermentation by gut microflora [15,16]. During this process,plenty of unknown compounds were generated, which revealed that gut microflora could metabolize and utilize carotenoids [17].The above studies suggested that gut microflora have an important influence on absorption and metabolism ofβ-carotene. In addition,relevant studies had shown that dietary carotenoids supplementation can regulate the composition of gut microflora. For example,lycopene can change the structure of gut microflora by promoting the growth ofBifidobacteriumandLactobacillus, and inhibiting the reproduction of Proteobacteria, maintaining the harmony of intestinal immunity and ameliorate the dextran sulfate sodium (DSS)-induced colitis and depression and anxiety-like symptoms [18]. Astaxanthin has been reported to decrease the abundance of Bacteroidetes and Proteobacteria, and increase the abundance of Verrucomicrobia andAkkermansiain an alcoholic fatty liver mouse model, which was related to relieve inflammation and decrease lipid accumulation [19].
Therefore, this study intends to explore the interaction betweenβ-carotene and gut microflora using anin vitrofermentation model.The retention rate ofβ-carotene and the main metabolites was analyzed. The shift of gut microflora was also explored. The results would provide a new way to understand howβ-carotene works in human body with gut microflora.
2. Materials and methods
2.1 Chemicals and materials
β-Carotene,trans-β-apo-8’-carotenal, retinol, retinoic acid,salivary amylase, pepsin, porcine pancreatic lipase, hemoglobin,resazurin, bile salt and VK1were purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). Cholesterol,oleic acid, monoglyceride, glucose, yeast extract, peptone, agar powder were purchased from Sangon Biotech Co., Ltd. (Shanghai,China).L-cysteine monohydrochloride was purchased from Hangzhou Jiecheng Biotechnology Co., Ltd. (Hangzhou, China).Methanol (chromatographic grade), methyl tert-butyl ether (MTBE)(chromatographic grade) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China).L-cysteine hydrochloride was obtained from J&K Scientific Co., Ltd. (Beijing,China). Acetic acid, propionic,i-butyric acid,n-butyric acid,i-valeric acid,n-valeric acid, 2-ethylbutyric acid (chromatographic grade) were purchased from Sigma-Aldrich Co., Ltd. (St Louis, USA). Unless otherwise stated, all other chemicals and supplies were purchased from Nanjing wanqing glass instrument Co., Ltd. (Nanjing, China) or Sinopharm Chemical Reagents Co., Ltd. (Shanghai, China).
The lactic acid kit was purchased from Nanjing Jiancheng Biotechnology Co., Ltd. (Nanjing, China).
MGC AnaeroPark®-Anaero anaerobic cartridges and anaerobic gas production packs were purchased from Mitsubishi Chemical Co.,Ltd. (Tokyo, Japan).
2.2 In vitro digestion of β-carotene
The digestion ofβ-carotene was studied by simulated oral and gastrointestinal digestion modelin vitroaccording to the reported method with some modifications [20]. Theβ-carotene, cholesterol,oleic acid and monoglyceride were dissolved in chloroform with final concentrations of 0.9, 0.5, 2.5 and 2.5 mmol/L, respectively. The mixture was dried by N2and then re-suspended in phosphate puffer solution (PBS) with 12 mmol/L sodium taurocholate. The mixture was dissolved at ultrasonic environment (240 W) for 30 min, and then centrifuged at 5 000 r/min for 10 min. The supernatant was obtained,and filtered by 0.22 µm organic membrane to obtainβ-carotene micelle.
The simulated saliva contained 50 mmol/L NaCl, 10 mmol/L KH2PO4, 42 mmol/L Na2HPO4·12H2O and 1 g/L salivary amylase(52.7 U/mg). The simulated gastric fluid contained 3.5 mmol/L KH2PO4, 10 mmol/L CaCl2·2H2O, 3.6 mmol/L MgCl2·6H2O,14 mmol/L KCl and 20 mmol/L NaCl. The simulated intestinal fluid contained 100 mmol/L NaHCO3, 30 g/L porcine pancreatic lipase(30 U/mg) and 68.5 mmol/L bile salt (bile acid > 60%).
An aliquot of 5 mL ofβ-carotene micelle was mixed with 3 mL simulated saliva, and adjusted pH value to 6.8 with 0.1 mol/L H3PO4solution. The mixture was incubated at 37 °C for 30 s to simulated oral digestive phase. Then, the mixture was added with 10 mL simulated gastric fluid and 2 mL 50.25 g/L pepsin solution(> 250 U/mg), and adjusted pH value to 2.0 by 0.1 mol/L HCl solution, incubated at 37 °C, 120 r/min for 60 min. After gastric digestive phase, the mixture was adjusted pH value to 7.0 by 0.1 mol/L NaHCO3immediately, and added with 20 mL simulated intestinal fluid, incubated at 37 °C, 120 r/min for 120 min. Samples of different digestive stages were taken for further analysis.
2.3 In vitro fermentation of β-carotene
The interaction betweenβ-carotene and gut microflora was studied by simulated anaerobic fermentation modelin vitroaccording to the reported method with some modifications [21]. Fecal samples were provided by 4 healthy volunteers (2 men and 2 women, aged 20-30) who had regular life, healthy diet, without gastrointestinal disease history and not received antibiotic treatment for at least for 3 months. The fresh fecal sample was mixed with sterile modified saline solution (0.5 g/LL-cysteine monohydrochloride, 9.0 g/L NaCl).The larger fecal particles were removed by low-speed centrifugation(4 °C, 1 000 r/min, 10 min) and obtained 10% fecal slurry.
The autoclaved basal nutrient medium contained 10 g/L glucose,2 g/L peptone, 2 g/L yeast extracts, 2 g/L NaHCO3, 0.5 g/LL-cysteine hydrochloridet, 0.5 g/L bile salt, 0.1 g/L NaCl, 0.04 g/L K2HPO4,0.04 g/L KH2PO4, 0.02 g/L hemoglobin, 0.01 g/L MgSO4, 0.01 g/L CaCl2, 1 mg/L resazurin, 2 mL/L Tween-80 and 10 µL/L VK1,adjusting pH value to 7.0 with 0.1 mol/L HCl solution.
β-Carotene was dissolved in dimethyl sulfoxide (DMSO),filtered by 0.45 µm organic membrane and obtained the 50, 250 and 500 mg/mL solution. An aliquot of 20 µL of the aboveβ-carotene solution was added with 9 mL autoclaved basal nutrient medium and 1 mL 10% fecal slurry to prepare 0.1, 0.5 and 1.0 mg/mLβ-carotene treatments (BCL, BCM, BCH), respectively. The blank control group(BLK) withoutβ-carotene was added with the same volume of DMSO(0.2%). All treatments were incubated at 37 °C for 24 h in an Anaero Pack system which the oxygen pressure in box was below 0.1%. The box was shaken gently every 4 h during the fermentation. Samples were taken at 0, 6, 12 and 24 h for further analysis.
2.4 Determination of β-carotene
The measurement ofβ-carotene was carried out according to the
reported method with slight modifications [22]. An aliquot of 1 mL sample solution was added with 1 mL trichlormethane:methanol(2:1,V/V) mixture and vortexed for 1 min. The mixture was then added with 2 mLn-hexane, and then centrifuged at 4 °C,3 000 r/min for 5 min. Collected the supernatant, and the lower layer was extracted twice. The supernatant was combined and then dried under N2, dissolved with 1 mL of methanol (chromatographic grade), filtered by 0.45 µm organic membrane for high performance liquid chromatography (HPLC) analysis. 0.1 mg/mLtrans-β-apo-8’-carotenal was used as an internal standard.
An Agilent 1260 HPLC system equipped with a YMC-C30 reversed phase column (4.6 mm × 250 mm, 5 µm, YMC Co., Ltd.,Kyoto, Japan) and a UV-visible diode array detector (DAD) was applied to detectβ-carotene. The column temperature was set at 2 5 °C. T h e m o b i l e p h a s e s w e r e c o m p o s e d o f (A)water:MTBE:methanol (5:25:70,V/V), and (B) water:MTBE:methanol(5:85:10,V/V) with linear gradient elution (0-4.5 min, 98%-80% A;4.5-12.5 min, 80%-40% A; 12.5-18.0 min, 50%-25% A; 18-24 min,25%-5% A; 24-30 min, 5%-95% A). A volume of 20 µL sample was injected into the column. The flow rate was set at 0.6 mL/min, and the elute was detected at 450 nm by DAD. All measurements were performed in triplicate.
2.5 Determination of retinol and retinoic acid
The measurement of retinol and retinoic acid was carried out according to the GB 5009.82-2016. An aliquot of 1 mL sample solution was added with 1 mL petroleum ether: diethyl ether (1:1,V/V)mixture and vortexed for 1 min. The mixture was added with 2 mLn-hexane, and then centrifuged at 4 °C, 3 000 r/min for 5 min.Collected the supernatant, and the lower layer was extracted twice.The supernatant was combined and dried under N2, and then dissolved with 1 mL of methanol (chromatographic grade) and filtered by 0.22 µm organic membrane for HPLC analysis.
An Agilent 1260 HPLC system equipped with a YMC-C30 reversed phase column (4.6 mm × 250 mm, 5 µm, YMC Co., Ltd.,Kyoto, Japan) and a UV-visible DAD was applied to detect retinal and retinoic acid. The column temperature was set at 25 °C. The mobile phases were composed of (A) water and (B) methanol with linear gradient elution (0-4.5 min, 1%-4% A; 4.5-12.5 min, 4% A;12.5-15.0 min, 4%-1% A). A volume of 20 µL sample was injected into the column. The flow rate was set at 0.8 mL/min, and the elute was detected at 325 nm by a DAD. All measurements were performed in triplicate.
2.6 Determination of short-chain fatty acids (SCFAs)
The measurement of SCFAs inin vitroanaerobic fermentation was carried out according to the reported method with slight modifications [23]. An aliquot of 1 mL sample solution was centrifuged at 4 °C, 5 000 r/min for 10 min, and the supernatant was collected and mixed with 2-ethylbutyric acid (an internal standard,25 mmol/L) in equal volume, and then filtered by 0.22 µm organic membrane for gas chromatography (GC) analysis. An Agilent 6 890 N GC system equipped with a HP-INNOWAX column (30 m ×0.25 mm, 0.25 µm, Co., Ltd., Kyoto, Japan) and a flame ionization detector (FID) was applied to detect acetic, propionic,n/i-butyric andn/i-valeric acids. N2as carrier gas at 19.0 mL/min. Flows of air,H2and N2were 260, 30 and 30 mL/min. Respectively, GC column has an initial column temperature of 100 °C and was maintained for 1 min. The column temperature raised to 180 °C at a rate of 5 °C/min and remained 4 min. Lactic acid was tested with commercial kits. An aliquot of 1 mL sample solution after fermented was centrifuged at 4 °C, 5 000 r/min for 10 min. After that, the supernatant was collected and mixed with detection reagent for 30 s, the absorbance was detected by spectrophotometer at 550 nm.
2.7 Gut microflora analysis
The total fecal microflora DNA was extracted using TIANamp a Stool DNA kit (Tiangen, China). The V3-V4 regions of 16S rDNA gene were amplified by PCR using Primer F (Illumina adapter sequence 1 + CCTACGGGNGGCWGCAG) and Primer R (Illumina adapter sequence 1 + GACTACHVGGGTATCTAATCC). Agarose gel electrophoresis (AGE) was used to detect the amplification products. Mixed the three parallel amplification products of the same sample, and each sample was added with the same volume of AgencourtAMpure XP purified magnetic beads to purify the product.Added sample-specific Index Sequence and detected insert size by Agilent 2100 Bioanalyzer to confirm that there was no non-specific amplification found between 120 and 200 bp. Fecal microflora composition was evaluated using Illumina MiSeq sequencing based on QIIME microflora analysis [24]. The sequences were binned into operational taxonomic units (OTUs) based on 97% identification by Usearch.
2.8 Statistical analysis
Statistical analysis was performed using a SPSS 24.0 software(IBM, USA). The Rarefaction curve, Shannon Index curve and Alpha-diversity index, including Chao, ACE, Shannon and Simpson indices were calculated by Mothur. The PCA and CLUST analyze were calculated by Vegan package of R (Vegan package, V 3.5.0,https://www.r-project.org/). The linear discriminant analysis effect size (LEfSe) and the LDA score and Metastats analysis was analyzed by Galaxy (http://huttenhower.sph.harvard.edu/). Data were shown as mean ± SD. Statistical differences were evaluated by one-way analysis of variance (ANOVA) followed by a Tukey’s multiple comparison test. A false discovery rate (FDR) adjustedPvalues < 0.05 were considered significant. The drawing software use Origin 2020 and HemI, etc.
3. Results and discussion
3.1 Effect of gut microflora on β-carotene
Carotenoids released from food were chewed and catalyzed by salivary enzymes, and then mixed with lipids to form lipid-soluble micelles, then they converted into water-soluble micelles after gastrointestinal digestion, finally, they were absorbed by intestinal epithelial cells and used for biological utilization. As shown in Fig. 1A,the bio-accessibility ofβ-carotene was (0.19 ± 0.02)% after the simulated oral digestion, the bio-accessibility ofβ-carotene was(1.08 ± 0.03)% after the simulated gastric digestion, and the bio-accessibility ofβ-carotene was up to (7.61 ± 0.54)% after the simulated intestinal digestion. The results showed similarities with other reports [25,26], and indicated that more than 90% ofβ-carotene are not absorbed and enter into large intestine.
The retention rate ofβ-carotene after 24 h incubation of gut microflora was analyzed. As shown in Fig. 1B, the retention rates ofβ-carotene in the BCL, BCM and BCH groups were (44.34 ± 2.97)%,(35.72 ± 6.23)% and (22.13 ± 3.69)%, respectively, the degradation rates ofβ-carotene in the BCL, BCM and BCH groups were(55.66 ± 2.97)%, (64.28 ± 6.23)% and (77.87 ± 3.69)%, respectively,which were 1.44, 1.46 and 1.46 times that of the BLK group,respectively. Retinal and retinoic acid are the main metabolites ofβ-carotene,β-carotene can be converted into two-molecule retinal byβ-carotene-15,15’-oxygenase (BCMO1) enzyme, and retinal can be hydrolyzed into retinoic acid. The production of retinal and retinoic acid in BCM group were further investigated (Fig. 1C). The concentration of retinal and retinoic acid increased with the increase of fermentation time. After 24 h, the concentrations of retinal and retinoic acid were (0.24 ± 0.00) and (0.80 ± 0.00) μg/mL, which were 1.99 and 1.06 times that of the BLK group, respectively. The BCMO1 have only been reported in human and animals, and have not been found in gut microflora yet [27,28]. In this study, more than 55% ofβ-carotene was degraded in simulated gut microflora environment,and the production of retinal was nearly 2 times of the group without gut microflora. The results indicated that the gut microflora can metabolizeβ-carotene into retinal and retinoic acid.
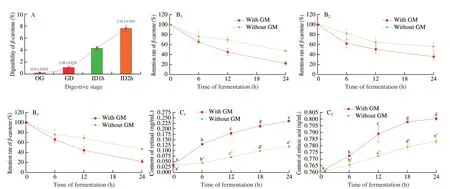
Fig. 1 (A) The digestibility of β-carotene in different simulated digestion stage. OG: Oral digestion; GD: Gastric digestion; ID1h: Intestinal digestion for 1 h;ID2h: Intestinal digestion for 2 h. (B) The retention rate of β-carotene in fermentation solutions during 24 h with different doses of β-carotene. The retention rate of 0.1 (B1), 0.5 (B2), 1 mg/mL (B3) β-carotene. GM: Gut microflora. (C) The concentration of retinal (C1) and retinoic acid (C2) in fermentation solutions during 24 h.
3.2 Effect of β-carotene on SCFAs production
As shown in Table 1, the concentration of SCFAs in different groups increased with the increase of fermentation time. After 24 h of fermentation, the total SCFAs concentration of BCL, BCM and BCH groups were (36.25 ± 5.51), (44.00 ± 1.16), (39.80 ± 5.56) mmol/L,which were 1.87, 2.26 and 2.05 times that of the BLK group,respectively. Among them, acetic acid, propionic acid and lactic acid were the main SCFAs. The production of acetic acid, propionic acid and lactic acid in BCL, BCM and BCH groups were all significantly higher (P <0.05) than the BLK group. When comparing the three different dose-groups, the BCM group showed significantly higher (P <0.05) SCFAs production than other two groups, and the general trend of different SCFAs concentration was in the order: BCM > BCH > BCL. However, butyric acid and valeric acid occurred in all groups without significant differences between different groups.

Table 1Concentrations of SCFAs in fermentation solutions at different time points of fermentation.
SCFAs, especially lactic acid, acetic acid, propionic acid, butyric acid are the main metabolites by gut microflora, they play important roles in maintaining the normal function of the large intestine and the morphology and function of colon epithelial cells [29]. For example,BifidobacteriumandLactobacilluscan metabolize dietary nutrients to produce acetic acid, propionic acid, butyric acid and lactic acid,which can inhibit the reproduction of intestinal spoilage bacteria and pathogenic bacteria, and improve immune function [30,31]. Butyric acid can increase the production ofLactobacillus, and reduce the number ofEscherichia coli, and benefit the intestinal micro-ecology [32].In this study,β-carotene significantly promote the production of SCFAs, strongly suggested that gut microflora could utilizeβ-carotene and secrete SCFAs. Meanwhile, the production of SCFAs among different groups showed difference, which might due to that different dose ofβ-carotene could shift the composition or abundance of gut microflora in different extent by increasing the abundance of some acetic acid, propionic acid-producing bacteria, such asBifidobacterium, and then led to the different production of SCFAs [24].
3.3 Effect of β-carotene on gut microflora structure
Alpha-diversity, beta-diversity and species composition were analyzed to explore the pattern of microbial variation [22,33]. In this experiment, 700 000 valid reads were obtained from 12 samples. As shown in Fig. S1, the rarefaction curve and Shannon index curve were balanced as the number of reads increases, which indicated that enough sequencing data were obtained and the sequencing depth were reasonable.
Alpha-diversity indexes of each groups were shown in Table 2. The Chao and ACE indexes showed no significant difference (P> 0.05)among different groups, which indicated that the richness of bacterial community did not change obviously with the addition ofβ-carotene. The Shannon indexes ofβ-carotene groups were higher than the BLK group, among them, BCH group showed significant difference (P< 0.05). Meanwhile, the Simpson index of BCH group was significantly lower than BLK group. The Shannon and Simpson indexs indicated that the addition ofβ-carotene can improve the uniformity of bacterial community [33].
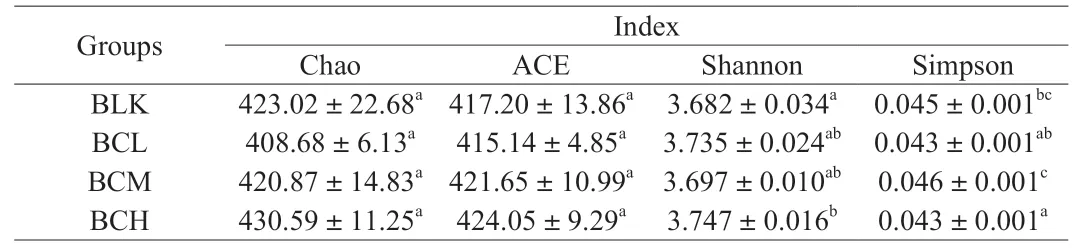
Table 2Alpha-diversity of gut microbiota including Chao, ACE, Shannon, Simpson index.
Beta-diversity analysis was used to evaluate the species complexity among different groups. As shown in Fig. 2, the same treatment was clustered in PCA and CLUST analysis. PC1 and PC2 axis in PCA diagram explained 86.367% of the overall difference ofin vitrofermentation microflora. Supplementation ofβ-carotene had significant effects on gut microflora (PC1 interpretation rate was 76.433%). In addition, allβ-carotene groups showed significant distance to the BLK group. These results indicated thatβ-carotene significantly change the structure and composition of gut microflora.Among the threeβ-carotene groups, PC2 interpretation rate was 9.934%, and the distance between the BCL and BCM group was closer than the BCH group. The results showed that the doses ofβ-carotene also affect the structure of gut microflora in a certain degree, while BCL and BCM groups showed more similarities.
The differences of gut microflora composition were further analyzed. Fig. 3 showed the distribution of gut microflora at the phylum and genus level in different treatments. At the phylum, the gut microflora was mainly composed of Bacteroidetes, Firmicutes,Proteobacteria and Actinobacteria, which was consistent with the results of other studies [34]. Compared with the BLK group, the abundance of Bacteroidetes, Firmicutes and Proteobacteria in allβ-carotene treatments were increased, and the Firmicutes showed significant differences. Meanwhile, Actinobacteria was decreased in allβ-carotene treatments. A study has shown that the proportions of Bacteroidetes and Proteobacteria in mice with intestinal diseases were significantly lower than normal mice, while astaxanthin supplementation significantly increased the level of bacteria [19].Supplement withβ-carotene also increased relative abundance of Bacteroidetes and Proteobacteria, which might support that theβ-carotene play positive roles in intestinal health. At the genus,β-carotene treatment increased the abundance ofParasutterella,Lachnospiracea,RoseburiaandDoreaetc., while decreased the abundance ofDialisterandEnterobateretc. In addition, low and medium doses ofβ-carotene treatment increased the abundance ofBifidobacteriumandCollinsella, while high dose ofβ-carotene treatment reduced the abundance ofLactobacillus. This might due to the optimal concentration ofβ-carotene to promote the growth of bacterial species is different, and high doses ofβ-carotene even has a certain inhibitory effect onLactobacillus.
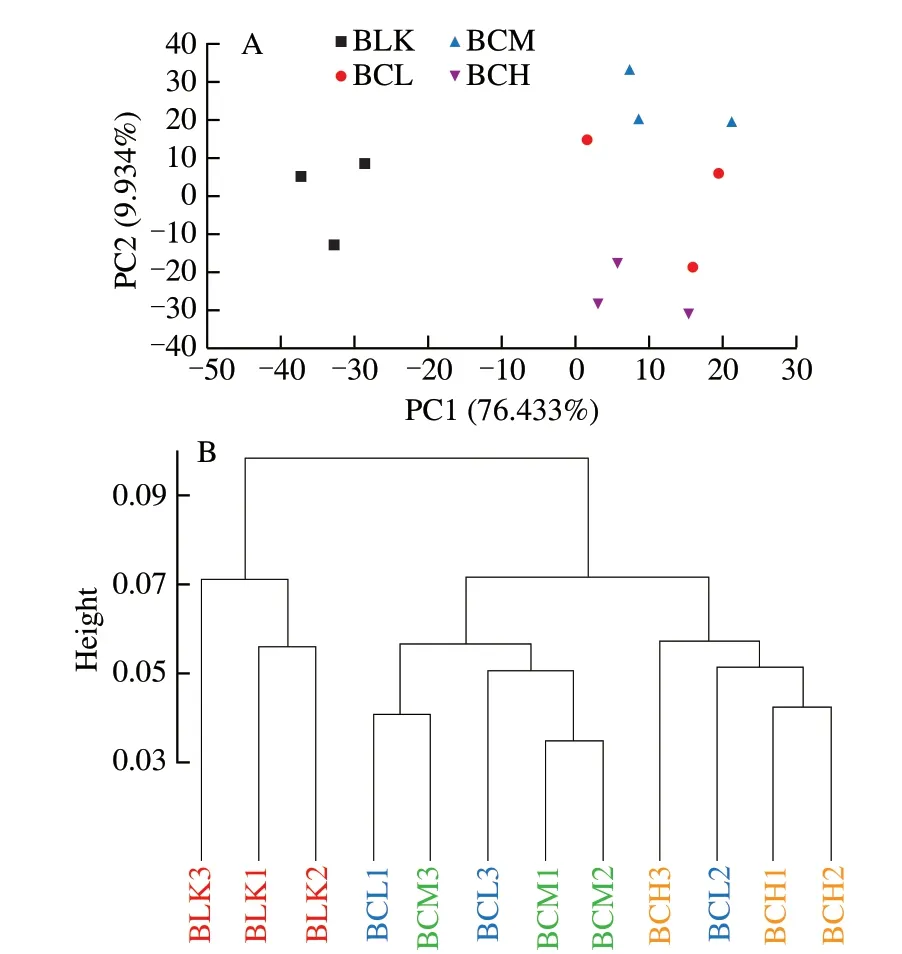
Fig. 2 Beta-diversity of gut microbiota including PCA and CLUST analysis.(A) The PCA analysis of gut microflora; (B)The CLUST analysis of gut microflora.

Fig. 3 Species composition analysis of gut microbiota. (A) Phylum level;(B) genus level.
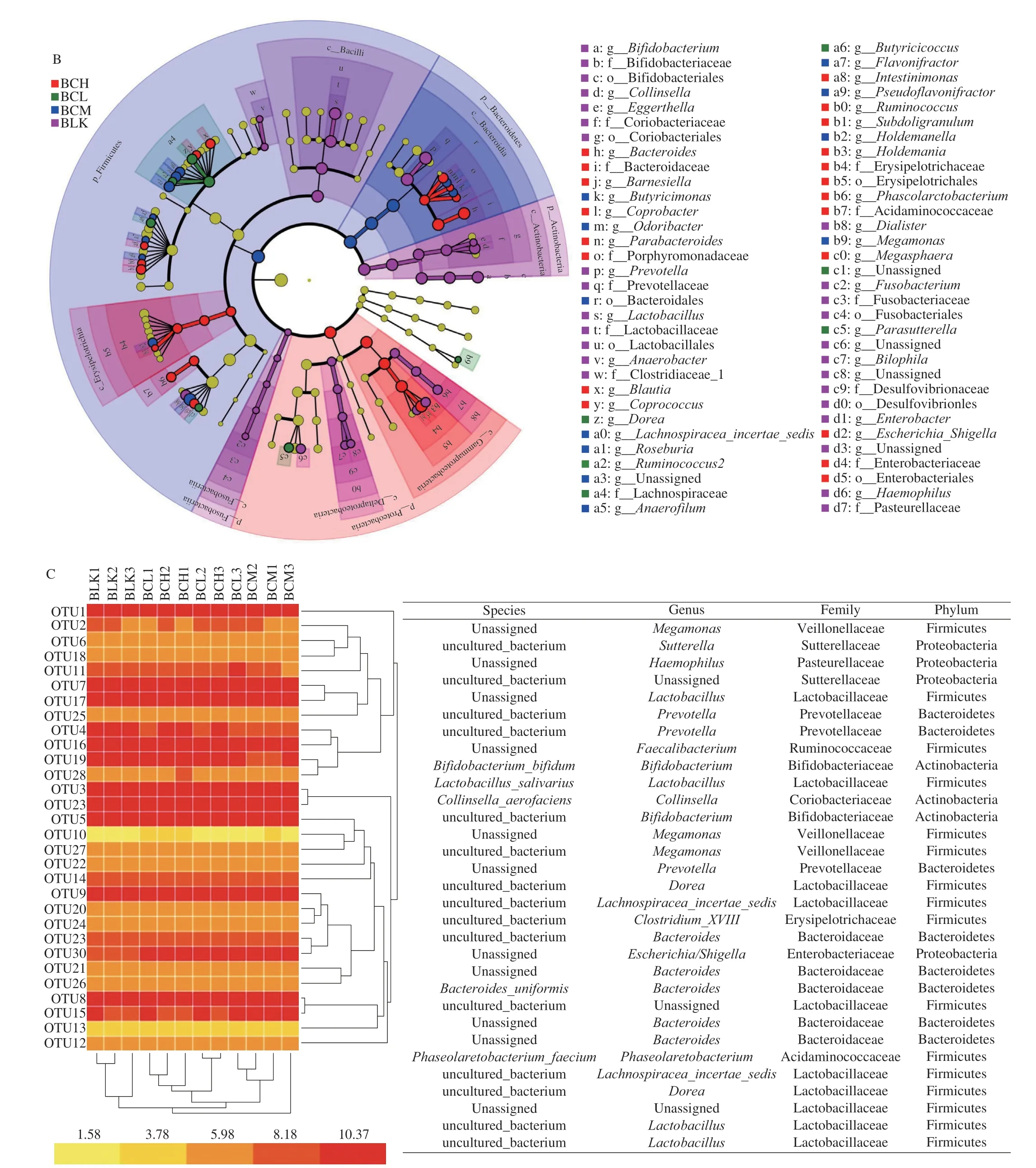
Fig. 4 (Continued)
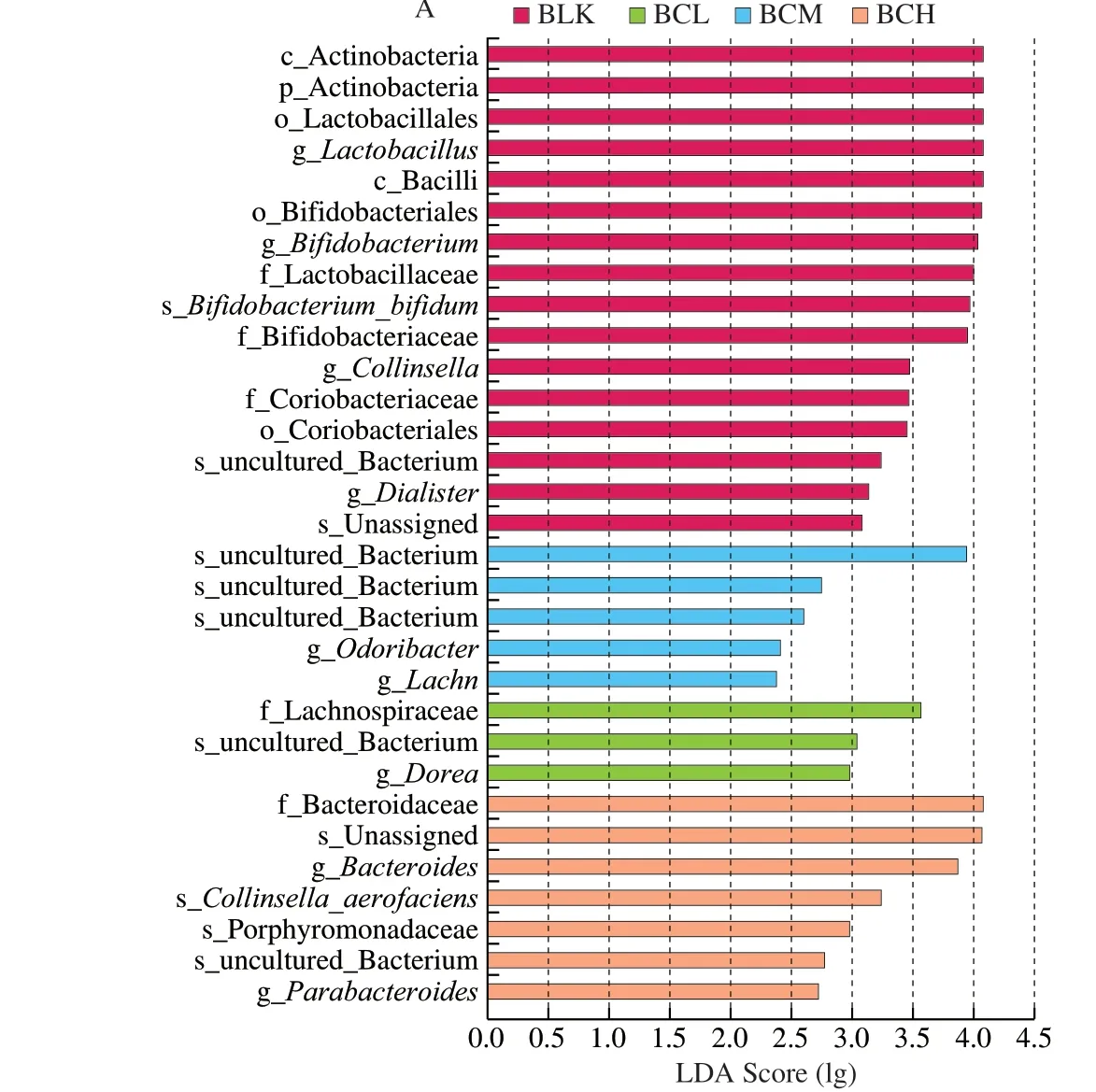
Fig. 4 The species composition analysis of gut microbiota. (A) Distribution histogram based on LDA; (B) Heat map based on LDA analysis; (C) Bacteria taxonomic information regulated by β-carotene (genus, family, phyla).
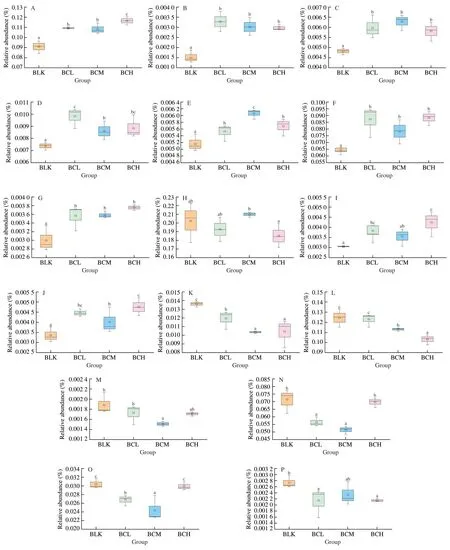
Fig. 5 Predominance species profiles based on Metastats analysis. (A) Bacteroides, (B) Parasutterella, (C) Roseburia, (D) Dorea, (E) Lachnospiracea_incertae_sedis,(F) Bifidobacterium, (G) Blautia, (H) Megamonas, (I) Phaseolaretobacterium, (J) Subdoligranulum, (K) Dialister, (L) Lactobacillus, (M) Enterobater,(N) Escherichia_Shigella, (O) Collinsella, (P) Haemophilus.
To research the key gut strains, the Lefse and Metastats analysis was further conducted. OTUs with relative abundance greater than 0.5% were screened for analysis based on LDA score (lg). As shown in Fig. 4, there were 3 higher OTUs in BCL group, 5 higher OTUs in BCM group and 7 higher OTUs in BCH group. Lachnospiraceae,Ruminococcus,DoreaandButyricicocouswhich belong to Firmicutes andParasutterellawhich belongs to Proteobacteria were key genus in BCL groups.Roseburia, Lachnospiraceae,AnaerofilumandPseudoflavonifractorwhich belong to Firmicutes and Bacteroidetes were key genus in BCM groups.Erysipelotrichaciaand Bacteroidetes were key genera in BCH group. These key genera were the main factors that cause differences between different groups. As shown in Fig. 5, the Metastats analysis showed thatβ-carotene significantly promoted the abundance ofRoseburia,Parasutterellaand Lachnospiraceae. Previous studies have shown thatRoseburiais a common butyric acid-producing bacterium, and can reduce inflammatory markers [35]. Lachnospiraceae is a potentially beneficial bacteria participates in the production of acetic acid [36].In this study,β-carotene significantly promoted the abundance ofRoseburia,Bacteroidesand Lachnospiraceae, also increased the production of butyric acid and acetic acid, which was consistent with the SCFAs results. Another study also reported that supplement with lycopene significantly increase the abundance of Lachnospiraceaein mice model [24].β-Carotene, lycopene, and other carotenoids have polyisoprene structures. We inferred that the utilization ofβ-carotene by gut microflora might begin with this structure. In this study, the production of VA increased with all treatments, which proved that the utilization of carotenoids by gut microflora might started at C15-C15’double bond. Meanwhile,β-carotene significantly inhibited the abundance ofDialister, which is a microbial marker of inflammatory bowel disease [37], indicating thatβ-carotene could protect the body by inhibiting the proliferation of some harmful bacteria in the gut.
4. Conclusion
In this work,in vitroanaerobic fermentation model was used to study the interaction betweenβ-carotene and gut microflora.The results showed that the response value ofβ-carotene decreased gradually with the increase of fermentation time, and the content of retinal, retinoic acid and SCFAs inβ-carotene groups increased gradually, indicating that gut microflora could degrade and utilizeβ-carotene. Meanwhile,β-carotene can change the composition of gut microflora by increasing the abundance ofRoseburia,Parasutterella,and Lachnospiraceae. In addition, different doses ofβ-carotene had different manifestations. Our results indicated that the gut microflora might be a potential target forβ-carotene’s utilization, which could provide further evidence for its molecular mechanism.
Declaration of conflict of interest
The authors declare no competing financial interests.
Acknowledgements
This work was supported by the project of the National Natural Science Foundation of China (31801541), the Independent Innovation Fund Project of Agricultural Science and Technology in Jiangsu Province (CX(20)3045), and Major Scientific and Technological Achievements Transformation project of Taizhou (SCG 202105).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.10.030.
杂志排行
食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents
