Immunomodulatory effect of ethanol-soluble polypeptides from Atlantic cod (Gadus morhua)
2023-01-03ZhenYunMeilinYngDongyngZhuDiWuShuzhenChengChoWuHeshmElSeediMingDu
Zhen Yun, Meilin Yng, Dongyng Zhu, Di Wu, Shuzhen Cheng,b,Cho Wu, Heshm R. El-Seedi, Ming Du,*
a School of Food Science and Technology, National Engineering Research Center of Seafood, Dalian Polytechnic University, Dalian 116034, China
b Beijing Advanced Innovation Center for Food Nutrition and Human Health, College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China
c Pharmacognosy Group, Department of Medicinal Chemistry, Uppsala University, Biomedical Centre, Uppsala 75123, Sweden
Keywords:Gadus morhua Immunomodulation Peptides TLR2 Molecular docking
A B S T R A C T There are many active substances in Atlantic cod (Gadus morhua) explaining the variety of biological activities. In order to study the immunomodulatory activity and the mechanism of Atlantic cod peptides at the cellular level. In this study, cod peptides were isolated by 80% ethanol extraction method, the isolated ethanol-soluble cod peptides (CP-ES) were investigated and their immunomodulatory activity was verif ied.Additionally, CP-ES showed lower molecular weight and more hydrophobic amino acids. CP-ES could promote the proliferation of spleen lymphocytes and T lymphocytes in mice, suggesting that CP-ES may regulate adaptive immunity. It promoted the release of NO and the expression of iNOS, TNF-α, IL-6 and IL-1β genes in macrophages, suggesting that CP-ES may regulate innate immunity. CP-ES could promote the expression of TLR2 gene, and the peptides identif ied in CP-ES were docked with TLR2 to predict the peptides playing a major role in CP-ES. These results suggested that CP-ES may regulate the immune activity of both innate and adaptive lines.
1. Introduction
The immune system plays an important role in maintaining homeostasis under normal physiological conditions [1]. Immune system imbalances can lead to immunodeficiency, infectious,hypersensitivity, and autoimmune diseases, and malignant tumors [2].Adaptive immunity is highly specif ic and can be divided into cellular immunity and humoral immunity. T lymphocytes (T cells) and B lymphocytes (B cells) play important roles in adaptive immunity [3].Toll-like receptors (TLRs), as innate immune receptors of microbial molecules, are the f irst responders to infection, initiating inf lammatory signals and helping to activate adaptive immune responses [4]. TLRs are type I transmembrane glycoproteins [5], and a member of the PRRs family which are important in both innate and adaptive immune responses [6].
In the North Atlantic, the Atlantic cod (Gadus morhua) is one of the most important species. Like many temperate species, Atlantic cod is an excellent breeder that has recently been the target of aquaculture and is widely consumed by humans. The low-fat content and size of the f ish make the Atlantic cod meat ideal for frozen f ish products such as f ish f ingers [7-10].
Studies have shown that fish protein hydrolysates have a variety of biological activities, such as antioxidants, anti-hypertension,anti-diabetes and antibacterial agents [11]. Similarly, cod has high nutritional value and contains a variety of active ingredients. Li et al. [12]found that the enzymatic peptides of Atlantic cod bladders could effectively remove DPPH·, HO· and O2-·, and has high Fe2+chelating activity, and improve cell viability, inhibit SA-β-gal activity and inhibit 2BS cell apoptosis rate induced by hydrogen peroxide (H2O2). Farvin et al. [13]found antioxidant active peptides in cod protein hydrolysates. Niu et al. [14]suggested that cod skin collagen could cure the symptoms of gastric ulcer.Chen et al. [2] found that cod skin collagen oligo-peptides could enhance both cellular immunity and specific humoral immunity in mice with CTX-induced immunosuppression. Although Chen et al. [2] studied the immunomodulatory activity of cod skin collagen oligo-peptides in mouse models, the immunomodulatory activity of cod hydrolysate peptides at the cellular level and the mechanism of action have not been studied.
In this study, 80% ethanol was used to separate ethanol-soluble cod peptides and ethanol-insoluble cod peptides from Atlantic cod. The molecular weight distribution and amino acid content of ethanol-soluble cod peptides were analyzed. To evaluate the immunomodulatory activity of ethanol-soluble cod peptides and identify the peptides sequence of the active part, molecular docking was carried out and the mechanism of action was highlighted.
2. Materials and methods
2.1 Materials and chemicals
Atlantic cod peptides were purchased from Qingdao Yihexing Food Co., Ltd. (Qingdao, China). Cytochrome C, L-lysine, LPS,ConA, MTT, neutral red stain from Solarbio Science and Technology(Beijing, China). Aprotinin and vitamin E were purchased from Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). NO detection Kit and Cell Counting Kit-8 were purchased from Beyotime Biotechnology (Shanghai, China). Trifluoroacetic acid (TFA) was purchased from Sigma Chemical Co., Ltd. (St. Louis, MO, USA).RPMI 1640 and DMEM High Glucose were purchased from Gibco Life Technologies (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from Pan-Biotech GmbH Medtrack Company (Edenbach, Bagoria, Germany). Penicillin-Streptomycin was purchased from Hycone (Logan, Utah, USA). The RNA extract was purchased from Wuhan Servicebio Technology Co., Ltd. (Wuhan,China). Other chemical reagents used in this article were analytical grade or HPLC grade.
2.2 Preparation of Atlantic cod peptides (CP)
CP were purchased from Qingdao Yihexing Food Co., Ltd.The preparation process of Atlantic cod peptides was as follows:Firstly, the cod meat was cut into small pieces about 5 cm in size and grounded with water according to the mass ratio of 1:1.5. The pH value was adjusted to 8.0, the temperature was maintained at 58 °C,and alkaline protease was added at a ratio of 0.20%, with enzymatic hydrolysis of 2 h. Then, the enzyme was inactivated at 100 °C for 15 min. After cooling, the first pressure filtration, and then through 120 mesh vibrating screen centrifugal screen, membrane filtration separation. Finally, the supernatant was spray-dried to obtain CP.
2.3 Preparation of ethanol-soluble (CP-ES) and ethanol-insoluble (CP-EI) of Atlantic CP
Slightly modified according to the method reported by He et al. [15].CP were separated. 20 g CP (5%,m/m) in 400 g 80% ethanol. The mixture was stiring at room temperature for 1 h, centrifuge at 8 000 ×gand 4 °C for 20 min. The ethanol in each section was removed by a rotary evaporator. The part obtained after rotary evaporation was redissolved and lyophilized. The supernatant part was CP-ES. The precipitated part was CP-EI. CP, CP-ES and CP-EI were treated with 100 Da dialysis bag for desalting. Store in refrigerator at -20 °C for later use.
2.4 Determination of peptide content and amino acid analysis
The peptide content of cod peptides was determined by the Kjeldahl method. The hydrolyzed amino acid and free amino acid contents of cod peptides were measured according to the method of Xu et al. [16]. Hitachi LA8080 amino acid analyzer was used for analysis. Hitachi high-performance cation exchange column was used.The separation column temperature was 57 °C, and the derivative column temperature was 135 °C. Injection volume was 20 µL. The peptides were dissolved in 6 mol/L hydrochloric acid and hydrolyzed at 110 °C for 22 h to prepare hydrolyzed amino acids. Before the determination of free amino acids, the peptides were dissolved in 0.02 mol/L hydrochloric acid, followed by the addition of the same amount of acetone and centrifugation at 10 000 ×gfor 10 min to remove large proteins. After rotary evaporation, 0.02 mol/L hydrochloric acid was redissolved in an equal volume, and the samples were filtered by 0.22 µm water filtration membrane. After dilution, samples were loaded.
2.5 Molecular weight distribution determination
Some modifications were made in accordance with the method
described by Tu et al. [17]. The molecular weight distribution of cod peptides was measured. Agilent 1260 HPLC system equipped with TSKgel G2000SWXL (7.8 mm × 300 mm) column was used to determine the samples. The column temperature was set at 25 °C, the flow rate was set at 0.5 mL/min, and the detection was performed with a 214 nm UV detector. Phase A was 0.1% trifluoroacetic acid aqueous solution, and phase B was 100% acetonitrile. The injection volume was 10 µL. After balancing the column with 45% B phase, the samples were eluted in the same proportion for 30 min. Cytochrome C (12 384 Da), aprotinin (6 511 Da), octapeptide (1 072 Da),vitamin E (431 Da) and L-lysine (146 Da) were used as the standard.According to the standard curve, the molecular weight range was divided into 5 parts (≤ 300, 300-600, 600-1 000, 1 000-3 000,and ≥ 3 000 Da).
2.6 Measurement of stimulation index in mouse spleen lymphocytes
The spleen lymphocyte stimulation index was measured by the CKK-8 method. In simple terms, splenic lymphocytes were obtained from the spleens of female BALB/c mice under sterile conditions.Mouse spleen lymphocytes were cultured in RPMI 1640 medium
supplemented with 10% FBS and 1% penicillin-streptomycin. Splenic lymphocytes of mice were inoculated 100 µL per well on 96-well plates, cell density was 1 × 106cell/mL, and treated with different samples. After incubating at 37 °C and 5% CO2for 24, 48 and 72 h,added 10 µL CKK-8 to each well, and incubated for another 3 h under the same conditions. The absorbance value was detected at 450 nm. The same amount of medium was used as blank control [3].Stimulation indices of peptides were calculated by the equation:

2.7 Measurement of stimulation index with mitogen in spleen lymphocytes
As mentioned above, the mitogen stimulation index of peptides was determined by CKK-8 staining. In brief, mouse spleen lymphocytes were treated with different concentrations of samples except for the blank group. The final concentration of LPS and ConA was 10 and 1 µg/mL, respectively. After incubating for 48 h in the incubator, added 10 µL CKK-8 to each well, and incubated for another 3 h under the same conditions. Absorbance value detected at 450 nm.
2.8 Cell culture
RAW264.7 cell was purchased from the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM high glucose containing 10% FBS and 1% penicillin streptomycin. The cells were stored in a humid incubator containing 5% CO2at 37 °C.
2.9 Assay of RAW264.7 cell viability
Macrophage RAW264.7 cell viability was detected by MTT assay [18,19]. RAW264.7 cells were inoculated with 100 µL cells per well on 96-well plates, cell density was 5 × 105cell/mL, and incubated in an incubator for 24 h, then incubated with different sample concentrations for 24 h. Added 20 µL 5 mg/mL MTT to each well. Incubated in the incubator for 4 h, discard the supernatant, added 150 µL DMSO, and measured the absorbance at 490 nm.
2.10 Assay of pinocytosis activity and NO of RAW264.7 cells
Some modifications were made according to the method described by Wen et al. [20]. The pinocytosis activity of macrophage RAW264.7 was measured. In simple terms, RAW264.7 cells were inoculated with 100 µL cells per well on a 96-well plate, cell density was 5 × 105cell/mL, and incubated in an incubator for 24 h, then incubated with different samples for 24 h. The supernatant was collected and the kit was used to measure the amount of NO in the cell supernatant. Then, added 100 µL 0.075% neutral red solution. After the cells were incubated for another 1 h under the same conditions, the cells were washed twice with PBS. Added 100 µL cell lysate (ethanol:glacial acetic acid(0.1 mol/L) = 1:1,V/V) to each well. After shaking, the absorbance value at 540 nm was detected with a microplate reader. 2 µg/mL LPS was used as positive control. The blank control group received the same amount of medium. The phagocytic index of macrophages phagocytic neutral red was calculated as follows:

2.11 Gene expression analyses
RAW264.7 cells were inoculated with 3 × 105cells/mL, 2 mL in each well of 6-well plates, and treated with different concentrations of peptides for 24 h. After incubation, total cell RNA was prepared using RNA extract (Servicebio). Total RNA was used for cDNA synthesis.BioRad real-time PCR system (Bio-RAD, Inc., USA) was used for qPCR. Denaturation at 95 °C for 10 min, denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s. GAPDH was selected as an internal reference, and the specificity of the primers was verified by melting curve. Data were calculated using 2-ΔΔCtmethod. The qPCR primers were as follows: GAPDH: 5’-CCTCGTCCCGTAGACAAAATG-3’(forward), 5’-TGAGGTCAATGAAGGGGTCGT-3’ (reverse);iNOS: 5’-AGCTCGGGTTGAAGTGGTATG-3’ (forward),5’-CACAGCCACATTGATCTCCG-3’ (reverse); TNF-α:5’-CTCTTCTGTCTACTGAACTTCGGG-3’ (forward),5’-GGTGGTTTGTGAGTGTGAGGGT-3’ (reverse); IL-6:5’-C C C C A A T T T C C A A T G C T C T C C-3’ (f o r w a r d),5’-CGCACTAGGTTTGCCGAGTA-3’ (reverse); IL-1β:5’-G C A T C C A G C T T C A A A T C T C G C-3’ (f o r w a r d),5’-T G T T C A T C T C G G A G C C T G T A G T G-3’ (r e v e r s e);TLR2: 5’-CCAAAGTCTAAAGTCGATCCGC-3’ (forward),5’-AGCCCATTGAGGGTACAGTCGT-3’ (reverse); TLR4:5’-TGAGGACTGGGTGAGAAATGAGC-3’ (forward),5’-CTGCCATGTTTGAGCAATCTCAT-3’ (reverse).
2.12 Nano-HPLC-MS/MS
The sample was desalted with MonoSpin C18column before testing. Sample was analyzed using Q Exactive™ coupled to an EASY-nanoLC 1200 system (Thermo Fisher Scientific, MA, USA)with Acclaim PepMap C18(75 µm × 25 cm, 1.9 μm). The flow rate of the instrument was set at 300 nL/min, the column temperature was set at 40 °C, and the electrospray voltage was set at 2 kV. Phase A was 0.1%formic acid (FA) aqueous solution, and phase B was 80% acetonitrile(ACN) plus 0.1% formic acid (FA). The sample was dissolved in phase A before loading. Sample loading was 3 µL. The gradient started at 2% B phase and balanced for 3 min. The B phase increased from 2% to 35% during 0-47 min, from 35% to 100% during 47-48 min, and 100% during 48-60 min. Them/zscanning range of MS was set asm/z200-1 500, the resolution was set as 70 000, and the automatic gain control target was 3 × 106, the maximum injection time was 60 ms. HCD-MS/MS resolution was set at 17 500, the automatic gain control target was 5 × 104, the maximum injection time was 50 ms, the normalized collection energy was 27, and the dynamic exclusion time was 20 s.
2.13 Molecular docking
Discovery Studio 2017 R2 software was used to analyze the binding mode of TLR2 and peptides. TLR2 receptor structure (PDB ID: 6NIG) downloaded from the website (https://www.rcsb.org/structure/6NIG). Before the calculation begins, the water in the TLR2 receptor was removed and the ligand peptides were minimized. The location of the original ligand KQD in TLR2 was taken as the binding site, and CDOCKER algorithm was used for molecular docking. After calculation, the binding ability, action site and binding mode of the peptides to TLR2 receptor were analyzed [18].
2.14 Statistical analysis
All experiments were repeated three times. Significance was analyzed by univariate ANOVA test in IBM SPSS Statistics 23 software. A post-hoc comparative assessment was performed using LSD, Tukey, and Dunnettt-tests. The significance level was set at 0.05. Data were expressed as mean ± standard deviation (SD). The images in this article were created using Origin 2018.
3 Results and discussion
3.1 Molecular weight distribution of CP, CP-ES and CP-EI
Molecular weight is an important parameter for evaluating bioactive peptides, which is closely related to biological activity [20].Studies have shown that low molecular weight peptides are easy to digest and bioavailable, and can promote the effects of peptides on various physiological functions of the human body [21]. He et al. [15] found that more small molecular weight peptides could be obtained by ethanol extraction, so we used the ethanol extraction method to separate CP into CP-ES and CP-EI. The molecular weight distribution results of CP, CP-ES and CP-EI were shown in Fig. 1.Fig. 1A showed the HPLC of CP, CP-ES and CP-EI, and Fig. 1B showed the molecular weight distribution of CP, CP-ES and CP-EI. As can be seen from Fig. 1B, compared with CP and CP-EI,CP-ES had a smaller molecular weight, below 1 000 Da accounting for 94.28%, among which, peptides with molecular weight between 600-1 000 Da accounted for 12.08%, those with molecular weight between 300-600 Da accounted for 29.22%, and those with molecular weight below 300 Da accounted for 52.97%. The proportion of CP with molecular weight less than 1 000 Da before separation was 83.42%, and that of CP-EI with molecular weight less than 1 000 Da after separation was 71.41%. The proportion of molecular weight above 3 000 Da in CP, CP-ES and CP-EI was all less than 5%. This result indicated that CP-ES obtained by ethanol separation contained more peptides with small molecular weight, which was consistent with the research results of He et al. [15]. So we speculated that CP-ES might have higher bioactivity.
3.2 Determination of peptide content and amino acid content of CP, CP-ES and CP-EI
The most common residues of immunomodulatory peptides were hydrophobic amino acids [20,22]. He et al. [15] found that more peptides containing hydrophobic amino acids could be separated by ethanol extraction. The CP was separated by this method, and the contents of hydrolyzed and hydrophobic amino acids obtained were shown in Table 1, and the contents of free amino acids were shown in Table 2. A total of 17 amino acids were detected. As shown in Table 1, the hydrophobic amino acid contents of CP, CP-ES and CP-EI accounted for 28.80%, 38.80%, and 20.71% of the total amino acid content respectively. These findings confirmed that CP-ES contained a higher proportion of hydrophobic amino acids, and was significantly higher than CP and CP-EI (P< 0.001). The results in this paper were consistent with those of He et al. [15], so it can be inferred that CP-ES has high biological activity. As shown in Table 2, the total free amino acid contents of CP, CP-ES and CP-EI were 110.67, 145.62 and 98.53 mg/g, respectively. It could be seen that the total free amino acid contents in CP-ES was significantly higher than CP and CP-EI (P< 0.001). As can be seen from Fig. 1B, the proportion of CP-ES less than 300 Da was 52.97%, higher than that of CP and CP-EI. This was consistent with the experimental results of total free amino acid contents. The peptide content of CP-ES measured by Kjeldahl nitrogen was 87.56%. Therefore, these results showed that the method of ethanol extraction can obtain samples with higher peptide content, and can effectively enrich peptides with small molecular weight and more hydrophobic amino acids, so we speculated that CP-ES might have better immunomodulatory activity.

Table 1Hydrolyzed amino acid contents of CP, CP-ES, CP-EI.
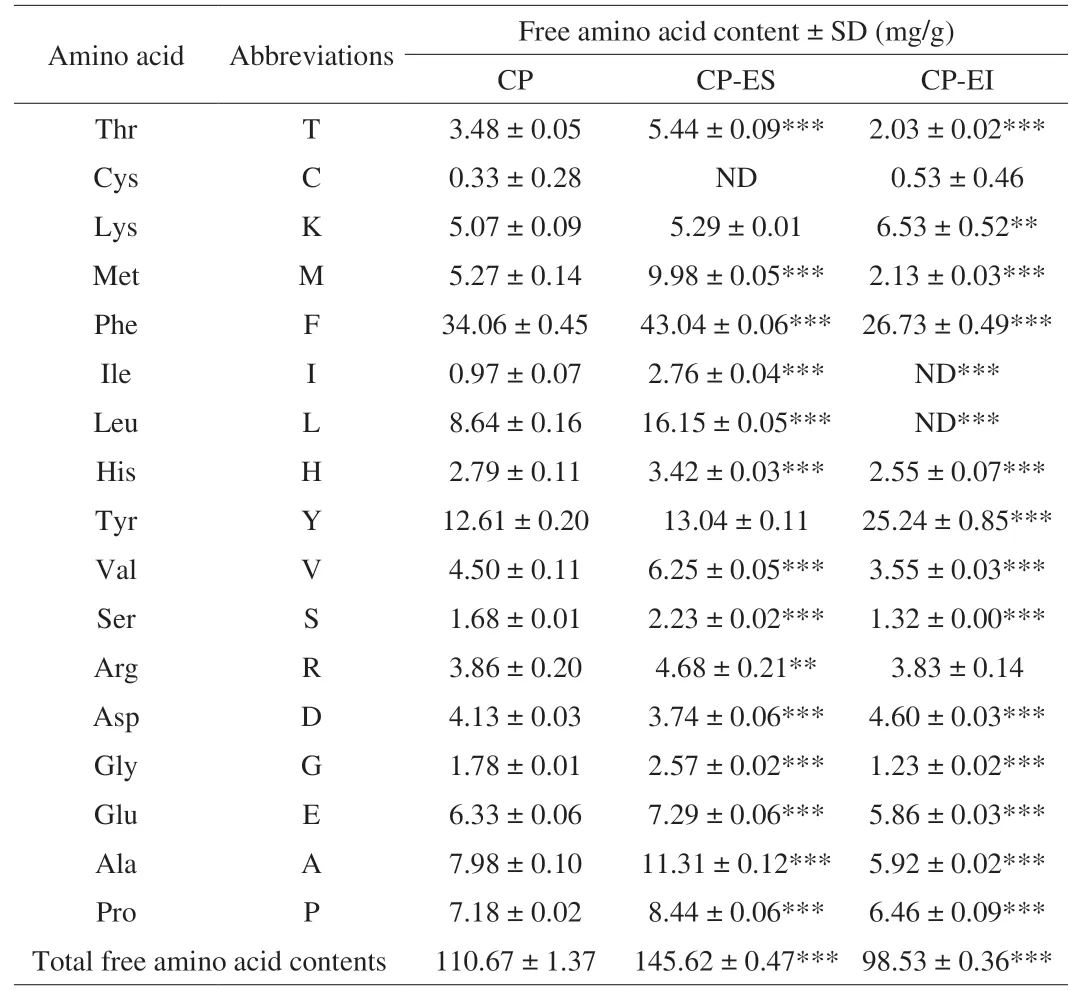
Table 2Free amino acid contents of CP, CP-ES, CP-EI.

Fig. 1 Molecular weight distribution. (A) HPLC and (B) molecular weight distribution of CP, CP-ES and CP-EI. **P < 0.01; ***P < 0.001 versus the CP group.CP-ES obtained by ethanol separation contained more peptides with small molecular weight and might have higher bioactivity.
3.3 Screening of immunomodulatory peptides
To confirm our hypothesis and select a more active component,we used mouse spleen lymphocytes and macrophages to study the immunomodulatory activities of CP, CP-ES and CP-EI. Spleen is the largest secondary lymphoid organ in human body. It plays a central role in host defense and has a wide range of immune functions [23,24].As shown in Fig. 2, we measured the stimulus indices of CP,CP-ES and CP-EI at different stimulation time (24, 48 and 72 h). As shown in Fig. 2A, compared with the blank control group, CP did not significantly enhance the mouse spleen lymphocytes ability of proliferation after 24 h of treatment (P< 0.05). CP-ES significantly promoted the proliferation of spleen lymphocytes manner in a concentration-dependent (P< 0.001). Equally interesting, CP-EI had more obvious effect on spleen lymphocyte proliferation than CP, but it was not dose-dependent. It has been reported that an appropriate amount of ethanol is beneficial to immune regulation [25], so we speculated that some ethanol molecules were introduced into the separation of CP-ES and CP-EI with ethanol, leading to the effect of CP-EI on promoting the proliferation of spleen lymphocytes in mice. As shown in Fig. 2B, CP showed certain ability to promote the proliferation of spleen lymphocytes in mice after treatment for 48 h,which was different from the result of CP treatment for 24 h. After 48 h treatment, CP-ES showed the strongest proliferation of spleen lymphocytes in a concentration-dependent manner. Although CP-EI also had a certain proliferation effect, the promotion effect of CP-EI on splenic lymphocyte proliferation was not as good as that of CP-ES, and there was no concentration dependence. This may because CP-ES has the characteristics of smaller molecular weight and more hydrophobic amino acids, which enhanced the increment ability of CP to mouse spleen lymphocytes. As shown in Fig. 2C,CP-ES kept promoting the proliferation activity of mouse spleen lymphocytes after 72 h culture, but at a slower rate compared with 48 h, which may be due to the high cell density and consumption of the peptides. Hence, it was concluded that CP-ES has the strongest promoting effect on the proliferation of spleen lymphocytes in mice,which was consistent with our previous speculation.

Fig. 2 Screening of immunomodulatory peptides. Mouse spleen lymphocytes had proliferative activity for (A) 24 h, (B) 48 h, (C) 72 h. (D) The cytotoxicity of CP, CP-ES and CP-EI on RAW264.7 cells and (E) the effect on NO release. *P < 0.05; **P < 0.01; ***P < 0.001 versus the control group. CP-ES had the strongest ability to promote the proliferative activity of spleen lymphocytes in mice. At 24, 48 and 72 h, it had a certain activity to promote value-added. CP,CP-ES and CP-EI have no toxicity to RAW264.7 cells at 100, 200 and 500 µg/mL. CP-ES could promote NO production by macrophages, which may activate the defensive mechanism and kill pathogens. Therefore, we selected CP-ES for further investigation.
Before using RAW264.7 cells to study the immunoregulatory activities of CP, CP-ES and CP-EI, the toxicity of CP, CP-ES and CP-EI on RAW264.7 cells should be detected first. Therefore,macrophage RAW264.7 was treated with three concentrations of 100,200 and 500 µg/mL to detect cell viability. As shown in Fig. 2D, CP,CP-ES and CP-EI showed no significant difference in the RAW264.7 cell number and shape compared with the blank control group at 100,200 and 500 µg/mL (P< 0.05). Therefore, it could be considered that CP, CP-ES and CP-EI have no toxicity to RAW264.7 cells at these three concentrations, and the next test could be carried out. NO is one of the paramount mediators of the immune response. It kills pathogens by activating macrophages [26]. As shown in Fig. 2E, the blank control group was treated with the same amount of medium,and the concentration of NO in RAW264.7 cells was 7.19 µmol/L.The positive control group was treated with 2 µg/mL LPS, and the concentration of NO in RAW264.7 cells was 16.22 µmol/L. CP-ES treatment of macrophages significantly increased the amount of NO produced by the cells in a concentration-dependent manner (P< 0.01).NO production in RAW264.7 cells reached 10.60 µmol/L at CP-ES of 500 µg/mL. CP and CP-EI had little effect on NO production in macrophages, which was also predictable. This result was analyzed because CP-ES extracted with ethanol had more small molecule peptides and hydrophobic amino acids. These results indicated that CP-ES is more conducive to the production of NO by macrophages than CP, and the increase of NO release can activate the defensive mechanism and kill pathogens.
In summary, we screened the immunoregulatory activities of CP,CP-ES and CP-EI by mouse spleen lymphocytes and RAW264.7 cells. The results showed that CP-ES could not only promote the proliferation of spleen lymphocytes in mice, but also promote NO production in macrophages. Therefore, we selected CP-ES for further investigation. This was consistent with our previous prediction that CP-ES with small molecular weight and more hydrophobic amino acids have stronger immunomodulatory activity. This was not only consistent with our previous prediction, but also with the results of He et al. [27] and Wen et al. [20], CP-ES has the characteristics of small molecular weight and more hydrophobic amino acids, and has better immunomodulatory activity.
3.4 Effects of CP-ES on proliferation of spleen lymphocytes (T and B cells) in mice
T and B cells are the two paramount types of cells in adaptive immunity [22]. ConA, induces T cell mitosis and LPS induces B cell mitogen, promoting cell growth and immunoglobulin release [28]. The effects of CP-ES on the proliferation of spleen lymphocytes, ConA-induced proliferation of T lymphocytes and LPS-induced proliferation of B lymphocytes at 10-700 µg/mL were shown in Fig. 3. As shown in Fig. 3A, CP-ES can significantly promote the proliferation of spleen lymphocytes in mice, and the stimulation index increased with the increase of CP-ES concentration(P<0.001). At 700 µg/mL supplied for 48 h, the splenic lymphocyte stimulation index was the highest, which was 1.18, and it continued to increase. As shown in Fig. 3B, CP-ES can significantly promote the proliferation of T lymphocytes (P<0.01). At 500 µg/mL, the stimulation index of CP-ES on T lymphocytes was the highest, which was 1.11. However, when CP-ES concentration increased to 700 µg/mL,the stimulus index decreased. No proliferation of CP-ES on B lymphocytes was observed at 0-700 µg/mL. These results showed that CP-ES encouraged the proliferation of spleen lymphocytes and T lymphocytes, but did not stimulate the proliferation of B lymphocytes to the same extent. Similar to the results of our study, RGPPP and YGPSSYGYG found by Yang et al. [3,29] can promote the proliferation of T lymphocytes, but not B lymphocytes.
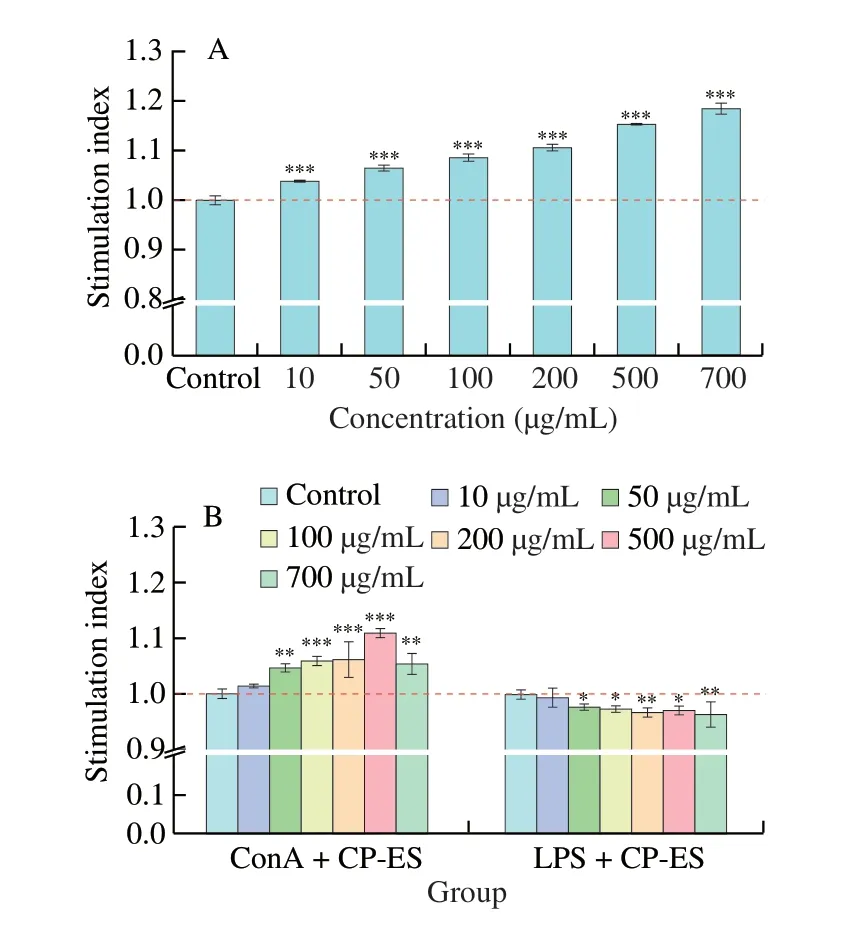
Fig. 3 Immunomodulatory activity of CP-ES in splenic lymphocytes. The effect of CP-ES on the proliferation of (A) spleen lymphocytes,(B) T lymphocytes and B lymphocytes in mice. *P < 0.05; **P < 0.01;***P < 0.001 versus the control group. CP-ES encouraged the proliferation of spleen lymphocytes and T lymphocytes, but did not stimulate the proliferation of B lymphocytes to the same extent.
3.5 Effect of CP-ES on phagocytic activity of RAW264.7 cells
Phagocytosis is the main characteristic function of macrophages,including recognition and engulfment of foreign bodies [30].Phagocytosis is defined as the mechanism for internalizing large particles of approximately 1 mm or more [31]. Phagocytosis is very effective as a central regulator of innate immunity [32]. As shown in Fig. 4A, CP-ES had no significant effect on macrophage cell viability in the concentration of 0-700 µg/mL (P<0.05). As shown in Fig. 4B, compared with the blank control group, the phagocytic activity increased by 36.07% in the positive control group. As can be seen from the figure, CP-ES significantly enhanced the phagocytic activity of macrophage in a concentration-dependent manner within the concentration range of 10-500 µg/mL (P<0.001). The phagocytic activity was the highest at 500 µg/mL, which increased by 25.04%compared with the blank control group. Subsequently, the phagocytic activity remained basically unchanged. These results suggested that CP-ES may play an important role in immune regulation by increasing the phagocytic activity of macrophages, which was consistent with the results of Wen et al. [20].
3.6 Effects of CP-ES on NO and iNOS gene expression in RAW264.7 cells
NO, a gaseous molecule in cells, is the smallest biologically active molecule and can be produced by a variety of cells. It plays a crucial role in the immune system and is an important pro-inflammatory mediator affecting the immune system. NO is synthesized by nitric oxide synthase (NOS). The iNOS in NOS can be induced by a variety of cytokines or other stimuli. The function of iNOS not only helps to kill pathogens, but also has immunomodulatory effects [33,34]. As shown in Fig. 4C, compared with the blank control group, CP-ES had no significant effect on promoting NO production by macrophages at 10 µg/mL (P<0.05), and could significantly promote NO production by macrophages with the increase of concentration from 50 µg/mL(P<0.001). As shown in Fig. 4D, CP-ES could significantly upregulate the expression ofiNOSmRNA in macrophage within the concentration range of 100-400 µg/mL (P<0.001). Taken together,CP-ES may induce NO production by activating the immune reaction of macrophages and increasing the expression ofiNOSmRNA. Wen et al. [20] had similar research results before, and they found that soybean peptides not only promoted the expression ofiNOSmRNA,but also increased the level of NO.
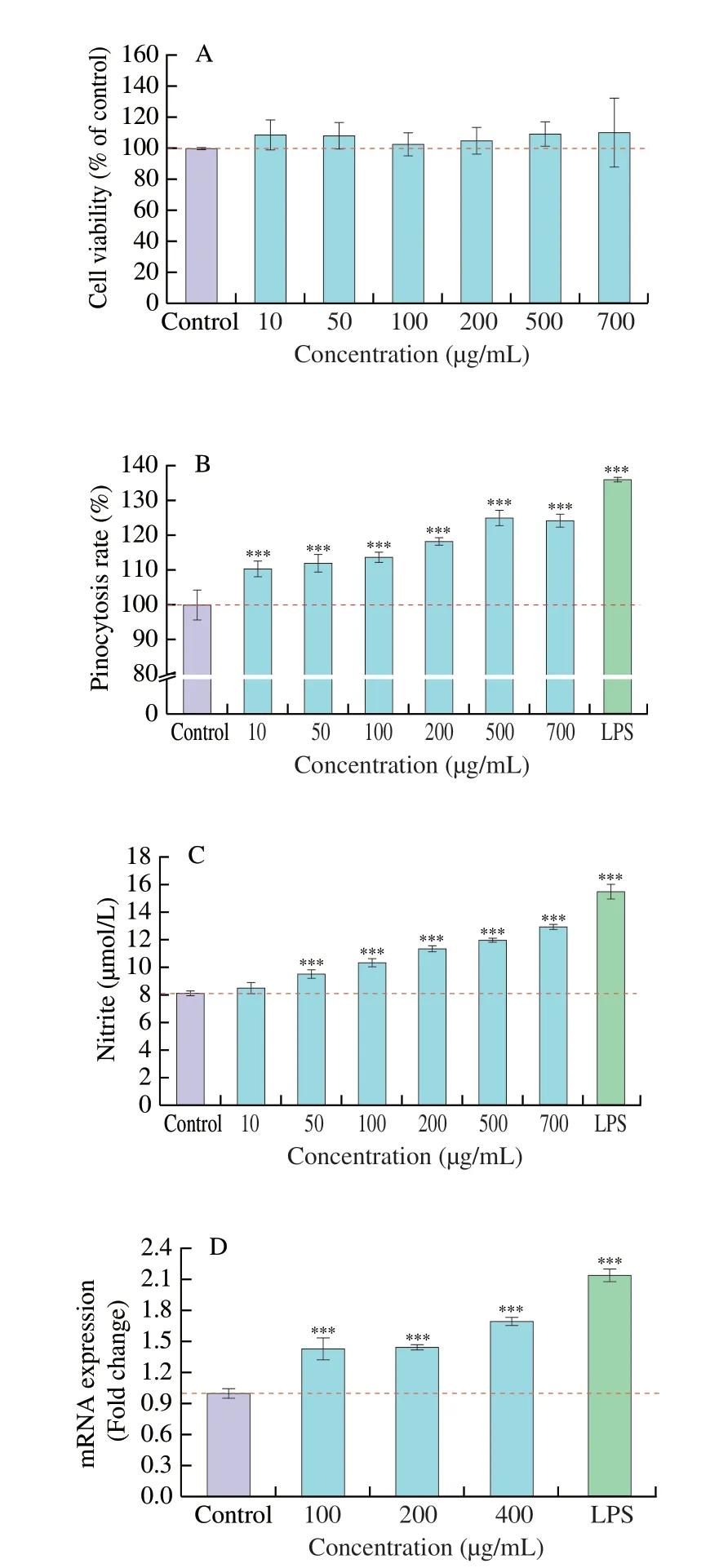
Fig. 4 mmunoregulation of CP-ES on RAW264.7 cells. (A) The cytotoxicity of CP-ES on RAW264.7 cells, (B) the effect of CP-ES on phagocytic activity of RAW264.7 cells, (C) the effect of CP-ES on NO release amount of RAW264.7 cells, (D) the effect of CP-ES on iNOS gene expression level of RAW264.7 cells. ***P < 0.001 versus the control group. CP-ES showed no toxicity to RAW264.7 cells in the concentration range of 0-700 µg/mL.CP-ES may play an immunomodulatory role by enhancing the phagocytosis of RAW264.7 cells. CP-ES may induce NO production by activating the immune reaction of RAW264.7 cells and increasing the expression of iNOS mRNA.
3.7 Effects of CP-ES on expression of TNF-α, IL-6, IL-1β,TLR2 and TLR4 genes in RAW264.7 cells
Macrophages can release pro-inflammatory cytokines such as TNF-α, IL-6 and IL-1β, which play an important role in the immune system, and participate in the cytolysis function of macrophages to regulate immunity [35-38]. Fig. 5 showed the effects of CP-ES on mRNA expression ofTNF-α(Fig. 5A),IL-6(Fig. 5B),IL-1β(Fig. 5C),TLR2(Fig. 5D) andTLR4(Fig. 5E) in macrophages. As shown in Fig. 5A, compared with blank control group, CP-ES did not significantly promote the expression ofTNF-αmRNA at 100 µg/mL,but significantly increased the expression ofTNF-αmRNA in macrophages at 200 and 400 µg/mL (P<0.01). As shown in Fig. 5B, CP-ES significantly promotedIL-6mRNA expression in a concentration-dependent manner at 100-400 µg/mL compared with the blank control group (P<0.01). As shown in Fig. 5C,CP-ES could promote the expression ofIL-1βmRNA at 100 and 200 µg/mL, but the effect was not significant, when the concentration was increased to 400 µg/mL, CP-ES could significantly improve the expression ofIL-1βmRNA in macrophages (P<0.01).These results suggested that CP-ES fostered the expression ofTNF-α,IL-6andIL-1βgenes at a certain concentration, suggesting that CP-ES may regulate immunity by controlling these cytokines.
Innate immune responses depend on the recognition of pathogens by several receptors on phagocytes. TLR receptor is an important receptor in innate immune recognition [39]. It has been documented that activation of TLR2 and TLR4 can induce or terminate the immune response. Activation of TLR2/4 leads to the production of multiple immune mediators [40]. Jiang et al.[41] measured the expression ofTLR2andTLR4mRNA simultaneously to investigate the activation mode of fucoidan from sea cucumberStichopus chloronotus. As shown in Fig. 5D, CP-ES did not significantly promoteTLR2mRNA expression at the concentration of 100 µg/mL compared with the blank control group. At 200 and 400 µg/mL,TLR2mRNA expression was significantly promoted (P<0.05). As shown in Fig. 5E, the expression ofTLR4mRNA in positive control group was significantly increased compared with blank control group, but the effect of CP-ES on the expression ofTLR4mRNA was not obvious within the concentration range of 100-400 µg/mL (P<0.05). The results suggested that CP-ES can promote the release of NO by macrophages and up-regulate the expression ofiNOS,TNF-α,IL-6andIL-1βmRNA, possibly due to the activation of TLR2 receptors on the surface of macrophages. TLR2 recognizes pathogens and triggers MyD88 dependent signaling pathways, prompting cells to release cytokines to kill pathogens [39].

Fig. 5 Effects of CP-ES on gene expression in RAW264.7 cells. (A) TNF-α, (B) IL-6, (C) IL-1β, (D) TLR2 and (E) TLR4 mRNA expression. *P < 0.05; **P < 0.01;***P < 0.001 versus the control group. CP-ES fostered the expression of TNF-α, IL-6, IL-1β and TLR2 genes, but did not affect the expression of TLR4 genes,suggesting that CP-ES may regulate immunity by controlling these cytokines. CP-ES may regulate immune activity by activating TLR2 receptor.
3.8 Identification of peptides sequence
Due to small molecular weight peptides, peptides with PeptideRanker score close to 1, are more likely to be active [42].Therefore, CP-ES were identified by nano-HPLC-MS/MS. Table 3 listed 27 peptides with high matching, which have 10 or fewer amino acids and PeptideRanker greater than 0.8. The most common residues of immunomodulatory peptides reported in the literature are hydrophobic amino acids Gly, Val, Leu, Pro and Phe, negatively charged amino acid Glu and aromatic amino acid Tyr. Studies have shown that hydrophobic amino acids and one or more residues such as Gln, Glu, Tyr, Trp, Cys, Asp, Asn can promote the immune regulatory activity of polypeptides [22]. Wen’s study showed that 46 of the 51 peptide sequences identified were immunomodulatory peptides [20]. We used a similar approach to predict peptides with immunomodulatory activity and found that 18 of the 27 peptides listed in Table 3 had this characteristic. Therefore, it could be speculated that the peptides with these characteristics may play the immunomodulatory function in CP-ES.
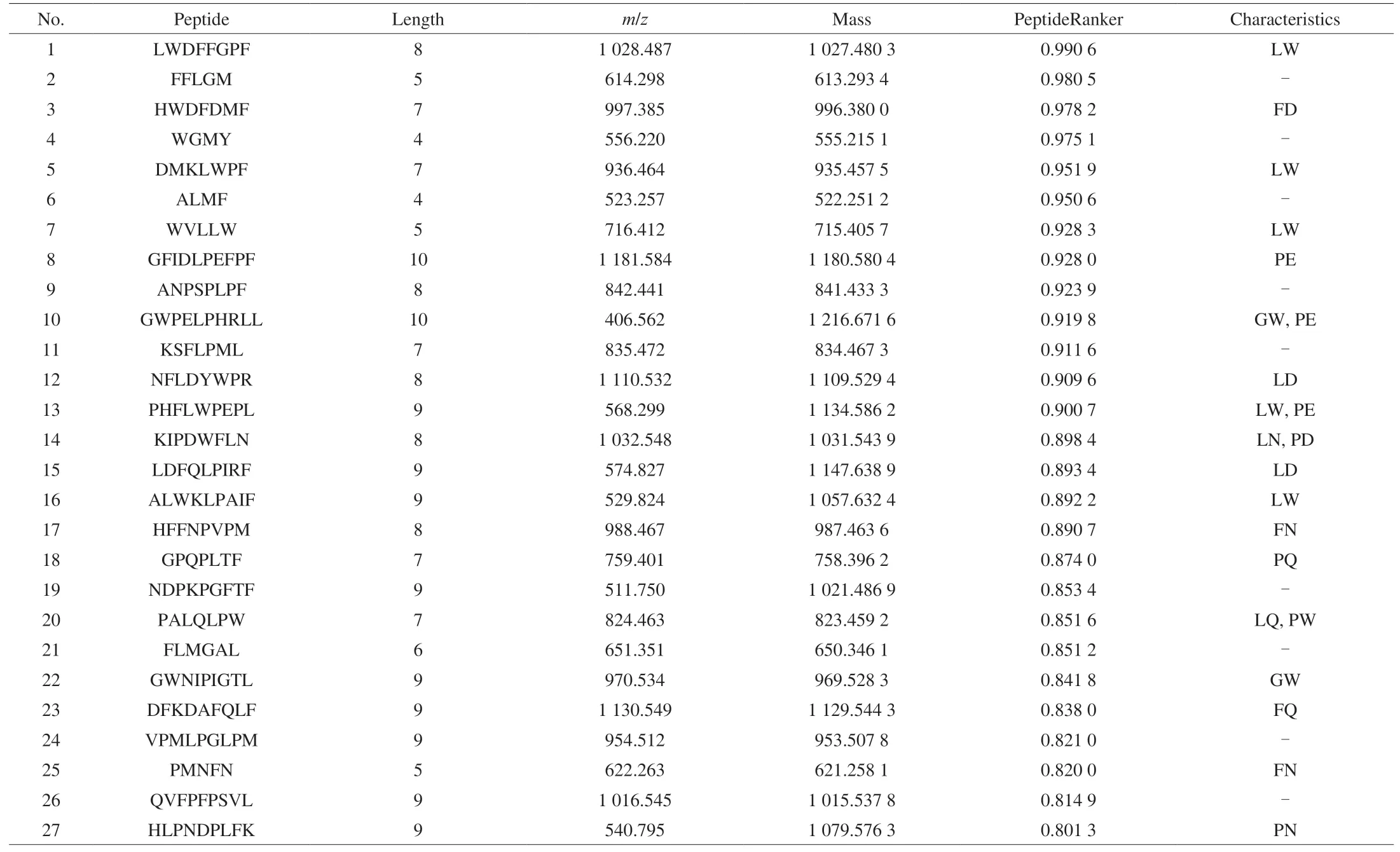
Table 3Peptide sequences identified by nano-HPLC-MS/MS.
3.9 Molecular docking
In the above study, we found that CP-ES could promote the expression ofTLR2mRNA in macrophages, but could not promote the expression ofTLR4mRNA. We speculated that CP-ES played its role through TLR2 receptor. Next, TLR2 was selected as the receptor, and the peptides were identified as the ligand. DS was used for molecular docking to explore the mechanism of the immune function of the peptides. TLR2 can act by forming heterodimers with TLR1 or TLR6 [39]. Activation of TLR2 leads to the production of a large number of immune mediators to promote the production of inflammatory cytokines, thus regulating immune function [40].The 18 identified and screened peptides were selected for molecular docking with TLR2 receptor, and the docking results were shown in Fig. 6 and Table 4. TLR2 receptor selected the protein with ID 6NIG from the PDB database. The PDB structure and binding site (represented by the transparent red sphere) were shown in Fig. 6A. After docking, the six peptides with the highest fraction of“-CDOCKER_ENERGY” were selected for interaction analysis.As shown in Fig. 6B, DFKDAFQLF and TLR2 receptor Ile319,Tyr326, Asp327, Leu328, Leu331, Tyr332, Val343, Val348, Phe349,Val351, Pro352 and Leu355 residues were used for van der Waals,conventional hydrogen bond, carbon hydrogen bond, Pi-Pi T-shaped,Alkyl and Pi-Alkyl interactions. As shown in Fig. 6C, LDFQLPIRF and TLR2 receptors Leu289, Ile314, Leu317, Ile319, Tyr323, Tyr326,Ser346, Phe349, Leu350, Pro352 and Tyr376 residues were used for van der Waals, conventional hydrogen bond, Pi-Pi Stacked,Pi-Pi T-shaped, Alkyl and Pi-Alkyl interactions. As shown in Fig. 6D,GFIDLPEFPF and TLR2 receptor Phe284, Ile314, Leu317, Phe325,Tyr326, Leu328, Leu331, Val343, Ser346 Lys347, Val348, Leu350,Val351, Pro352 and Leu355 residues were used for van der Waals,conventional hydrogen bond, carbon hydrogen bond, Pi-Anion, Alkyl and Pi-Alkyl interactions. As shown in Fig. 6E, NFLDYWPR and TLR2 receptor Ile319, Tyr323, Tyr326, Leu328, Tyr332, Ser346,Lys347 Val348, Phe349, Leu350 and Leu355 residues were used for van der Waals, conventional hydrogen bond, carbon hydrogen bond, Pi-Pi T-shaped, Alkyl and Pi-Alkyl interactions. As shown in Fig. 6F, HWDFDMF and TLR2 receptor Tyr323, Phe325, Tyr326,Tyr332, Val348, Leu350, Val351 and Pro352 residues were used for van der Waals, conventional hydrogen bond, carbon hydrogen bond, Pi-Anion, Pi-sigma and Pi-Alkyl interactions. As shown in Fig. 6G, GWPELPHRLL and TLR2 receptors Leu266, Leu289,Leu312, Ile314, Ile319, Tyr323, Phe325, Tyr326, Leu328, Leu331,Tyr332, Val343, Ser346, Lys347, Val348, Phe349, Leu350 and Leu355 residues were used for van der Waals, conventional hydrogen bond, carbon hydrogen bond, Pi-sigma, Pi-Pi Stacked, Alkyl and Pi-Alkyl interactions. Pam3CSK4 has been shown to be an effective TLR2 agonist. On TLR2, residues of Asp327, Leu328, Tyr332 and Leu350 are essential for Pam3CSK4 activity [4]. CP-ES could also act on similar sites with Pam3CSK4, so it could be speculated that CP-ES can activate TLR2 in a similar way to Pam3CSK4. These results suggested that CP-ES may act as effective agonists of TLR2 and regulate immune function by activating macrophages.
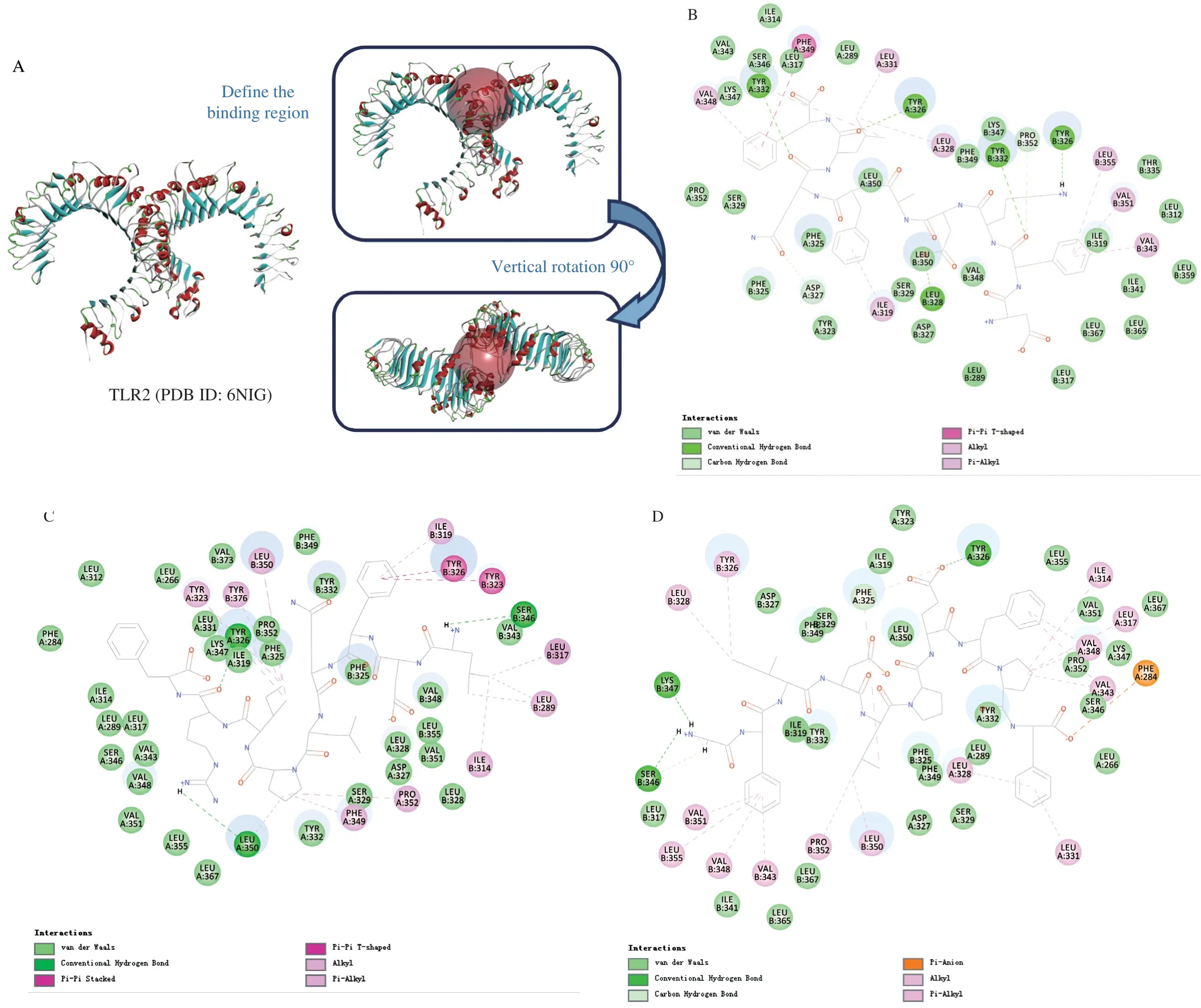
Fig. 6 Results of peptides docking with TLR2 receptor (PDB ID: 6NIG) molecule. (A) Three-dimensional structure diagram of TLR2 receptor and docking site(red sphere is the docking site), (B-G) two-dimensional structure diagrams of interaction between DFKDAFQLF, LDFQLPIRF, GFIDLPEFPF, NFLDYWPR,HWDFDMF, GWPELPHRLL and TLR2 receptor, respectively. CP-ES may act as effective agonists of TLR2 and regulate immune function by activating macrophages.

Fig. 6 (Continued)
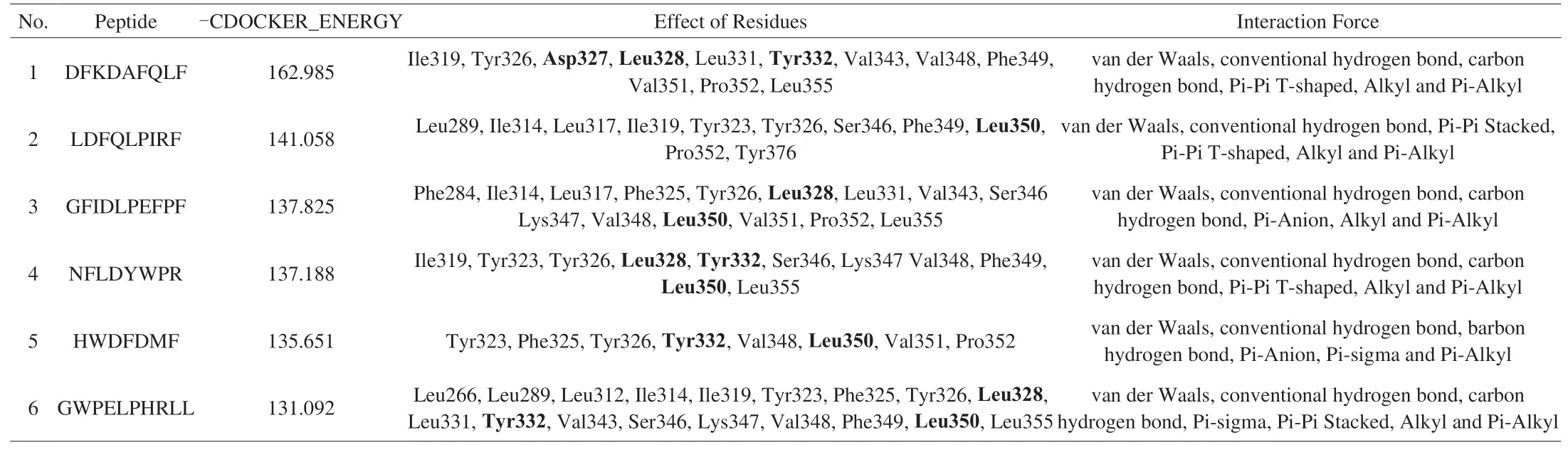
Table 4DFKDAFQLF, LDFQLPIRF, GFIDLPEFPF, NFLDYWPR, HWDFDMF, GWPELPHRLL and TLR2 receptor molecules docking interaction residues and interaction forces.
4. Conclusions
This study showed that the peptide content of CP-ES extracted by ethanol was 87.56%, which was high. The molecular weight of CP-ES was small. CP-ES with molecular weight below 1 000 Da accounted for 94.28%, among which, peptides with molecular weight between 600-1 000 Da accounted for 12.08%, those with molecular weight between 300-600 Da accounted for 29.22%, and those with molecular weight below 300 Da accounted for 52.97%. The hydrophobic amino acid proportion and free amino acid contents of CP-ES were higher than those of CP and CP-EI. The ethanol separation could obtain samples with higher peptide content, and could effectively enrich peptides with small molecular weight and more hydrophobic amino acids. The immunomodulatory peptides were screened from CP,CP-ES and CP-EI by mouse spleen lymphocytes and RAW264.7 cells. The results showed that CP-ES can not only promote the proliferation of spleen lymphocytes in mice, but also promote NO production in macrophage RAW264.7, therefore, CP-ES was tested further. CP-ES attributed to the proliferation of spleen lymphocytes and T lymphocytes in mice, and may modulate adaptive immunity. In the meanwhile, CP-ES was not equally effective with B lymphocyte proliferation. CP-ES enhanced the phagocytosis of macrophage RAW264.7, increased the expression ofiNOSmRNA and induced the production of NO in macrophage RAW264.7. CP-ES upregulated the expression ofTNF-α,IL-6,IL-1βandTLR2genes, but did not affect the expression ofTLR4genes. It was speculated that CP-ES may regulate innate immunity by upregulating the expression of cytokine genes and activating TLR2 receptors. According to the amino acid characteristics of immunomodulatory peptides derived from food protein reported in the literature, 18 of the 27 peptides were screened out and docked with TLR2 receptor molecules. Six peptides DFKDAFQLF, LDFQLPIRF, GFIDLPEFPF, NFLDYWPR,HWDFDMF and GWPELPHRLL with the highest score of“-CDOCKER_ENERGY” were selected to analyze further the acting residues and interaction forces. It was speculated that CP-ES can act as an activator of TLR2 in a manner similar to Pam3CSK4. These results suggested that the mechanism of CP-ES action may regulate the immune function by initiating the innate and adaptive cascades.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This work was financially supported by the Project of Xingliao Talent Plan “Science and Technology Innovation Leader”(XLYC1802047).
杂志排行
食品科学与人类健康(英文)的其它文章
- Emerging natural hemp seed proteins and their functions for nutraceutical applications
- A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
- Modulatory effects of Lactiplantibacillus plantarum on chronic metabolic diseases
- The role of f lavonoids in mitigating food originated heterocyclic aromatic amines that concerns human wellness
- The hypoglycemic potential of phenolics from functional foods and their mechanisms
- Insights on the molecular mechanism of neuroprotection exerted by edible bird’s nest and its bioactive constituents
