Characterization and discrimination of fermented sweet melon juice by different microbial strains via GC-IMS-based volatile prof iling and chemometrics
2023-01-03ZhaolingWangSiMiXianghongWangKeminMaoYuweiLiuJieGaoYaxinSang
Zhaoling Wang, Si Mi, Xianghong Wang, Kemin Mao, Yuwei Liu, Jie Gao, Yaxin Sang*
College of Food Science and Technology, Hebei Agricultural University, Baoding 071000, China
Keywords:Sweet melon Fermented juice Volatiles Headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS)Multivariate analysis
A B S T R A C T The main purpose of this study was to investigate the effect of different lactic acid bacteria and yeast strains on the volatile composition of fermented sweet melon juice. Headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS) coupled with chemometrics was performed to identify the potential volatiles for the discrimination of different fermented sweet melon juice. In total, 70 volatile compounds were found in the fermented sweet melon juices. Of them, 45 compounds were annotated according to the GC-IMS database and classif ied into esters, alcohols, aldehydes, ketones and furans. Results from the multivariate analysis reveal that sweet melon juice fermented by different combinations of microbial strains could be distinctly separated from each other. A total of 15 volatiles with both variable importance in projection value > 1 and P < 0.05 were determined as potential markers for the discrimination of fermented sweet melon juice. This study conf irms the effect of microorganisms on the f lavor of the fermented sweet melon juice and shows the potential of HS-GC-IMS combined with chemometrics as a powerful strategy to obtain volatile f ingerprints of different fermented sweet melon juice.
1. Introduction
Sweet melons (CucumismeloL.) belong to Cucurbitaceae. They are popular among consumers due to their organoleptic attributes(e.g. pleasant taste, fresh f lavor) as well as abundant nutrients such as carotenoids, minerals, and phenolic constituents [1-4].
Sweet melons are widely distributed all over the world. China is the largest producer and consumer of sweet melons in the world.According to the data released by the National Bureau of Statistics of China, the planting area of sweet melon reached 0.39 million hectares in 2019, and the total output of sweet melon was around 1.36 million tons. Sweet melons are mainly used as fresh fruit [5]. Fermented fruit and vegetable juice has been widely welcomed because of its potential health function and good f lavor [6].
Microbial strain selection is crucial for the production of desired fermented juice. Lactic acid bacteria and yeasts are the most commonly used for fermentation [7]. Many studies have shown that microbial strains can exert a great inf luence on the f lavor of fermented juice [8-13].In recent years, headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS) analysis has been widely used to identify different sources and assess the authenticity of food samples [14].Through HS-GC-IMS analysis, high-speed, simple, inexpensive,direct, sensitive, and non-target-based volatile fingerprints can be established [15]. Chen et al. [8] evaluated the effect of four lactic acid bacteria strains,Lactobacillusacidophilus,L.rhamnosus,L.caseiandL.plantarum, on the f lavor of fermented apple juice by SPME/GC-MS. Most of the volatile compounds related to the typical aroma of apple juice were retained or enriched after fermentation.Among them,L.acidophilushad the greatest inf luence on the f lavor and organic acid of fermented apple juice. The contents of important aromatic compounds ethyl-acetate, 2-ethylhexanol, 2-methyl-1-butanol and 3-methyl-1-butanol were greatly increased. Alves Filo et al. [9]evaluated the volatile compounds ofL.caseifermented melon and cashew juice by HS-SPME-GC-MS. Some esters in melon and cashew juice were reduced by fermentation. At the same time, the contents of 3-methyl-2-butenyl can be used as the index of volatile compounds in the fermentation process of melon juice. Combining with fruit and lactic acid fermentation, fermented melon and cashew apple juice have slightly formation and reduction of aroma compounds. Corona et al. [10] studied kefir-like vegetable beverages,and the results showed that lactic acid bacteria and yeast can grow in fruit and vegetable juice and significantly increase the contents of volatile organic compounds, especially esters. Not only lactic acid bacteria but also yeast can ferment fruit and vegetable juice and improve the flavor of fruit and vegetable juice. Lorenzini et al. [11]studied the ability ofSaccharomycesand non-Saccharomycesstrains to ferment apple juice and their effects on the production of volatile compounds in cider by GC-MS. A large amount of 2-phenyl ethanol was found inS.uvarumcider.Hanseniasporauvarumwas the non-Saccharomycesyeast which produces the most hexyl acetate and isoamyl acetate. Yang et al. [12] studied the volatiles of fresh and thermally watermelon juice by HS-GC-IMS and GC-O-MS. Allyl methyl sulfide and 3-hydrox-2-butanone were identified in heated juice first and 2,6-dimethyl-5-heptenal was identified in fresh juice first as odor contributing compounds. Yu et al. [13] examined the differences of aroma profile of two different muskmelon by GC-IMS,in which fresh aroma decreased and off odor belonging to “sulfurous”and “fermented” volatile were found in both two muskmelon cultivars. Therefore, fermentation can improve the flavor of fruit and vegetable juice. The main objective of the present study was to compare the volatile profiles of sweet melon juice fermented by different combinations of lactic acid bacteria and yeast. The volatile fingerprint of melon fermented juice was established by HS-GC-IMS.Principal components analysis (PCA), partial least-squares discriminant analysis (PLS-DA) and other chemometric tools were used to process the non-targeted volatile data set, and the potential volatile markers for the discrimination of fermented sweet melon juice were screened. The data may be useful for the scale-up production of fermented sweet melon juice in the future.
2. Materials and methods
2.1 Microorganisms
The lactic acid bacteria strains ofL.plantarumC17 andL.pentosusLp-B were isolated from kimchi products, while the yeast strain ofPichiakluyveriNEER was kindly provided by Shijiazhuang Junlebao Dairy Co., Ltd. (Hebei Province, China). All of these strains were stored as stock solutions in glycerol at -80 °C until use.
2.2 Sample collection and preparation
The sweet melons used in this study were purchased from the local market (Cangzhou, Hebei Province, China). The variety of sweet melon shapes like a goat’s horn. When the fruit is ripe, the peel is greyish-white with a little light green at the bottom, the pulp is yellow-orange, about 25 cm long, 1-2 cm thick and 1-2 kg in weight.
After collection, the sweet melon samples were first cleaned to remove the surface impurities. Then, the sweet melon samples were homogenized using a food processor (Langbo Instrument Manufacturing Co., Ltd., China). The samples were filtered and pasteurized (65 °C, 30 min) in a water bath (Changfeng Instrument Co., Ltd., China) [16]. After cooled to room temperature, the juice was divided into 5 groups: unfermented group (Group 1), fermented with combined strains ofL.plantarumC17 andL.pentosusLp-B(Group 2),L.plantarumC17 andP.kluyveriNEER group (Group 3),L.pentosusLp-B andP.kluyveriNEER group (Group 4),L.plantarumC17,L.pentosusLp-B andP.kluyveriNEER group(Group 5). The fermentation was carried out in a 200 mL glass bottle containing 100 mL of the prepared sweet melon juice. The strains were mixed at a ratio of 1:1 and 3 mL of the different mixed strains were inoculated into the sweet melon juice and fermented at 30 °C for 24 h.
When the fermentation was completed, the sweet melon juice was centrifugated at 1 × 104r/min for 10 min at 4 °C, and then sterilized at 95 °C for 15 min. Then the juice samples were stored at -20 °C for subsequent analysis [17].
2.3 Untargeted headspace gas chromatography-ion mobility spectrometry analysis
Based on the slightly modified to the research method of Zhou et al. [18],the volatile analysis of sweet melon juice samples was carried out on Agilent 490 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) using a FS-SE-54-CB capillary column (15 m ×0.53 mm, 0.5 µm) and an ion mobility spectrometer (FlavourSpec®,Gesellschaft für Analytische Sensorsysteme mbH, Dortmund,Germany) equipped with an automatic sampler (CTC Analytics AG,Zwingen, Switzerland), and samples can be directly injected from headspace by using a 1 mL airtight heated syringe.
An aliquot of 3 mL of sweet melon juice (with/without fermentation) was added to a 20 mL headspace glass sampling vial.Subsequently, the sample was equilibrated at 40 °C for 15 min.At 85 °C, the headspace of the 500 μL sample was automatically injected into the GC-IMS instrument by heating the syringe. Then the samples were driven into the FS-SE-54-CB capillary column(isothermal condition at 60 °C) by nitrogen (99.999% purity) at the following program flow rate: 2 mL/min for 2 min, 10 mL/min for 8 min, 100 mL/min for 10 min, 130 mL/min for 10 min. The analyte was driven into the ionization chamber by a 3H ionization source with 300 MBq activity to ionize in a positive ion mode. The generated ions were driven to a drift tube (9.8 cm long), which works at a constant temperature of 45 °C and a voltage of 5 kV.
2.4 Data processing and statistical analyses
Raw IMS data were acquired and processed using Laboratory Analytical Viewer (LAV, G.A.S., Dortmund, Germany). The retention index (RI) values of the volatiles were calculated by usingn-ketones C4-C9 (Sinopharm Chemical Reagent Beijing Co., Ltd.,Beijing, China) as external standards. The volatile compounds were identified by comparing the RI values and IMS drift time (DT, the time it takes for ions to reach the collector through a drift tube, in milliseconds) with those of the authentic reference compounds in the GC × IMS Library (Gesellschaft für Analytische Sensorsysteme mbH,Dortmund, Germany).
All analyses were performed in triplicate and the data were presented as mean value ± standard deviation (n= 3). Statistical analysis was performed using Addinsoft XLSTAT-Premium v2019.4.1 (Barcelona, Spain). Analysis of variance (ANOVA) and Turkey-Dunnett multiple comparison test was performed to evaluate the differences in the abundance of volatile substances in fermented sweet melon juice samples. Unless specified,Pvalue of less than 0.05 was considered statistically significant. The unsupervised PCA was adopted to visualize the grouping of the fermented sweet melon juice, whereas the supervised PLS-DA was implemented to determine potential volatile makers. Volatile compounds which were responsible for the discrimination of fermented sweet melon juice were determined by the varying importance in projection scores(VIP > 1) calculated through the PLS-DA models.
3. Results and discussion
3.1 Volatile profiles of fermented sweet melon juice
A previous study in our lab indicated thatL.plantarumC17andL.pentosusLp-B could grow well in juice and produce the functional factor-gamma aminobutyric acid (GABA) during the fermentation process [19]. With the addition of NEER, the flavor of sweet melon juice was improved significantly. Therefore, the above three strains were selected to ferment sweet melon juice. After HS-GC-IMS analysis, a total of 45 volatile compounds were annotated in the fermented sweet melon juice samples, including 29,32, 33 and 32 respectively in Groups 2-5. The detailed information of these identified volatiles is summarized in Table 1. Furthermore,these volatile compounds can be generally classified into 5 groups,including esters (11/45), alcohols (12/45), aldehydes (13/45), ketones(8/45) and furans (1/45) (Table 1). Our results are consistent with the previous study, indicating that alcohols, aldehydes and esters especially acetic esters were the main volatile compounds of the two oriental sweet melon (Cucumismelovar.makuwaMakino) cultivars [20].

Table 1Volume of sweet melon juice fermented by different strains.
Among the annotated volatiles, 23 of them were commonly present in the fermented sweet melon juice (Fig. 1). Moreover,5 volatiles were uniquely detected in Group 2, namely, (E)-2-hexen-1-ol M, pentanal M, 3-methylbutanal D, 3-methylbutanal M and 2-methyl-1-propanol (Fig. 1, Table 1). In the unfermented group, the contents of these 5 volatile compounds were higher and the relative content of Group 2 decreased, while in other groups, these substances disappeared, which may be related to the addition of NEER (Fig. 2).
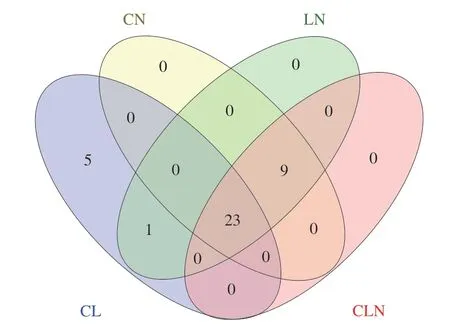
Fig. 1 Venn diagram of fermented sweet melon juice.

Fig. 2 Gallery plot of the selected signal peak areas obtained with sweet melon juice fermented with different strains. C17 + Lp-B: sweet melon juice fermented with L. plantarum C17 and L. pentosus Lp-B; C17 + NEER: sweet melon juice fermented with L. plantarum C17 and P. kluyveri NEER; Lp-B+ NEER: sweet melon juice fermented with L. pentosus Lp-B and P. kluyveri NEER; C17 + Lp-B + NEER: sweet melon juice fermented with L. plantarum C17, L. pentosus Lp-B and Pichia kluyveri NEER.
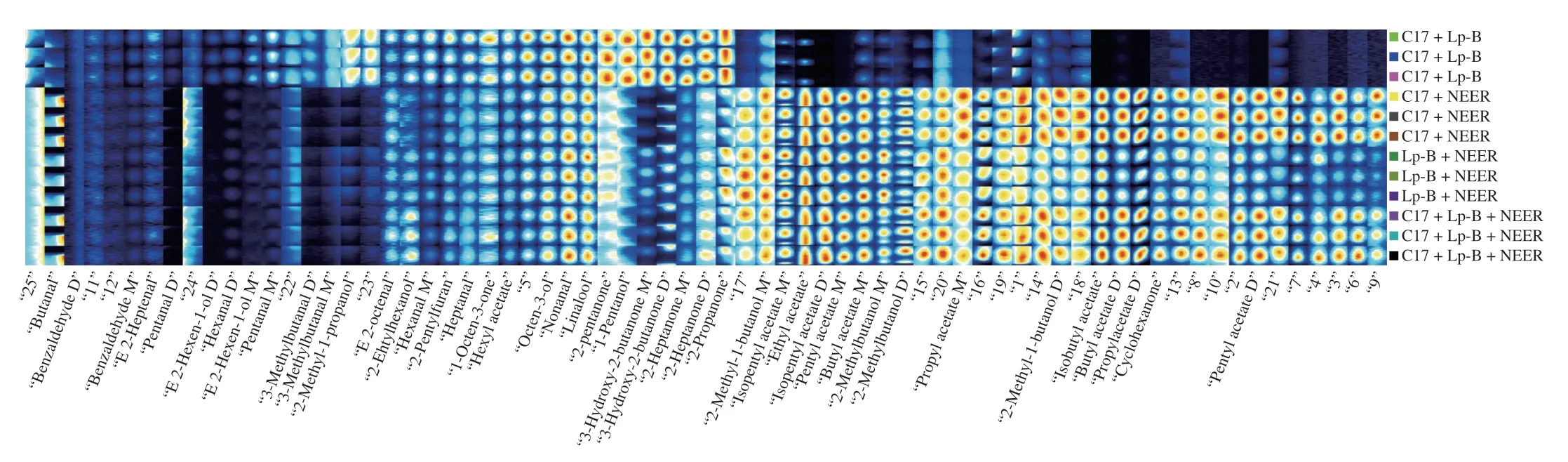
Fig. 3 Top view of GC-IMS 3D-topographic plot of fermented sweet melon juice. The topographic plot of unfermented sweet melon juice was selected as a reference (Fig. S1), and the topographic plot of other samples was deducted from the reference.
The differences of volatile compounds in fermented and unfermented sweet melon juice samples were analyzed by HS-GC-IMS.A top view of the HS-GC-IMS 3D-topographic plot of sweet melon juice samples is shown in Figs. 3 and S1. We can see that the retention time of most signals appears in 100-500 s, and the DT appears in 1.0-1.8. Taking one sample as a reference, if the volatile compounds in other samples are the same, the background after removing the same components is white. Red spots indicate that the concentrations of substances are higher than the reference value and blue spots indicate that the concentrations of substances are lower than the reference value. In addition to the samples fermented byL.plantarumC17 andL.pentosusLp-B, the signal peaks of other melon juices were significantly different. In contrast, whenP.kluyveriNEER was present in the fermentation strains, the volatile components increased significantly. It is consistent with previous reports.Non-S.cerevisiaecontributes to the increase of complex aroma components and has a positive effect on the overall aroma components [21].C.kluyveriN6 can produce caproic, butyric and octanoic acids and their corresponding ethyl esters, which has significant contribution to the flavor of Chinese strong-aroma type liquor [22].
3.2 Comparison of volatiles in sweet melon juice fermented with different strains
Due to different concentrations, some compounds may produce multiple signals or spots (dimers or even trimers). Dimer and trimer can be discriminated by DT, in which the DT of trimer is larger than that of dimer [23]. We selected the visual map and listed it through the gallery map for visual comparison. Therefore, the differences of volatile compounds of sweet melon juice before and after fermentation were observed, and the characteristic fingerprints of each strain combination were established. The differences of volatile compounds in sweet melon juice fermented by different strains are shown in Fig. 2.
As shown in Figs. 2 and S2, each row represents all selected signal peaks and each column represents the signal peaks of the same volatile compounds in different samples. The retention index,migration time and the peak volume data of all substances in each sample are detailed in Table S1.
As shown in Figs. 2 and S2, a total of 10 volatiles were commonly identified in all the juice samples. The data of unfermented melon juice are detailed in the Fig. S1. On the contrary, benzaldehyde D,benzaldehyde M, (E)-2-heptenal, pentanal D, (E)-2-hexen-1-ol D and hexanal D were uniquely detected in the unfermented juice, and isopentyl acetate D, pentyl acetate M, isobutyl acetate, butyl acetate D, propyl acetate D, cyclohexanone and pentyl acetate were uniquely in the juice fermented byL.plantarumC17,L.pentosusLp-B andP.kluyveriNEER. The abundance of some volatiles decreased in the fermentation process of different strain combinations. These compounds include benzaldehyde, butanal, (E)-2-heptenal, pentanal,hexanal, 3-methylbutanal, (E)-2-octenal, heptanal, 2-ethylhexanol,E-2-hexen-1-ol, 2-methyl-1-propanol. This may be attributed to the immature sweet melon. Liu et al. [24] reported that C6 alcohols and aldehydes, including hexanal,Z-2-hex-enal, andE-2-hexenol, were the main aromatic compounds in the early stage of fruit ripening. The aroma compounds transformed from “grass fragrance” to “fruity fragrance”.
Relatively, the combination ofL.plantarumC17 andL.pentosusLp-B had less weakening effect on the substances before fermentation than the combinations with NEER, while the concentration of octen-3-ol, nonanal and linalool floral aromas was equal to the unfermented sweet melon juice [24]. However, this combination produced more 3-hydroxy-2-butanone, 2-heptanone, 2-propanone,2-pentanone, 1-pantanol and other ketones in sweet melon juice, which contribute to the distinctive flavor of cheese and improve the flavor of the sweet melon juice [25]. Compared with the unfermented sweet melon juice, the combination ofL.plantarumC17 andP.kluyveriNEER,L.pentosusLp-B andP.kluyveriNEER,L.plantarumC17 andL.pentosusLp-B andP.kluyveriNEER produced a large number of esters, such as ethyl acetate, isopentyl acetate, isobutyl acetate, butyl acetate, propyl acetate and pentyl acetate in fermentation (Fig. 2),which may produce a stronger fruity aroma [24]. Ester components,such as (Z)-3-hexenyl acetate, ethyl butyrate and ethyl octanoate,have the greatest abundance in the mature stage of fruit development ofPsidium guajava[26]. The concentrations of nonanal and linalool were equal to those in the unfermented samples, but the concentrations of ketones produced in fermentation was much lower than those of the combination ofL.plantarumC17 andL.pentosusLp-B when compared to the 3 groups withP.kluyveriNEER. The effects of different combinations of microbial strains on volatile profiles also varied. The concentrations of isobutyl acetate, butyl acetate, propyl acetate and pentyl acetate in the fermented sweet melon juice produced withL.pentosusLp-B andP.kluyveriNEER were slightly lower than those in the other two combinations. Therefore, the fruit flavor is lighter than that of the other two combinations containing NEER.
Changes of volatile compounds in fermentation were analyzed.The results showed that 3-hydroxy-2-butanone was only found in fermented sweet melon juice which enhanced the creamy flavor of the original juice, while isopentyl acetate, pentyl acetate, butyl acetate and propyl acetate were identified only in sweet melon juice fermented withP.kluyveriNEER. The concentrations of 2-pentanone,1-pentanol, 3-hydroxy-2-butanone, 2-propanone and 2-heptanone in sweet melon juice fermented withL.plantarumC17 andL.pentosusLp-B were the highest among the 5 groups of sweet melon juice which increased the pleasant creamy flavor. However, the impact of ketone compounds was of especial considerations due to their practical contributions to meat products [27]. Therefore, the flavor of fermented melon juice in Group 2 was not harmonious. After fermentation, the signal intensities of volatile compounds (such as benzaldehyde, (E)-2-heptenal, 3-methylbutanal, etc.) were weaker than those in unfermented samples.
3.3 Discrimination of fermented sweet melon juice by PCA
The unsupervised PCA was applied to the data containing 45 volatiles. Fig. 4 shows a PCA score plot, and a clear separation was observed among the 5 groups of sweet melon juice. It showed the distribution of the first two main components determined by PCA, describing a cumulative variance contribution rate of 75% and 19%, respectively.
As shown in Fig. 4, flavors between fermented and unfermented sweet melon juice varied greatly. At the same time, flavors of different samples fermented by three strain combinations were also largely different, samples in Group 3 and Group 2 in particular. As seen from the cluster dendrogram of Fig. S3, among the fermented samples,the flavor of sweet melon juice fermented byL.plantarumC17 andL.pentosusLp-B was most similar to that of the unfermented sample.Samples in Group 3 and Group 5 almost have the same flavor. The flavor of sample in Group 4 was also similar to that of samples Group 3 and Group 5. The results revealed that the differences of fermented and unfermented sweet melon juice were obvious.
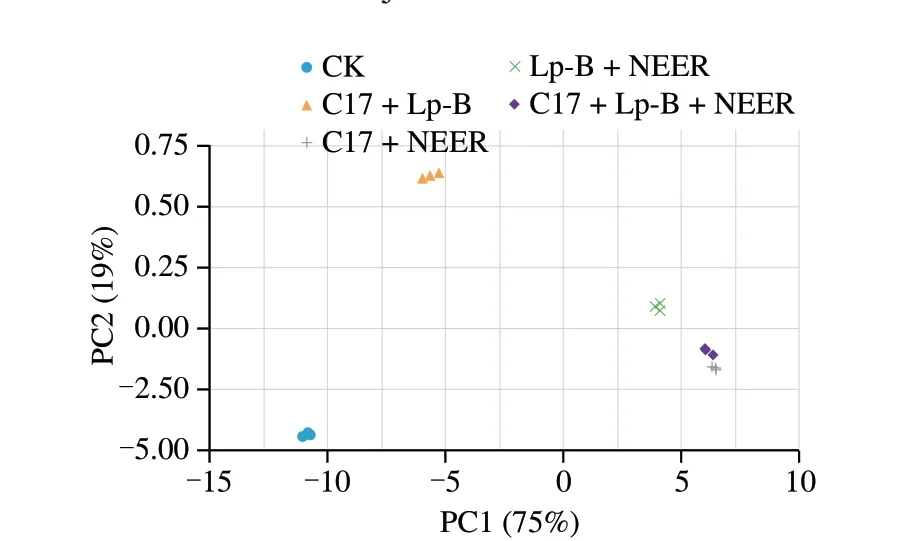
Fig. 4 Principal component analysis based on the signal intensity obtained with fermented and unfermented sweet melon juice by L. plantarum C17,L. pentosus Lp-B and P. kluyveri NEER.
3.4 Discrimination of fermented sweet melon juice by PLS-DA
In order to identify potential volatiles that could be used to discriminate fermented sweet melon juice by different combinations of microbial strains, we performed supervised PLS-DA on the volatile data set. Fig. 5a shows the PLS-DA score plots of the sweet melon juice fermented with different strain combinations. It can be seen that all groups of samples were clearly separated from each other.The overall fitness and predictive ability of the PLS-DA models were evaluated byR2XandQ2values. In this study, the cumulativeR2XandQ2values were 0.99 and 0.97, respectively.
Therefore, the established PLS-DA models have a good prediction ability (Q2> 0.50), and the new data set can be divided into several groups [28]. According to the PLS-DA model, VIP scores of volatiles were calculated and important volatile compounds were selected based on the VIP value, which is not less than 1 [29]. Univariate analysis was also carried out on the same data set to identify the significant volatiles which were helpful to distinguishing the 5 groups of sweet melon juice. A total of 15 with both VIP > 1 andP< 0.05 were determined in Fig. 5b.
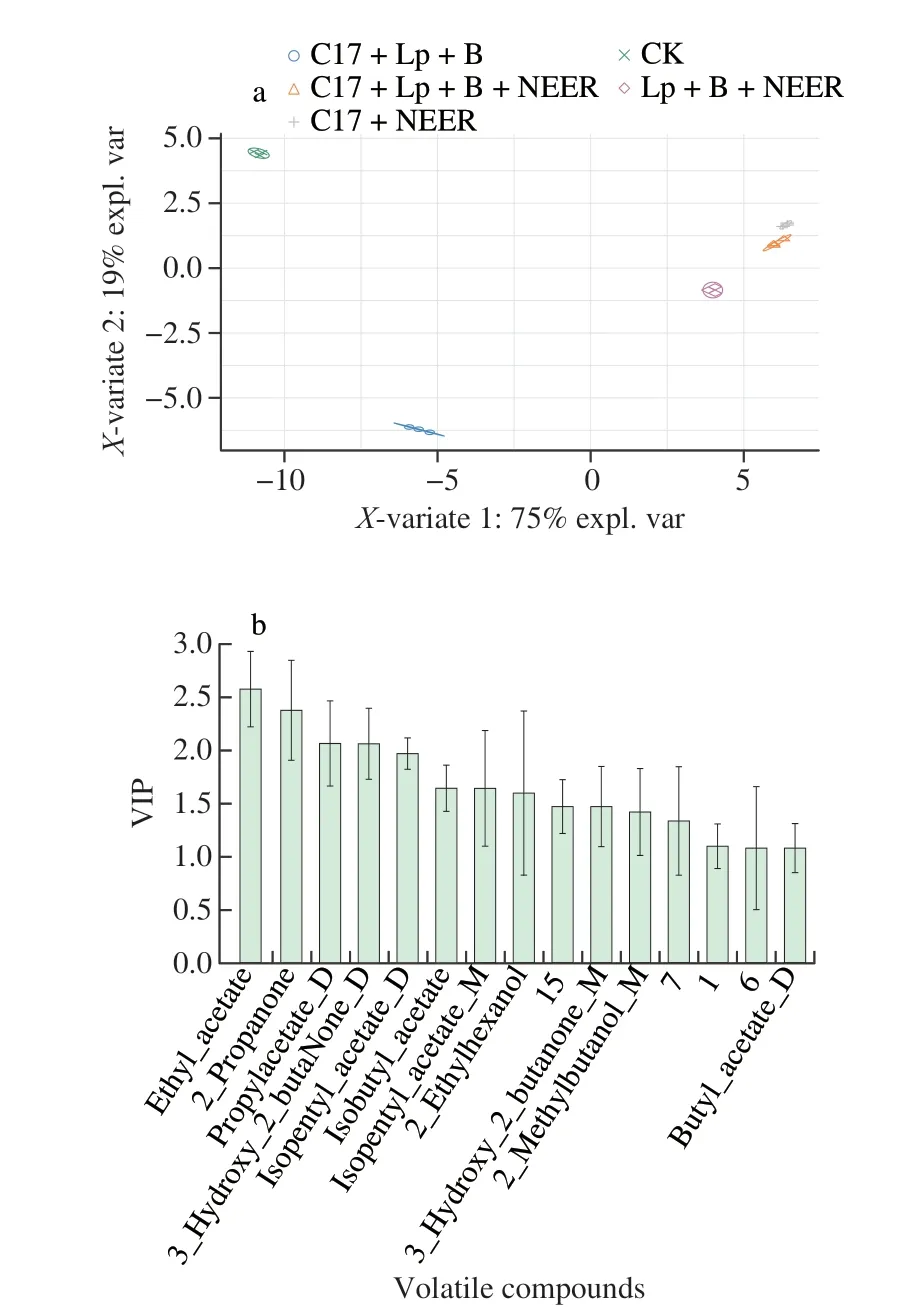
Fig. 5 Score chart of partial least squares discriminant analysis and diagram with chromatographic peaks of samples with VIP > 1 (P < 0.05) of fermented sweet melon juice.
As shown in Fig. 5b, ethyl-acetate has the highest VIP value of 2.58, and its content is most significant among all the samples,especially in the juice with or without NEER in fermentation.Ethyl-acetate is particularly important when producing products with a rich, light, rice-flavored and delicate flavor. Therefore, ethyl-acetate is a main beneficial ester in Baijiu, which has the aroma of pear and banana [30]. In this study, ethyl-acetate played an important role in the flavor of melon juice fermented with NEER, according to Figs. 2 and 5b. The VIP value of 2-propanone is 2.38. It is widely used to remove off-flavor from plant resources (such as soybean and lupin)and relevant products (such as protein isolates and flakes) [31].Propyl-acetate has a soft fruity aroma and its VIP value is 2.07. As effective solvents,n-propyl acetate and ethyl acetate are widely used in coating printing and dyeing, pharmaceutical perfume and papermaking industries [32]. With a VIP value of 2.06, 3-hydroxy-2-butanone D has a pleasant creamy fragrance. 3-Hydroxy-2-butanone is a valuable spice found in cauliflower, corn, peas and kidney beans [33].With a VIP value of is 1.97, isopentyl-acetate D has the aroma of ripe fruit like bananas. It can be extracted from plant or produced by extraction or fermentation, and is widely used as a solvent or perfume in cosmetics and pharmaceutical industry [34]. Another study found that the content of isopentyl-acetate was higher in bananas, with a relative content of 25.27% [35]. According to Fig. 2, isopentyl-acetate was only contained in fermented melon juice with “NEER”participation. Therefore, NEER increases the aroma of melon juice.With a VIP value of 1.64, isobutyl-acetate is considered as a major green solvent for the chemical industry. Isobutyl-acetate is widely used in cosmetics because of its fruity and floral fragrance, and also used as an odor component in the condiment and spice industry [36].With a VIP value of 1.60, 2-ethyl-hexanol can be used to improve the lubricating function of vegetable oil bio-lubricants [37].2-Methybutanal has great influence on the beer flavor. The flavor changes with the concentration of the compound, which reminds people of green grass, potatoes, cloves, cheese, etc. and usually reduces the freshness of beer [38]. This is only a trial of sweet melon juice fermentation. In the future, the sample size will be scaled up to confirm or validate the findings of the present study.
4. Conclusions
In the present work, we performed a comprehensive and comparative volatile analysis to characterize and distinguish sweet melon juice fermented by different combinations of lactic acid bacteria and yeast strains. The volatile profiles were established by the simultaneous identification of 45 flavor compounds using a HS-GC-IMS-based method. All the fermented sweet melon juice under investigation was distinct in terms of the composition and abundance of volatile compounds. In addition, our results indicate that the sweet melon juice fermented byL.plantarumC17,L.pentosusLp-B andP.kluyveriNEER had the most abundant aroma components, which were characterized by relatively higher levels of ethyl acetate, isopentyl acetate, pentyl acetate, butyl acetate, isobutyl acetate and propyl acetate. Despite these findings, future studies with a larger sample size and with scale-up production are needed to validate the discrimination performance of the volatile markers.
Conflict of interest
All the authors of this study have no relevant conflict of interest to declare.
Acknowledgments
This research was supported by Hebei Provincial Key Research Projects (19227114D), the Vegetable Industry Innovation Team Project of Hebei Modern Agricultural Industrial Technology System(HBCT2018030208).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.10.006.
杂志排行
食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents
