The benef icial effects of Tartary buckwheat (Fagopyrum tataricum Gaertn.) on diet-induced obesity in mice are related to the modulation of gut microbiota composition
2023-01-03YimingZhouHoyuLuShenZhoBeieiYnHongWngXioliZhouYingXio
Yiming Zhou, Hoyu Lu, Shen Zho, Beiei Yn, Hong Wng, Xioli Zhou,,*, Ying Xio
a School of Perfume and Aroma Technology, Shanghai Institute of Technology, Shanghai 201418, China.
b University Think Tank of Shanghai Municipality, Institute of Beautiful China and Ecological Civilization, Shanghai 201418, China
Keywords:Tartary buckwheat High-fat diet Biochemical parameters Gut microbiota Lipid metabolism
A B S T R A C T The gut is home to a large number of intestinal microbiota that play an important role in the metabolism and immune system of the host. A growing body of evidence suggests that a high-fat diet is closely associated with many metabolic disorders, including fatty liver and type 2 diabetes. According to reports, Tartary buckwheat extract has a positive effect on intestinal microbiota in animals. The effects of Tartary buckwheat on biochemical indexes and intestinal microflora in mice were studied. Tartary buckwheat protein (FGP),Tartary buckwheat resistant starch (FGS) and Tartary buckwheat flour (FGF) alleviated organ damage in mice and lowered the atherosclerotic index (AI) in plasma. Otherwise, principal coordinate analysis(PCoA) showed that intestinal bacterial structure of FGF were separated apparently from other groups. The Firmicutes/Bacteroidetes (F/B) value of the high-fat (HF)-FGF group was significantly lower than that of the HF-FGP and HF-FGS groups. FGF signif icantly increases the abundance of benef icial bacteria such as Bif idobacterium, while decreasing the abundance of lipopolysaccharide (LPS)-producing bacteria. Observation of blood lipid metabolism parameters and analysis of the intestinal microbiota suggested that FGF can be more effective than FGP and FGS to reduce the effects of a high-fat diet in mice, restoring the blood parameters to values similar of those in mice fed a low-fat diet. FGF may be used to prevent or treat blood lipid metabolism disorders and intestinal microbiota disorders in mice fed a high-fat diet.
1. Introduction
With the global economic development and the change of people’s lifestyle, the incidence of glucose/lipid metabolism diseases,such as obesity, hyperlipidemia, and type 2 diabetes mellitus (T2DM),is increasing yearly [1,2]. At present, people usually prevent and treat cardiovascular and cerebrovascular diseases by means of blood lipid-regulating drugs. However, long-term drug therapy can bring about many problems, such as a change in life quality, side effects of drugs, and a heavy economic burden of drug costs. Studies have shown that hyperlipidemia is closely related to people’s diet, and it is an effective way to prevent and treat hyperlipidemia by adjusting and improving people’s food structure [3]. Therefore, it is of great practical significance to effectively regulate the body’s energy balance, intervene with the occurrence of diabetes, and improve the human immune system by adjusting the dietary structure and
improving the human intestinal f lora [4,5].
The structure of the gut microbiome is relatively stable under normal conditions, but several studies have shown that nutrients such as protein, sugars, dietary fiber (DF), and polyphenols in the diet or dietary supplements can affect the gut microbiome. Studies have shown that the amount of protein intake is positively correlated with the diversity of intestinal microbes [6,7]. Compared to protein,the impact of carbohydrates such as oligosaccharides and DF,which cannot be directly digested and absorbed by the body, on gut microbes is higher. Such compounds can be fermented by intestinal microorganisms to provide energy for the host, changing the intestinal environment in the process, improving the abundance of intestinal flora and the number of beneficial bacteria such as bifidobacteria and lactic acid bacteria [8-10]. Given the link between the gut microbiome and diet, improving the gut microbiome through diet changes may have a preventive and therapeutic effect on some diseases. So, a lot of research revolves around finding the right dietary ingredients.
Tartary buckwheat (Fagopyrum tataricumGaertn.), which is a grain crop that is used both as food and as medicine, is widely grown in the high-altitude areas of Southwest China [11,12]. It contains a lot of starch, protein, and other nutrients and it is a good dietary raw material. Many studies have shown that Tartary buckwheat intake has protective effects against several chronic diseases, including hypertension, obesity, cardiovascular diseases, and T2DM [13,14].These effects are mainly attributed to the resistant starch (RS),protein, and phenolic substances in the grain.
The content of starch, the main component, in Tartary buckwheat is more than 60%. Moreover, it is rich in amylose, which becomes weakly alkaline after heat treatment and has a good relieving effect on the excessive production of stomach acid. Starch can be divided into rapidly digested starch (RDS), slowly digested starch (SDS),and RS. Generally speaking, digestible starch can be broken down in the small intestine into free glucose byα-amylase and glucosylase,which can be absorbed by the body. But not all starch is absorbed by the small intestine of the human body. The resistance of some starch to enzymatic hydrolysis is so strong that it cannot be digested and enter the large intestine. This kind of starch is called RS. RS is a kind of polysaccharide substance, which can be regarded as DF from the perspective of physiological function [15]. It cannot be digested and absorbed in the small intestine to provide glucose, but it can be utilized by microorganisms in the large intestine, mainly in the cecum and colon, to slowly ferment to produce short-chain fatty acids (SCFAs), such as acetic acid, propionic acid, and butyric acid [16,17]. Part of the SCFAs is absorbed through the intestinal tract and then enters the blood circulation system. The body utilizes SCFAs through a series of biochemical effects to exert certain physiological functions and affect the absorption of other substances to regulate intestinal microflora and lipid metabolism [18,19]. In addition, RS can also regulate blood glucose and lipid levels [20,21].
Protein accounts for about 15%-17% of the dry weight ofFagopyrum tataricumGaertn. (FG) grain, mainly including albumin,globulin, gluten, and gliadin. The contents of gliadin and gluten are lower, while the contents of albumin and globulin are higher.Different varieties of FG have different protein content, but the difference is not obvious. Tartary buckwheat protein (FGP) extract reduces total cholesterol (TC) levels, inhibiting the accumulation of fat in the body, improving superoxide Dismutase (SOD),catalase (CAT), and total antioxidant capacity (T-AOC) levels, and promoting excretion. The regulatory effects of FGP extract on blood lipid are more beneficial than those of soybean protein [22,23].Therefore, in recent years, more and more research has focused on: (i) the effects of FG on obesity and related diseases and (ii) the development of related products with preventive effects on lipid metabolism [24].
Most research on FG mainly focuses on the preparation, physical and chemical properties, and sensory quality, but the beneficial regulatory effects on intestinal flora and hyperlipidemia are rarely studied. In this paper, FG grains were used as raw materials and Tartary buckwheat (FGF), Tartary buckwheat RS (FGS) and FGP were added to mouse feed. Fresh blood and fecal samples were collected. Lipid analysis and high-throughput sequencing were carried out to explore the effects of FG intervention on lipid metabolism and intestinal microflora in mice fed a high-fat diet (HFD). Therefore, the value of FG as a functional food raw material was determined, which provided dietary guidance for obese people to improve their health.
2. Materials and methods
2.1 Materials and chemicals
Tartary buckwheat (‘Heifeng No. 1’, Zuoyun, Shanxi,China) was used to prepare diet supplements. Sodium hydroxide,hydrochloric acid, anhydrous ethanol, etc. (Sinopharmaceutical Group Shanghai Chemical Reagent Co., Ltd., China), TC determination kit, total triglyceride (TG) determination kit, high-density lipoprotein-cholesterol (HDL-C) determination kit, low-density lipoprotein-cholesterol (LDL-C) determination kit (Nanjing Jiancheng Bioengineering Institute, China), low-fat diet mouse feed.
2.2 Preparation of FGF, FGP, and FGS
Tartary buckwheat was ground into powder and sieved through 80-mesh sieves to obtain FGF.
The prepared FGF was defatted with petroleum ether for 24 h and air-dried naturally. The defatted FGF was dissolved in distilled water at a ratio of solid to liquid of 1:10, and the pH was adjusted to 8 with 1 mol/L NaOH. Magnetic stirring was performed at 4 °C and 50 r/min for 2 h. After stirring, samples were centrifuged at 5 000 r/min for 20 min. After centrifugation, the supernatant was taken, and the pH was adjusted to 4.5 with 1 mol/L HCl. The precipitate was taken after standing. The precipitation was washed with distilled water two times and neutralized with 0.1 mol/L NaOH. The FGP was obtained by freeze-drying for 24 h.
The preparation process of RS was as follows. The weighed 100 g starch sample was added to 500 mL distilled water to make a starch milk solution. The pH was adjusted to 6.0. After pre-gelatinization in a boiling water bath, added high-temperatureα-amylase (5 U/g starch) for enzymatic hydrolysis at 96 °C for 30 min. Took out and cooled. Then added a certain amount of distilled water and adjusted the pH to 4.5. Pullulanase (5 U/g starch) was added and enzymolyzed at 55.8 °C for 8 h. Then autoclave treatment was conducted at 121 °C for 30 min. Finally, the samples were taken out and cooled to room temperature, then placed in a 4 °C refrigerator for 24 h. FGS was obtained by taking out the recovered starch, drying it at 60 °C,crushing it, and sifting it through a 100-mesh sieve.
2.3 Animals and groups
Forty-five 3-week-old male SPF C57BL/6 mice (body weight 90-100 g) were purchased from Shanghai Slack Laboratory Animal Co., Ltd., certificate No.: SCXK (Shanghai) 2007-0005 (Shanghai,China). The mice were fed a basal diet for 2 weeks and randomly divided into 5 groups (n= 9 mice per group). The mice were kept in the same room and in separate cages, with free access to food and water throughout the experiment. The relative humidity of the room was kept at (50 ± 10)%, and the room temperature was kept at(22 ± 2) °C. Mice were kept under a 12 h/12 h light/dark cycle (lighting from 6:00 to 18:00).
The mice were randomly divided into the following five groups:the low-fat diet (LFD) group, the HFD group, the FGF intervention(HF-FGF) group, the FGS intervention (HF-FGS) group, and the FGP intervention (HF-FGP) group. The feed was prepared once every 3 days and stored in a refrigerator at 4 °C.
2.4 Preparation of FGF, FGP, and FGS
After feeding for 6 weeks, the mice were anesthetized with ether at 8:00 in the morning, and blood was collected from the eyes. The blood was immediately centrifuged in a centrifuge tube pretreated with heparin sodium (4 °C, 4 000 r/min, 15 min). After centrifugation,the supernatant (plasma) was stored at -80 °C until further analysis.
The liver, duodenum and colon of the mice were dissected immediately, and each tissue was weighed and fixed in 10%
formalin solution for about 0.1 g. After fixation, tissue sections were
prepared and hematoxylin-eosin (HE) staining was performed, and the histopathological changes of tissue sections in each group were observed under microscope.
2.5 Preparation of FGF, FGP, and FGS
Plasma TG, TC, HDL-C, and LDL-C contents were determined using kits following the manufacturers’ instructions.
The atherosclerotic index (AI) was calculated as follows:

2.6 Fecal DNA extraction and amplification
The feces of mice in each group were collected and stored at -80 °C for analysis. The bacterial DNA from the mouse feces was extracted using the E.Z.N.A.®soil DNA Kit (Omega Bio-tek,Norcross, GA, USA) according to manufacturer’s instructions.The V3-V4 region of 16S rRNA genes was amplified by polymerase chain reaction (PCR) using the forward primer 338F(5’-ACTCCTACGGGAGGCAGCA-3’) and the reverse primer 806R (5’-GGACTACHVG GGTWTCTAAT-3’). PCR reactions were performed with the following protocol: pre-denaturation at 95 °C for 3 min, 27 cycles of denaturing at 95 °C for 30 s, annealing at 55 °C for 30 s, elongation at 72 °C for 45 s and a final annealing extension step at 72 °C for 10 min. Amplicons were purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City,CA, USA) and quantified using QuantiFluorTM-ST (Promega, USA).Purified amplicons were sequenced on the Illumina MiSeq platform(Illumina, San Diego, USA) by Majorbio Bio-Pharm Technology Co.,Ltd. (Shanghai, China).
2.7 Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM).The statistical significance of the differences was analyzed using one-way ANOVA, along with Tukey’s multiple comparison test.SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) was used for all analyses andPvalues below 0.05 were considered to be statistically significant.
3. Results and discussion
3.1 Morphological analysis of tissue sections
3.1.1 Effects of FG on liver histopathology
Microscopic analysis of liver sections of mice showed that liver cells in the LFD group were spherical, evenly distributed, and full and complete (Fig. 1). The livers of mice from the other 4 groups(fed a HFD) had different degrees of lipidosis and inflammatory cell infiltration, among which the observed effects were strongest in the HFD group. There were many large granules of cavities in the liver cells of the mice, including adipose tissue, which proved that a HFD could cause liver damage in mice. However, this phenomenon was alleviated in the HF-FGP, HF-FGS, and HF-FGF groups to varying degrees. Liver cell fat cavities in the HF-FGP group were significantly reduced, followed by the FGP group. No cavity was found in the HF-FGF group, but the cell distribution was uniform, and the morphology was basically the same as that in the LFD group. The results indicated that the three supplements had certain inhibitory effects on liver injury in mice, among which FGF had the best inhibitory effect, followed by FGP, and FGS had the weakest effect.
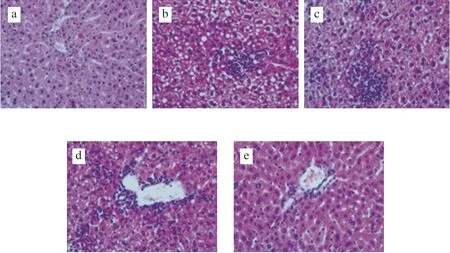
Fig. 1 Microscopic examination of mouse liver sections (40×). (a) LFD,(b) HFD, (c) HF-FGP, (d) HF-FGS, (e) HF-FGF group.
3.1.2 Effects of FG on colon histopathology
A HFD can cause colitis. We analyzed the changes in the colon tissue of mice and the influence of different food supplements. As can be seen in Fig. 2, the colon epithelium of mice in the LFD group was intact, and no inflammatory cell infiltration was found. Different degrees of inflammatory cell infiltration and increased vascular density in the lamina propria were observed in the colon tissue of the other 4 groups.

Fig. 2 Microscopic examination of mouse colon sections (20×). (a) LFD,(b) HFD, (c) HF-FGP, (d) HF-FGS, (e) HF-FGF group.
Inflammatory cell infiltration and severe edema were found in the sections of the HFD and HF-FGS groups, while this situation was well relieved in the HF-FGF and HF-FGP groups. The colon tissue in the HF-FGF group was more complete and more normal. These results indicate that FGP and FGF can alleviate colonic inflammation caused by a HFD in mice, and FGF can better inhibit colonic lesions.
3.1.3 Effects of FG on duodenum histopathology
Compared with the control group (Fig. 3b), duodenal villi in the HFD group (Fig. 3a) were disintegrated and broken, with more inflammatory secretions in the lumen and tissue fluid exudated into the space to form edema. As shown in Fig. 3, duodenal inflammation of mice in different intervention groups was improved to varying degrees,and the number of goblet cells and inflammatory cells was reduced.
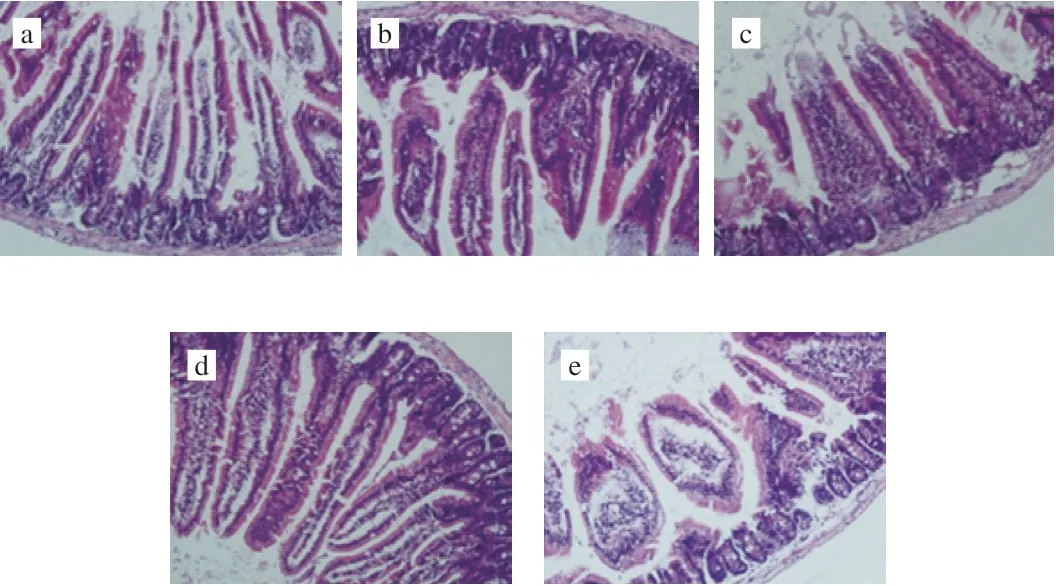
Fig. 3 Microscopic examination of mouse duodenum sections (20×).(a) LFD, (b) HFD, (c) HF-FGP, (d) HF-FGS, (e) HF-FGF group.
As can be seen from Fig. 3c, FGS alone did not exert significant protective effects on the duodenum. Previous studies have shown that FGP can promote the growth of beneficial intestinal bacteria, inhibit the growth of pathogenic bacteria, and promote the production of intestinal SCFAs to protect the intestinal environment. Microscopic analysis of duodenal tissue sections (Fig. 3d) showed that the rupture of duodenal villi in mice fed a HFD with FGP was well alleviated,which also confirmed the claim that FGP could protect the intestinal tract, as previously reported [25]. As shown in Fig. 3e, FGF can inhibit duodenal inflammation and villus rupture caused by a HFD in mice, and edema was also improved.
3.2 Blood lipid parameters
The plasma lipid levels and AIs of mice are shown in Table 1.The contents of TC and LDL-C in the HFD group were increased by 61% and 280% compared with those in the LFD group (P< 0.05),respectively. Plasma TC and LDL-C contents in the HF-FGS,HF-FGF, and HF-FGP groups were significantly lower than those in the HFD group (P< 0.05); in the HF-FGF group, TC and LDL-C levels were 27.33% and 35.67%, lower than in the HFD group, respectively.TG and HDL-C levels in the HFD group were significantly lower than those in the LFD group (P< 0.05). Plasma TG levels in the HFD,HF-FGS, and HF-FGP groups were significantly lower than those in the LFD group (P< 0.05), but there was no significant difference among the three groups (P> 0.05). These results indicated that a HFD could decrease plasma TG levels in mice, and the three intervention methods had no obvious alleviating effect on this decrease.
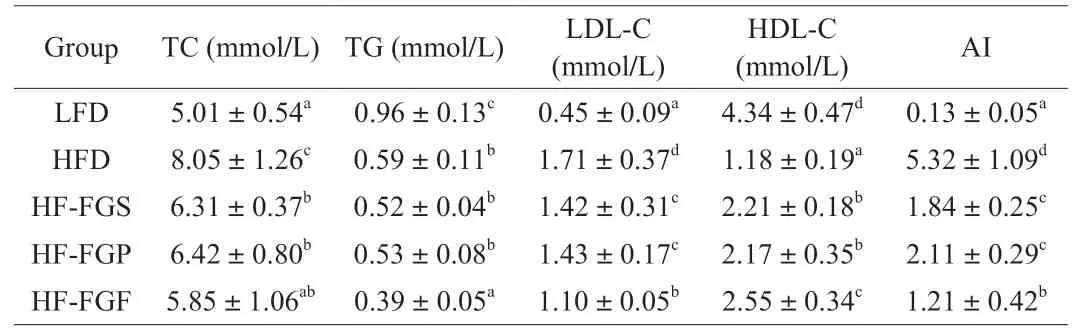
Table 1Plasma lipid levels of mice in each group.
The HDL-C levels were significantly lower in the LFD group than in the other 4 groups (P< 0.05), and there was no significant difference between the HF-FGS and HF-FGP groups (P> 0.05).The plasma HDL-C content of the HF-FGF group was more similar to that of the LFD group, but there was still a significant difference(P< 0.05). Plasma LDL and HDL levels are commonly used clinical lipid indicators. LDL is responsible for transporting endogenous cholesterol to peripheral tissues. High levels of LDL increase the probability of atherosclerosis due to its small particles, which easily enter arteries and are easily oxidized. High levels of HDL can inhibit atherosclerosis because it reversely transports cholesterol from peripheral tissues to the liver for metabolism. The plasma HDL-C content of mice fed a HFD was significantly decreased and LDL-C levels were increased, indicating that a HFD is more likely to lead to atherosclerosis. FGP, FGS, and FGF can inhibit the development of atherosclerosis caused by a HFD in mice to different degrees, and FGF exhibited the strongest inhibitory effects.
Consistent with previous research, the plasma AI (Table 1) of the HFD group was significantly higher than that of the LFD group(P< 0.05) and was the highest among the five experimental groups [26].There was no significant difference between the HF-FGS and HF-FGP groups (P> 0.05), but the AI in these groups was significantly lower than in the HFD group (P< 0.05). The AI in the HF-FGF group was more similar to that of the LFD group.
These results indicated that a HFD could significantly increase the AI in mouse plasma. FGS and FGP could both reduce the elevation of the AI in mice fed a HFD, but the effect of FGF was the strongest.Based on the above analysis, a HFD can significantly increase the TC content, LDL-C content, and AI of blood lipid in mice, leading to dyslipidemia [27]. The three intervention methods in this paper all exert different regulatory effects on dyslipidemia in mice fed a HFD.Generally speaking, the best intervention method is FGF intervention.
3.3 Effects of FG on microbial community richness and diversity
3.3.1 General OTU distribution
The 338F_806R region in gut microbiota was amplified. In the 45 fecal samples tested, the total number of optimized sequences was 2 198 194, the number of optimized base pairs was 961 249 214, and the average length of optimized sequences was 438 bp. As we can see, Table 2 is the basic information for sequencing.composition of the sequencing samples between and within the groups can be analyzed. The closer the sample points are in the figure,the more similar the sample species composition is. The sample community composition was studied by PCoA. It was found that the samples of the LFD, HFD, HF-FGP, HF-FGS, and HF-FGF groups were aggregated, respectively, and the 5 groups were distributed at different coordinates, indicating that the microbial composition of the samples in each group was similar, but the microbial composition among the groups was different to some extent.

Table 2Basic sequencing information.
According to the PCoA results, the distance between the HF-FGS and HF-FGP groups was relatively short, indicating that the species composition difference was small. The HF-FGS group and the HFD group showed most overlap, indicating that the sample species composition between the two groups was similar, which was consistent with the results in Fig. 5a. Combined with the analysis of Fig. 5a, a HFD can cause changes in the species composition of intestinal flora of mice. FGP can inhibit the effects of a HFD on intestinal flora structure, and FGF has a stronger effect, but FGS has almost no effect on the flora structure. Therefore, we speculate that FGP plays a major role in regulating the intestinal flora structure and promoting intestinal health.
Rarefaction curve analysis can be used to judge the quality of sequencing data. As shown in Fig. 4a, the Shannon index of each sample increases as the amount of sequencing data randomly selected from the 5 groups’ increases. The rank-abundance curve can explain the species abundance and species evenness of the intestinal microflora, and the smoothness of the curve can reflect the species evenness in the sample. The flatter the curve, the more even the species distribution is. According to the rank-abundance curve(Fig. 4b), as the number of OTUs increases, the relative abundance of each group of samples reaches a plateau. Therefore, the sequencing depth of the 5 groups of samples in this experiment is acceptable.
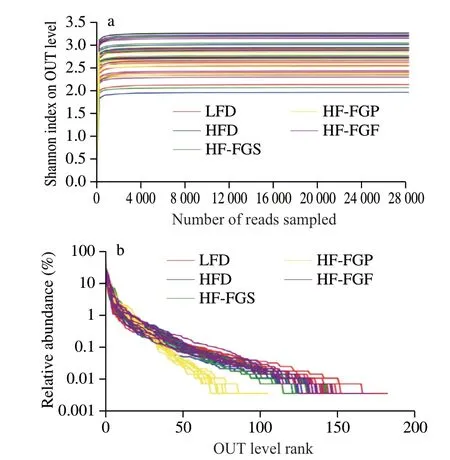
Fig. 4 Rarefaction curve (a) and rank-abundance curve (b).
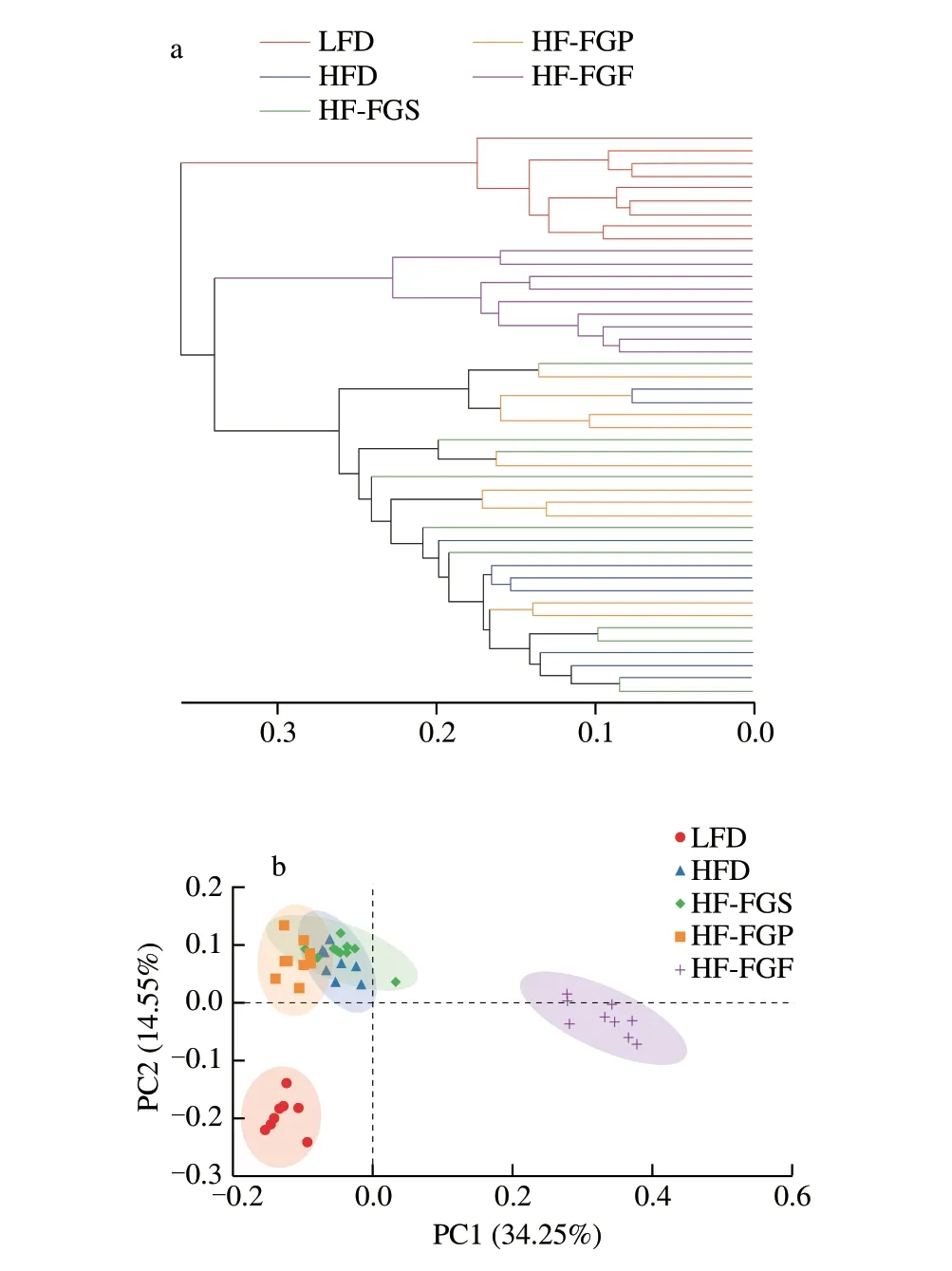
Fig. 5 Hierarchical clustering analysis and PCoA of samples. (a) Hierarchical clustering tree at the OTU level. (b) PCoA at the OTU level.
3.3.2 Comparative analysis of fecal samples in mice
PCoA is a non-restrictive data dimensionality reduction analysis method. Through PCoA, differences of the bacterial community
3.3.3 Species composition analysis of intestinal flora in mice
We created a Venn diagram to visualize the numbers of common OTUs assigned at a 97% sequence similarity threshold among the 5 groups. As can be seen from Fig. 6a, the total number of OTUs in the five groups was 233, among which 125 OTUs (53.65%) were shared by all fecal samples. The number of unique OTUs in each group was as follows: 11 (4.72%) in the LFD group, 3 (1.29%) in the HFD group, 5 (2.15%) in the HF-FGS group, 6 (2.58%) in the HF-FGP group, and 23 (9.87%) in the HF-FGF group. The proportion of the number of unique OTUs was very small, indicating that there were some differences in the bacterial community composition among the samples, but the difference was small.

Fig. 6 Venn diagram (a) and heatmap (b) of intestinal flora in mice.
Heatmaps can be used to visualize information in a two-dimensional matrix or a table with different colors. It can intuitively express data values in the defined color depth. As can be seen from Fig. 6b, the LFD group and the HFD groups have inter-group differences, and the 4 HFD groups are at the same level. Among the 4 HFD groups, the community composition of HF-FGF and LFD was the most similar at the phylum level, indicating that FGF can alleviate the interfering effects of a HFD on intestinal flora, making the intestinal tract healthier and at the same time reducing obesity.
From Fig. 7, it can be seen that the results are consistent with previous studies of fecal samples of mice at the phylum level:Firmicutes, Bacteroidetes, and Actinobacteria accounted for the majority in the HF-FGS, HF-FGP, HF-FGF, and HFD groups,while Firmicutes, Bacteroidetes, and Proteobacteria accounted for the majority in the LFD group [28,29]. Compared with the HFD group (342.9%), the abundance of Firmicutes and Bacteroidetes was significantly decreased in all intervention groups (78.3% in the HF-FGF group, 328.6% in the HF-FGS group, and 233.3% in the HF-FGP group). As can be seen from Fig. 8, FGP exerted stronger regulatory effects on the intestinal flora than FGS. There was no significant difference in Firmicutes and Bacteroidetes abundance between the LFD and HF-FGF groups, while the abundance of Firmicutes and Bacteroidetes in the HF-FGS group was significantly higher than that in the LFD group, and Bacteroidetes abundance was significantly lower than that in the LFD group.
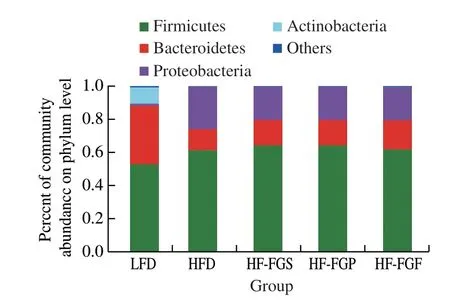
Fig. 7 Analysis of intestinal flora at the phylum level.
Firmicutes can decompose saturated fatty acids in the intestines.Bacteroidetes are a group of bacteria that can ferment carbohydrates and participate in carbohydrate metabolism, bile acid metabolism,and steroid metabolism, and may have an inhibitory effect on energy absorption of the body. The ratio of intestinal Firmicutes to Bacteroidetes (F/B) has an important influence on the energy metabolism of the host. For example, the F/B value in the intestine of obese people and obese mice is positively correlated with the degree of obesity. By studying the proportional structure of the flora,we can effectively analyze the energy metabolism of the body, and even improve the metabolic mechanism of energy metabolism, fat metabolism, endocrine function, and inflammation of the host by regulating the proportion of the two kinds of bacteria [30,31].
The results show that the F/B value of the HF-FGF group was significantly lower than that of the HF-FGP and HF-FGS groups(P< 0.05), indicating that carbohydrate fermentation and saturated fatty acid decomposition in the FGS group were increased and decreased, respectively, possibly because the large number of gram-negative bacteria in the intestines of the HFD group necessitates the mass synthesis of lipopolysaccharide (LPS), the main component of the cell wall of gram-negative bacteria. LPS is a compound of lipids and polysaccharides that is toxic to the host.
From Table 3 and the above results, it can be seen that FGF can significantly improve the intestinal bacterial structure of hyperlipidemic mice; especially at the genus level, it can be seen that FGF has the best regulatory effects.

Table 3Ratio of F/B in the gut.
Fig. 9 shows that the intestinal microbial community structure at the genus level has high diversity (more than 20 genera). The following genera were most abundant:Bacteroides,Escherichia-Shigella,Romboutsia,Faecalibaculum,Blautia,Erysipelatoclostridium, andTuricibacter.
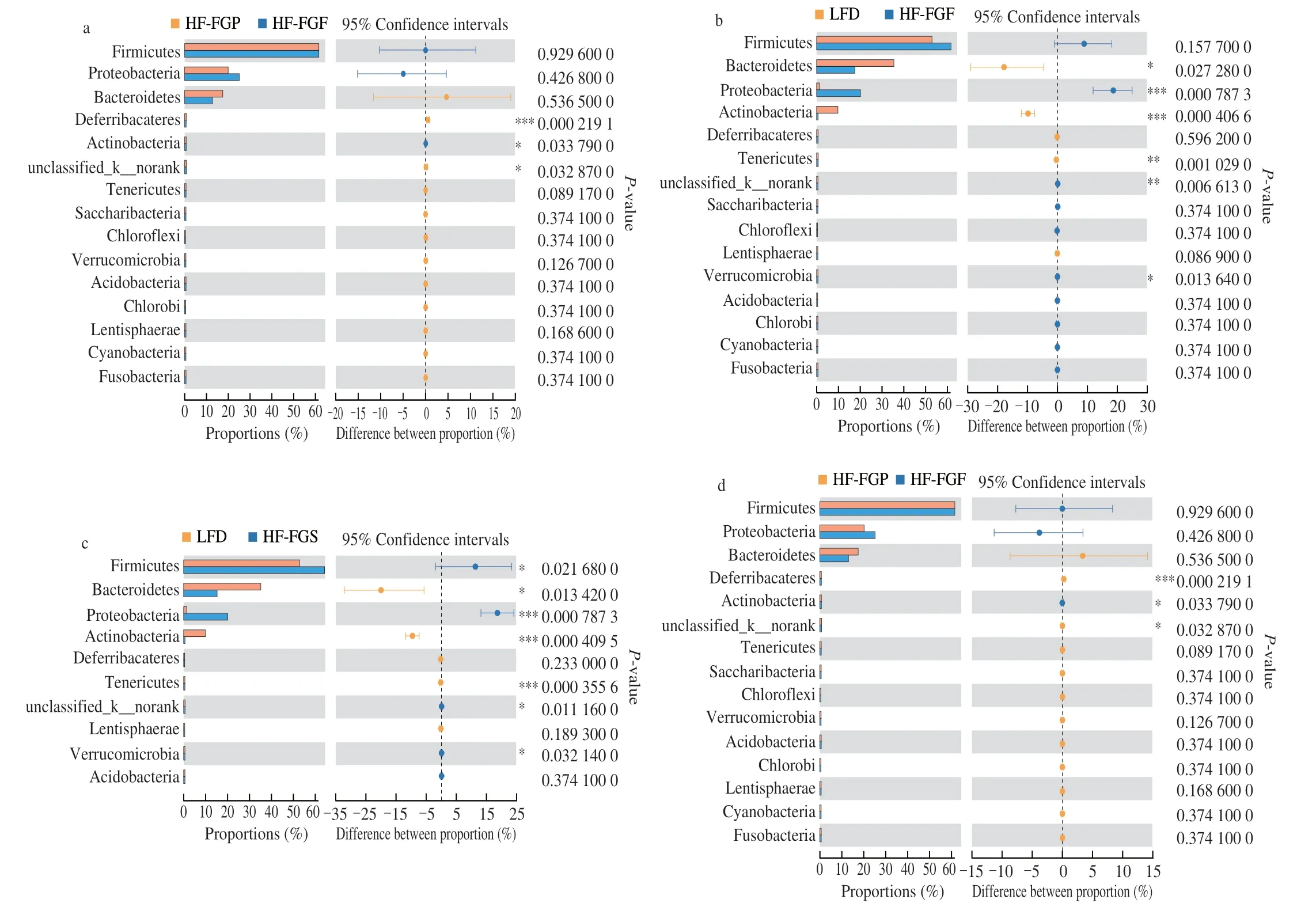
Fig. 8 Difference analysis of gut microbiota at the phylum level among 5 groups.
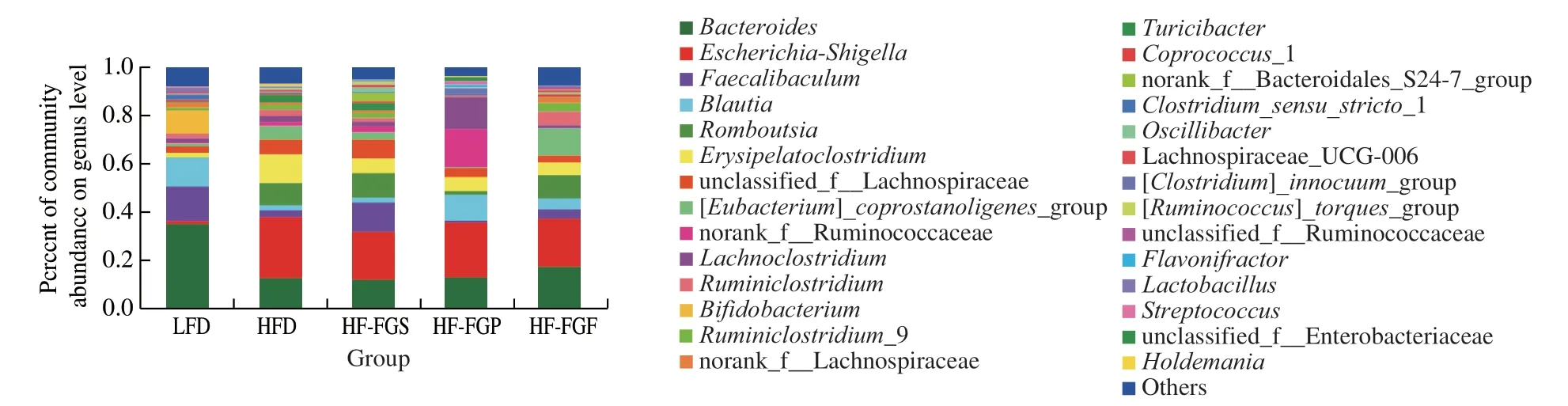
Fig. 9 Analysis of intestinal flora at the genus level.
Among them, the abundance ofEscherichia-Shigellain the 4 HFD groups (especially in the HFD, HF-FGP, and HF-FGS groups)was higher than that in the LFD group, which was consistent with previous studies. It has been proved that in patients with non-alcoholic fatty liver, the abundance ofEscherichia-Shigellawas significantly increased. In the intervention groups, it can be seen that the intervening effects of FGF are strongest. Second,ErysipelatoclostridiumandTuricibacter, both of which are related to metabolic syndrome, were more abundant in the HFD, HF-FGP, and HF-FGS groups.
A HFD significantly decreases the abundance of beneficial bacteria such asBifidobacterium, while increasing the abundance of LPS-producing bacteria, which can lead to systemic inflammation and metabolic syndrome. As can be seen from Fig. 9, bifidobacteria almost disappeared in the HFD groups.
It was found that the following nine genera from the phylum Bacteroidetes all have the ability to produce SCFAs by fermentation in human intestines:Phascolarctobacterium,Roseburia,Blautia,Faecalibacterium,Clostridium,Subdoligranulum,Ruminococcus,Coprococcus, andBacteroides. It can be seen from Fig. 9 that the abundance of short-chain adipose-producing bacteria is higher in the LFD and HF-FGF groups than in the other 3 groups, which is consistent with the results in Table 3. As a result, we infer that FGF can promote the production of SCFAs, which is beneficial to intestinal health.
4. Conclusions
We found that FGF inhibits liver, colon, and duodenal inflammation and improves edema induced by a HFD in mice. In addition, it can promote the reproduction of beneficial bacteria in the intestine, inhibit the growth of pathogenic bacteria, and promote the production of SCFAs in the intestine, thus playing a protective role in the intestinal environment. Moreover, FGF can regulate the distribution of intestinal microbiota by increasing the relative abundance of Bacteroides and Firmicutes in mice fed a HFD. Compared with FGP and FGS, FGF could better promote the reproduction of beneficial intestinal flora in mice, improve the
intestinal environment, and make the structure of the intestinal flora
of mice fed with a HFD resemble that of control mice more closely.In addition, Tartary buckwheat has the important effect of regulating blood lipids. FGF can effectively regulate blood lipid disorders caused by a HFD and alleviate the formation of fatty liver.
In conclusion, Tartary buckwheat, an effective dietary supplement, can improve intestinal barrier and lipid disorders caused by a HFD, which are closely related to the improvement of health.Therefore, the value of Tartary buckwheat as a functional food raw material was determined, providing dietary guidance for obese people to further improve their health. However, more research is required to
elucidate its specific mechanism of action.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank Shanghai Natural Science Foundation(20ZR1455800),the National Science Foundation of China(31871805), Shanghai Municipal Education Commission (Plateau Discipline Construction Program) and China Agriculture Research System (CARS-08-D2). And we thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
杂志排行
食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents
