Evaluation of corrosion resistance,mechanical integrity loss and biocompatibility of PCL/HA/TiO2 hybrid coated biodegradable ZM21 Mg alloy
2022-12-30NvdeepSinghUmBtrKmlKumrAnilMhptro
Nvdeep Singh,Um Btr,∗,Kml Kumr,Anil Mhptro
a Department of Metallurgical and Materials Engineering,Punjab Engineering College,Chandigarh 160012,India
b Department of Mechanical Engineering,Punjab Engineering College,Chandigarh 160012,India
c Department of Biomedical Engineering,Wichita State University,Wichita,KS 67260,United States
Abstract A novel PCL/HA/TiO2 hybrid coating on ZM21 Mg alloy substrate has been investigated for corrosion resistance,biocompatibility and mechanical integrity loss in terms of bending,compressive and tensile strength in physiological media.The prepared hybrid coating was dip coated over ZM21 from HA/TiO2 and PCL solutions followed by creating a microporous PCL layer by utilizing Non-solvent Induced Phase Separation (NIPS) technique.The electrochemical measurement and in-vitro degradation study in SBF after 28 days showed that the PCL/HA/TiO2 hybrid coating reduced H2 evolution rate,weight loss,and corrosion rate by 64,116 and 118 times respectively,as compared to uncoated ZM21 samples.The surface studies carried out using SEM-EDX,FTIR and XRD revealed formation of highly stable 3d fl wer-like HA crystals with Ca/P ratio of 1.60 in the PCL micropores.This dense apatite growth effectively protected the PCL/HA/TiO2 hybrid coated samples to maintain the good mechanical integrity even after 28 days of immersion as compared to HA/TiO2 composite coated,As-polished(A/P) and As-machined (A/M) samples.The failure analysis of samples under mechanical loading were performed using SEM-BSE-EBSD.The in-vitro cellular viability of L929 fibroblas cells on PCL/HA/TiO2 hybrid coating was found 50.47%higher with respect to control group,whereas bacterial viability was supressed by 57.15 and 62.35% against gram-positive Staphylococcus aureus and gram-negative Escherichia coli bacterial models.The comprehensive assessment indicates PCL/HA/TiO2 hybrid coating as a suitable candidate to delay early degradation and mechanical integrity loss of Mg-based alloys for devising biodegradable orthopaedic implant.
Keywords: Magnesium alloy;Surface modification Hybrid coating;Mechanical integrity loss;Degradation rate;Biocompatibility.
1.Introduction
Biodegradable implant devices,necessary for providing structural support and appropriate degradability during healing phase of bone defects for orthopaedic and craniofacial applications,have gained increasing attention in recent years[1].Mg and Mg alloys are the high potential candidates for such implant devices because their mechanical properties are quite similar to those of natural bone.Nevertheless,in the presence of physiological media,these alloys undergo fast corrosion and abrupt H2release at their surface.The degradation process involve formation of localized pits,cavities,and cracks,which act as stress raisers under bending,tension and compression loading [2].This results in untimely loss of mechanical integrity over the designed service span leading to their premature failure.Various techniques such as alloying and surface modificatio have been employed to address these challenges [3].Nevertheless,addition of alloying elements form micro-galvanic cells in the Mg matrix due to their electronegativity difference with Mg and consequently promotes the corrosion rate.The chemical coatings may not directly influenc the mechanical properties of Mg alloys,but can aid in delaying their degradation in physiological media,thereby resulting in retention of adequate mechanical strength up to bone healing period [4].Previously various types of inorganic coatings such as HA,TiO2,ZnO,ZrO2have been deposited on Mg alloys but none of these could effectively restrict corrosion primarily because of the coating defects [5].Even though,near defect-free coatings of polymers like PCL,PLGA,PLA,etc.with good hydrolytic stability can be obtained,it is still likely for the aggressive ions of physiological media to penetrate through permeable matrix of these coatings and cause the blister formation or even delamination [6].Thus,existing alloying and surface modificatio techniques do not provide acceptable mechanical integrity for Mg-alloys in physiological environment.
It has recently been reported that inorganic-organic composite and duplex coatings on Mg alloys have provided good corrosion resistant and enhanced endurance of mechanical integrity in physiological media as compared with their uncoated counterparts.Abdal-hay et al.[7] have reported that the PCL/nHAp composite coated AM 50 Mg alloy could retain 34% more tensile strength than the uncoated alloy after twenty days of SBF immersion.Li et al.[8] have reported that MgF2coated Mg-Zn-Zr alloy possess higher yield strength and % El as compared to uncoated samples by 35.31% and 14%,respectively after thirty days of immersion in SBF.Bakhsheshi-rad et al.[9] have reported that nFHAPCL duplex coated Mg-2Zn-3Ce alloy resulted in 13% loss in compressive strength after ten days of SBF immersion,while the decline was 33% for nFHA coated and 25% for alone PCL coated Mg alloy,respectively.However,limited bio-functionalities and inadequate long-term stability restrain their capabilities required for clinical qualification Alternatively,in multi-layered hybrid coatings,the individual layers beside acting as an additional physical barriers against aggressive ions,effectively introduces several bio-functionalities such as osteo-induction,biocompatibility,biocidal response,self-healing,drug delivery etc.as summarized in Table 1 [10–16].Thus,the multi-layered hybrid coatings can be considered as promising substitute to the composite or duplex coatings.Apart from widely recognized bio-functionalities provided by hybrid coatings,literature does not provide adequate evidence on the mechanism by which the multi-layered hybrid coatings reduce the mechanical integrity loss of the Mg alloy under various loading conditions in the presence of physiological medium.
Polymer is one of the important constituent in the hybrid coatings studied in past.Among various biocompatible degradable polymers,PCL exhibit superior corrosion resistance andin-situmechanical features like elongation up to 80% at break-point [17],however,it lacks adhesion strength and biocompatibility when coated directly on Mg substrate[18].Literature shows the use of bioactive ceramic-like HA for polymeric coatings due to its good binding strength [19].However,HA-PCL composite results in a mechanically unstable coating due to difference in their elastic modulus [20].Moreover,the presence of HA particles in HA-PCL composite enhances its water uptake ability due to PCL hydrolysis leading to fast degradation of the composite.Alternatively,when an HA layer is applied at the Mg-PCL interface,adhesion strength is improved through hydrogen bonding and electrostatic interactions [21].
It is well known that the implants are exceedingly riskprone towards microbial pathogenic attacks that leads to the implant failure and sometimes the infections are so severe that it even causes the patient’s death [22].TiO2has been extensively recognized for its bactericidal tendency in bothin-vivoandvitroconditions.The chemical inertness of TiO2in HA matrix makes it a reasonable bactericidal agent when used with the HA coatings [23].The unique combination of PCL,HA/TiO2may impart their individual characteristics to provide better performance in terms of corrosion resistance,mechanical integrity,cell-viability and anti-bacterial response towards Mg alloys.But such work has not been reported yet.To fulfi the research gap,the potential of a novel PCL/HA/TiO2multi-layer hybrid coating has been investigated for the degradation behaviour and loss in mechanical integrity of the hybrid coated ZM21 during 28 days of SBF immersion.The loss in the bending strength,compression strength,tensile strength and % elongation of the coated ZM21 alloy has been correlated to the potentiostatic and EIS corrosion study.The SEMEDS-EBSD study has been used to reveal the plastic deformation mechanism during the course of degradation.The Biocompatibility of coatings has been examined using MTT assay for L929 cell-line,while the antibacterial response of the coatings against a gram-positive S.aureus and gram-negative E.coli bacteria has been investigated using XTT assay conditions.The performance of PCL/HA/TiO2hybrid coting has been compared with HA/TiO2composite coating,A/P (Aspolished) and A/M (As-machined) uncoated ZM21 Mg alloy over the immersion period up to 28 days in SBF.
2.Experimental details
2.1.Material and substrate preparation
An extruded plate of ZM21 Mg alloy with 7 mm thickness having elemental composition of 1.0Mn – 2.0Zn – 97.0Mg(wt.%) was used in the present study.The samples for the tensile(S.1a),compression(S.1b),three-point bend test(S.1c)and disc-shaped samples (S.1d) were prepared using a nonconventional electric discharge WEDM (ELPULS 15,Electronica,India).The A/M samples were produced by WEDM operations on ZM21 Mg alloy.The WEDM machining parameters controls the surface integrity of Mg alloys [24,25].Therefore,optimized WEDM parameters as given in Table 2,were utilized to obtain uniform surface finis on each A/M sample.The A/M substrates were grounded by 1200 grit-size SiC paper,followed by ultrasonication cleaning for 10 min.Thus obtained A/P samples were utilized for coating deposition.
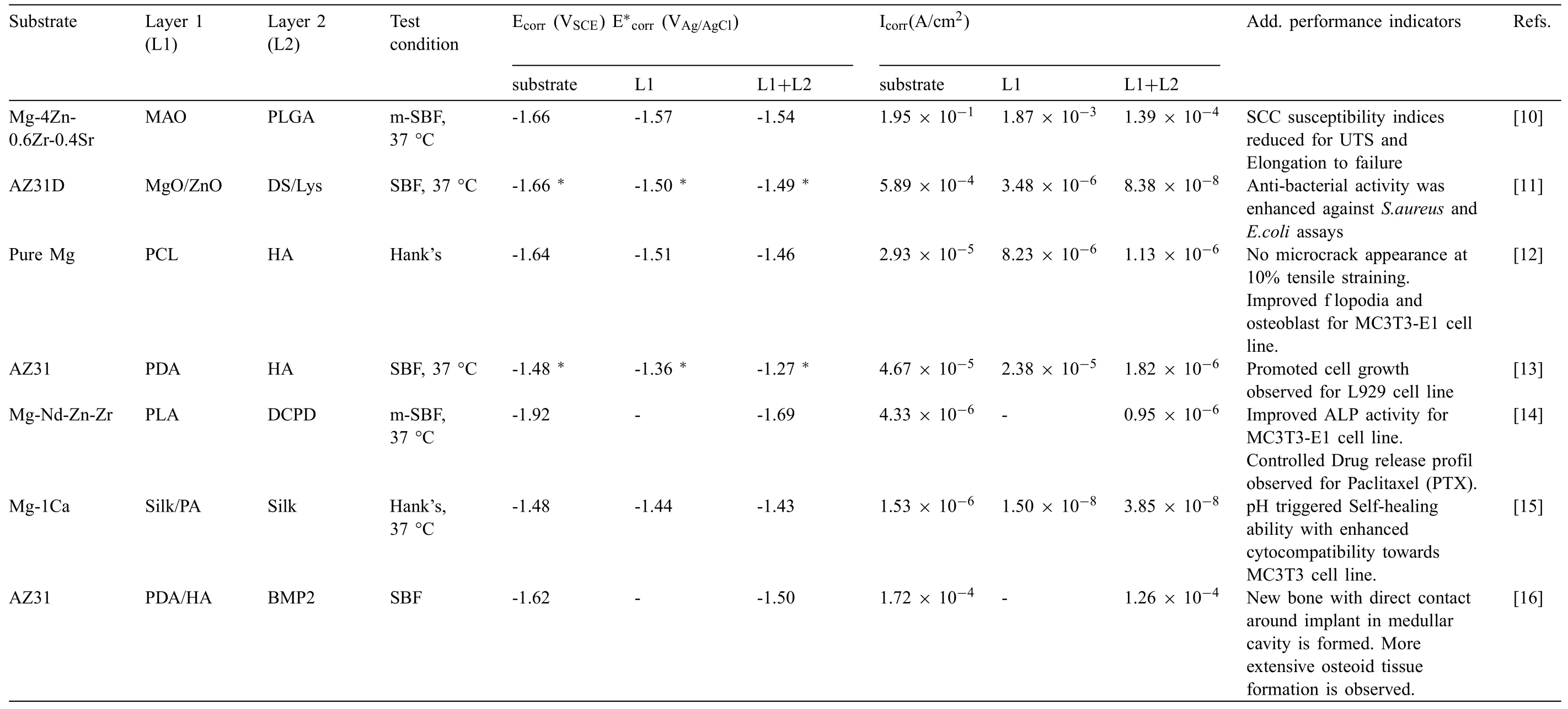
Table 1 Corrosion properties and additional performance indicators of various multi-layered hybrid coatings on Mg alloys

Table 2 Optimized WEDM parameters for machining of ZM21 Mg alloy.

Fig.1.A schematic process fl w diagram for coatings preparation on ZM21 Mg alloy.
2.2.Coatings preparation
2.2.1.HA/TiO2 coating preparation
Titanium (IV) n-butoxide (Alfa-Aesar,USA) and acetic acid (Sigma-Aldrich,USA) HAc was added dropwise in ethanol (Sigma-Aldrich,USA) EtOH by maintaining the molar ratio of 1:0.1:9 respectively for the preparation of TiO2sol that is pale yellow in color.The hydroxyapatite powder (HA)synthesis was carried out by sol-gel route as given by Batra et al [26].Thus obtained HA powder was blended with TiO2sol (1:1 w/v) by continuous stirring for 24 h to achieve a uniform HA/TiO2sol.The coating deposition was achieved by following the process parameters mentioned in Fig.1.The coated samples were sintered for 3 h at 350 °C to obtain anatase TiO2.
2.2.2.PCL/HA/TiO2 hybrid coating preparation
The fabrication of PCL (Sigma-Aldrich,USA) layer over HA/TiO2coated samples was carried out in two steps.Firstly,a PCL layer was dip coated at withdrawl speed of 10 mm/min from 5% w/v soln of PCL -DCM (Sigma-Aldrich,USA),followed by drying upto 1 h at 30 °C.The cycle was repeated fi e times.Then,the coated substrate is heat treated at 50 °C for 24 h to obtain uniformly densifie and pore free PCL layer.Secondly,an interconnected microporous layers of the PCL were obtained on pore-free dense PCL layer by Non-Solvent induced phase separation (NIPS) technique.The cycle was repeated three times followed by drying upto 1 h at 30°C after each cycle as shown in Fig.1.
2.3.Surface analysis and structure characterization
The morphologies of PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P,and A/M ZM21 Mg samples were characterized using a JSM-IT 500 scanning electron microscope,SEM (JSM-IT 500,JEOL,Japan) operated at 20 kV of acceleration voltage.Energy Dispersive X-ray,EDX(Ultim Max,Oxford instruments,UK)mapping and line scan was conducted to reveal the elemental distribution on the coated surface and cross section,respectively.The surface finis of samples were measured by portable surface roughness tester (SJ-310,Mitutoyo,Japan).A Cross-cut tape adhesion test was performed on PCL/HA/TiO2hybrid coated and HA/TiO2composite coated ZM21 Mg alloy according to ASTM D3359-17 standard.
The structural characteristics of apatite deposited on the PCL/HA/TiO2hybrid coated sample during 28 days of SBF immersion were carried out by FTIR and XRD analysis.The apatite deposited preferably in the pores of top PCLNIPSlayer.For the purpose of characterization of deposited apatite,the top PCL layer peeled-off carefully from the PCL/HA/TiO2hybrid coating,was used as the sample.The functional groups of molecular structures were detected using an infrared spectroscopy,FTIR (Tensor II,Bruker,Germany) in the spectral range of 400–4000 cm−1with 2 cm−1resolution.The phase detection and structure characterization of was analysed by X-ray diffraction (X’PERT Pro,Panalytical,Netherland) technique using Cu-Kαradiation for diffraction angles 10–99°.The texture variations after mechanical failure were examined using Electron backscattered diffraction,EBSD (Quanta 3D FEG,FEI,japan) in the tensile and compression zones of the samples failed during three-point bend test after 28 days of immersion.Due to the presence of coarser grains,automated EBSD scans were conducted over a sizeable scanned area (1200 × 1200 μm2) with a step size of 0.8 μm.Data analysis was carried out using TSL-OIM software(V8,EDAX Inc.,USA).
2.4.Electrochemical measurements
The Potentiodynamic polarization (PDP) and Electrochemical impedance spectroscopy (EIS) measurements were conducted on an electrochemical workstation (Autolab PG-STAT 302N,Metrohm,Switzerland) with a standard three-electrode cell configuration The Ag/AgCl (1M KCL),graphite rod and samples with circular exposed area (1 cm2) served as the reference electrode,counter electrode and working electrode,respectively.The SBF served as electrolytic soln.that was prepared as mentioned by Kokubo and Takadama [27].The electrolyte was buffered with 0.1 M HCl and tris-hydroxymethyl aminomethane to maintain pH at 7.4.PDP was performed at scan rate of 1 mVsec−1using the potential value of ±1V against the open circuit potential (OCP) with step potential of 1 mV.PDP scans were obtained at 0,7,14,21,and 28 days of SBF immersion.EIS was performed at OCP with an AC amplitude of 10 mV RMS in the frequency range of 100 KHz–0.01 Hz at an interval of 0,7,14,21,and 28 days.The EIS data were fitte by ZsimpWin software(Version 3.20,EChem Software,USA),While Nova software (Version 1.10,Metrohm,Switzerland) was used to fi the polarization curves.The obtained corrosion current density,Icorr(mA/cm2),was used to calculate corrosion rate (Pi) in mm/year by following Eqn.(1)[28]:in mm/year by following Eq.(1) [28]:

The corrosion inhibition efficien y (IE) of the samples was calculated upto 28 days of SBF immersion by employing Eq.(2) [29]:
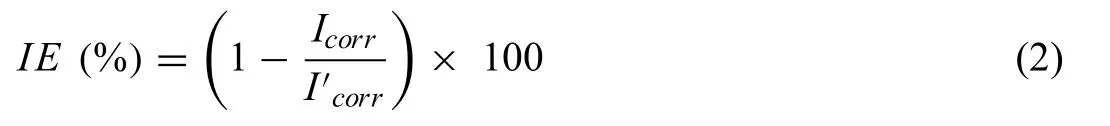
Where,Icorrrepresents measured current density of prepared PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P samples,whileI′corrrepresents current density of base A/M ZM21sample.
2.5.In-vitro degradation
The immersion test was conducted on the PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P and A/M ZM21 Mg samples according to ASTM G31-72 in SBF with pH 7.40 in order to investigatein-vitroweight loss and hydrogen evolution rate.The samples in triplicate having volume to area ratio 0.20 mL/mm2were immersed in SBF at 37 ± 1 °C for 7,14,21,and 28 days.The SBF was changed after every 72 h and pH change was recorded using a pH meter (pH 700,EUTECH Instruments,Singapore) prior to fresh SBF replacement.The sample were taken out of SBF at preselected time intervals and were cleaned with chromic acid,followed by DI water rinsing and vacuum drying.The corrosion rate,Pw(mm/year) from corresponding degradation rate,ΔW(mg/cm2/day) was calculated on a micro-weighing machine (SI-234,Denver instruments,USA) using Eq.(3) [30]:

Whereρrepresents density of material determined by the Archimedes method (ρ= 1.801 g/cm3).The amount of hydrogen evolved at the surface of sample during immersion was measured at the interval of 7 days up to 28 days.The corrosion rate,Ph(mm/year) based on the hydrogen evolution rate,Vh(mL/cm2/day) was calculated by Eq.(4)[28]:

2.6.Mechanical integrity after immersion
The mechanical tests including three-point bend,compression,and tensile test were performed on samples in triplicate obtained from thein-vitrodegradation study at 7-day interval up to 28 days using a servo-operated universal testing machine,UTM (HIC-100.25,HEICO,India).The threepoint bend test was performed according to ASTM E290-14 standard at a loading rate of 10−2N/sec,the compression and tensile tests were performed according to ASTM E9-19 and ASTM E8-16ae1,standards respectively.The ultimate strength (σUS),yield strength (σ0.2YS) and elongation(%El) were calculated from the stress-strain curves for all three mechanical tests and compared with a non-immersed sample,designated as Pristine ZM21(0 day).A stereozoom microscope (Stemi 305,Carl-Zeiss,Germany) was employed to measure the crack angle (CA) (i.e.angle between the direction of load application and crack axis),the critical bending angle (CBA) and the limiting bending depth (LBD) after completion of bend test.Apart from uniaxial tensile and compressive stresses,the distribution of shear stresses at tensile and compression fractured surfaces for PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P and A/M samples after 28 days of immersion was observed according to Eq.(5).[31,32]:

Where,σYstands for yield strength,∅represented the directional angle enclosed between normal to slip and applied load,andλis the inclination between slip and applied load direction.The termCos∅.Cosλis known as Schmid factor,‘m’.
2.7.Cell culture and antibacterial assessment
Thein-vitrocellular activity was performed by using the Mouse fibroblas cells (L929).The cells were cultivated at 37 in Roswell Park Memorial Institute 1640 (RPMI 1640)medium (Thermo-Fisher,USA) supplemented with 10% fetal bovine serum (FBS) and an antibiotic (1% streptomycinpenicillin) solution in an incubator (Heracell VIOS 160i,Thermo Scientific USA),in a humidifie 5%CO2atmosphere Confluen cells were treated with trypsin (0.25%)- ethylenediamine tetra acetic acid,EDTA (0.02%) solution and centrifuged at 2000 rpm for 3 min.The samples were sterilized by irradiation to UV light for 20 min.The sterilized samples were released in a 12-well tissue culture plates and 1 mL of L929 cellular suspension (5 × 105cells/mL) were seeded on each sample with surface area 78.5 mm2.The cultured samples were incubated for 24 and 48 h,separately at 37 °C in 5% CO2atmosphere.The well containing cell without cultured samples was taken as control.At each time point the supernatant medium including cell debris was replaced with fresh medium having 2 μM Calcein AM (1mg/mL in DMSO)(C3100,Invitrogen,USA).The cells were incubated with Calcein AM dye for 30 min in 5% CO2atmosphere in a cell incubator at 37 °C.The incubated samples were washed thrice using 1XPBS followed by the addition of fresh RPMI 1640 medium in each well.Cell images were taken using an upright fluorescenc microscope (80i,Nikon,Japan).
The cytotoxicity of the PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,and uncoated ZM21 Mg alloy samples was assessed using MTT assay (Sigma-Aldrich,USA)on L929(CCL-1)cell line procured from ATCC(American type culture collection).The MTT assay is a colorimetric test for measuring the enzymatic activity [33].The 1 × 104cells were seeded in 100 μL of RPMI medium in each well of a 96 well plate and incubated for 24 h at 37 °C in a humidifie 5% CO2cell incubator.The 100 μL of existing media in wells was replaced within-vitrodegradation media obtained after 24 h of immersion for each sample.The 96 well plate were incubated for 24 and 48 h.Thereafter,20 μL of MTT solution(5 mg mL−1dissolved in 1XPBS)was added in each well and incubated in dark for 3 h at 37 °C.After 3 h,80 μL of stop solution containing 50% (v/v) DMF and 20% (w/v) SDS was added to solubilize the formazan.The cell viability was spectrophotometrically examined by determining the absorbance at 570 nm using ELISA microplate reader (Epoch,BioTek Inc.,USA).The % cell viability was calculated employing the Eq.(6).[34]:

Where,Cells with only RPMI 1640 + 10% FBS was taken as control.
Anti-bacterial activity of multi-layered PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,and uncoated ZM21 Mg alloy samples were examined by using Gram-positiveStaphylococcus aureus(ATCC 25923) and Gram-negativeEscherichia coli(MTCC 1610) bacterial models.S.aureusandE.colistrains were grown in Mueller Hinton Broth (MHB,HiMedia) to its exponential phase (up to 1 O.D at 600 nm)under optimal conditions and were further serially diluted to reach a fina bacterial cell count of ∼1×106CFU/mL.Later,100 μL of prepared bacterial suspension was added to each well in 96 well plate and co-cultured within vitrodegradation media of each sample for 24 h and 48 h at 37 °C in 95% relatively humidifie atmosphere.After incubation for 24 h and 48 h,20 μL (5 mg/10 mL) of (sodium3′-[1-[(phenylamino)-carbony]-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzenesulfonic acid hydrate) XTT - Menadione (Sigma Aldrich,USA)was added to each well and incubated in dark for 30 min at 37 °C.The bacterial viability was spectrophotometrically quantifie by determining the absorbance at 490 nm using ELISA microplate reader.
3.Results
3.1.Surface analysis
The SEM-EDX analysis of A/M ZM21 Mg sample displayed in Fig.2(a) revealed the formation of overlapped craters by the bombardment of ions during WEDM.A high rise in temperature followed by rapid cooling during WEDM machining caused formation of micro-cavities and cracks on the A/M surface [25,35].SEM-EDX analysis of the A/P sample displayed in Fig.2(b).The presence of surface perturbances produced during machining of samples got completely removed on polishing.Fig.2(c)gives the SEM-EDX analysis of HA/TiO2composite coating deposited on the A/P ZM21 Mg sample.Various micro-cracks have formed probably due to evaporation of solvent residues during the calcination of coating[36].These micro-cracks are the potential sites for aggressive ions to reach the substrate and initiate corrosion.The EDX elemental mapping reveals the presence of Mg at the top layer of composite coating suggesting that Mg ion from substrate has interacted with HA and believed to have substituted Ca2+sites in HA [37].Fig.2(d) and (e) show the morphology of the PCL/HA/TiO2hybrid coating comprising intermediate PCL layer and the top PCLNIPSlayer,respectively.The intermediate PCL layer is dense and it consists of Ca and Mg,while the PCLNIPSlayer with homogenously distributed micro-pores consists of C and O only.As shown in SEMEDX micrograph in Fig.3,the HA/TiO2composite,PCL and PCLNIPSlayers of PCL/HA/TiO2hybrid coating have a thickness of 13.33 ± 0.83 μm,17.47 ± 0.97 μm and 22.03 ±1.59 μm,respectively.The coating stability of PCL/HA/TiO2hybrid and HA/TiO2composite coating was determined by adhesion test.As shown in Fig.4,a cross-cut tape adhesion test was performed on PCL/TiO2/HA hybrid and TiO2/HA composite coated ZM21 according to ASTM D3359-17 standard [38,39].The result indicates that PCL/HA/TiO2hybrid coating endured the tape test,demonstrating adhesion grade of 4B.Whereas,HA/TiO2composite coating got peeled-off,exhibiting localized patches of coating removal and adhesion grade of 2B.
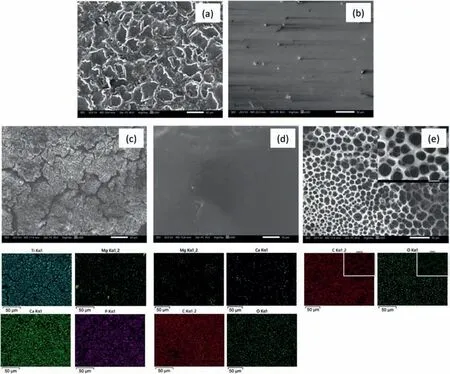
Fig.2.SEM analysis of (a) A/M,(b) A/P ZM21 and SEM-EDX analysis of (c) HA/TiO2 composite layer (d) Dense PCL layer,(e) PCLNIPS layer.

Fig.3.SEM-EDX line scan of the cross-sectional area of PCL/HA/TiO2 hybrid coating.
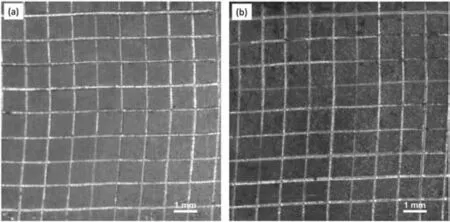
Fig.4.Tape test results of (a) PCL/HA/TiO2 hybrid coated and (b) HA/TiO2 composite coated ZM21 Mg alloy.
The FTIR and XRD results of the coatings,developed under similar conditions have been reported in our previous work [21].In this study,the FTIR and XRD studies were performed to observe the structural characteristics of apatite fille in the pores of PCLNIPSlayer of PCL/HA/TiO2hybrid coated sample.The FTIR spectra of apatite deposited on hybrid coating was shown in Fig.5.The characteristic peaks displayed at 3438 cm−1(OH−),2943 cm−1and 2864 cm−1(asym and sym stretched CH2),1720 cm−1(C=O),1470 cm−1(C-H bending),1376 cm−1(C-H scissoring),1290 cm−1(C-C stretch),1241 cm−1and 1172 cm−1(asym and sym C-O-C stretching) and 731 cm−1(CH2bending) from the PCL.The peaks at 1095 cm−1and 1033 cm−1(ν3PO43−),961 cm−1(ν1PO43−),565 cm−1and 603 cm−1(ν4PO43−) are from the HA.In addition,peaks at 836 and 452 represents Mg-O-Mg (stretching) and Mg-O (stretching).With prolonged SBF immersion up to 28 days,an increase in the intensity of peaks at 1095 and 1033 cm−1and a doublet formation at 603 and 565 cm−1were observed.Alternatively,the intensities of Mg-O and Mg-O-Mg peaks were reduced significantl with prolonged immersion,showing that the presence of chemically and structurally stable HA could effectively impede the release of Mg2+ions from the substrate.FTIR results show C=O peak shifts to 1720 cm−1,which ideally occurred at 1726 cm−1.The peak shift suggests the added hydrogen bonding due to the presence of HA at the Mg alloy-PCL interface which could results in the enhancement of the adhesion strength.The XRD analysis of apatite deposited on PCL/HA/TiO2hybrid coating after an interval of seven days up to 28 days of SBF immersion are shown in Fig.6.After seven days,various peaks of HA (JCPDS 96-900-2214),MgO (JCPDS 96-900-2214),Mg(OH)2(JCPDS 01-083-0144) and different Mg-PO43−compounds (JCPDS 96-201-7954,96-900-1027,96-220-7380) appeared in addition to the PCL peak (JCPDS 0-1431).With prolonged SBF immersion up to 28 days,the intensities of HA characteristic peaks at 2θ= 31.8°,32.1°,32.9° and 46.7° were augmented.Whereas,the intensities of peaks for MgO,Mg(OH)2,and Mg-PO43−compounds reduced after 14 days of immersion and continued to even suppress up to 28 days of immersion.The bottom most layer of coating comprising HA/TiO2remained intact on the Mg alloy substrate,when the upper PCL layer was peeled off.Therefore,the TiO2is not seen in the FTIR spectra and XRD patterns.
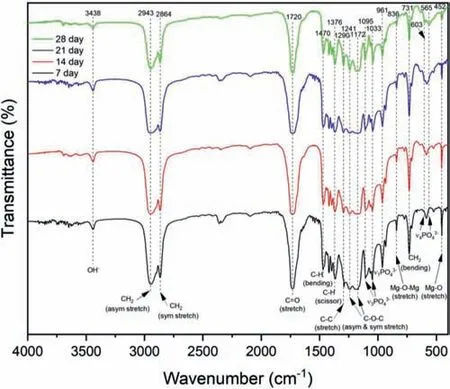
Fig.5.FTIR spectra of apatite deposited on PCL/HA/TiO2 hybrid coating after 7,14,21,and 28 days of immersion in SBF.

Fig.6.XRD analysis of apatite deposited on PCL/HA/TiO2 hybrid coating after 7,14,21,and 28 days of immersion in SBF.
3.2.Electrochemical measurements
Fig.7(i–v) and Fig.7(a–j),respectively,give the potentiodynamic polarization and the EIS curves for PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P,and A/M ZM21 Mg alloys after SBF immersion at intervals of 7,14,21,and 28 days.The fitte results of PDP curves are given in Table 3.Initially,the PCL/HA/TiO2hybrid coated sample has highest value of Ecorrat -0.407 V and lowest value of Icorrat 7.3×10−8among all the samples.After seven days of SBF immersion,Ecorrfor PCL/HA/TiO2hybrid coated sample dropped to -0.694 V in addition to an increase in Icorrto 4.8 × 10−6A/cm2,however post seven day results showed only marginal change in both the values up to 28 days.The corrosion resistance of HA/TiO2composite coating is considerably inferior to the PCL/HA/TiO2hybrid coating.A/P and A/M sample show extremely poor corrosion resistance.

Table 3 The fitte parameters for PDP curves for PCL/HA/TiO2 hybrid coated,HA/TiO2 composite coated,A/P,and A/M ZM21 Mg alloy up to 28 days of SBF immersion.
According to Nyquist plots,the PCL/HA/TiO2hybrid coated sample consists a high-frequency capacitive loop followed by a diffusional tail in medium and low-frequency regions.HA/TiO2composite coated,A/P and A/M samples showed three-time constants in the Nyquist plot.Two conductive loops at high and medium frequency regions and one inductive loop in the low-frequency region.To precisely understand the corrosion behavior of PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P,and A/M samples,equivalent circuits (ECs) as shown in S2 were used to fi the data according to the EIS spectra’s characteristics.In EC,Rs denotes the solution resistance,while CPE is a constant phase element.The circuit elements Rp,Rct,and Radsare referred to as coating resistance,charge transfer resistance,and inductive resistance,respectively.CPEcoatand CPEdlrepresent the capacity of coating/deposited corrosion product and doublelayer,respectively.In EC of a PCL/HA/TiO2hybrid coated sample,Rpand CPEcoatcharacterize the dielectric properties of coating in a high-frequency conductive loop.In medium and low-frequency regions along with Rctand CPEdl,a Warburg impedance (Zw) is added to describe the diffusional impedance that occurred due to ionic diffusion through the micro-pores in the PCL coating [29,40].The variation in Rpand Rctfor PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P,and A/M samples are shown in S3.

Fig.7.Electrochemical measurements of PCL/HA/TiO2 hybrid coated,HA/TiO2 composite coated,A/P,and A/M ZM21 Mg alloy: PDP curves (i-v),Nyquist Plots (a–j).
The Rpfor PCL/HA/TiO2hybrid coated sample was 1.28 × 107Ωcm2on the day zero,which is higher in magnitude by the order of 105and 106when compared to HA/TiO2composite coated (7.44 × 102Ωcm2) and A/P(9.11 × 101Ωcm2),A/M samples (4.52 × 101Ωcm2),respectively.Consequently,the Rctof PCL/HA/TiO2hybrid coating (7.32 × 109Ωcm2) is also 107and 108times higher than HA/TiO2composite (5.17 × 102Ωcm2) and A/P (5.36 × 101Ωcm2),A/M samples (2.35 × 101Ωcm2).After 7 days of SBF immersion,Rpand Rctvalues for the PCL/HA/TiO2hybrid coating declined to 6.12 × 105Ωcm2,which was still 103and 104times higher when compared to HA/TiO2composite coated and A/P,A/M samples.On the fourteenth day of SBF immersion,Rpfor PCL/HA/TiO2hybrid increased to 6.36 × 105Ωcm2due to apatite deposition in the coating layers,which in turn increased the Rctvalue also of PCL/HA/TiO2hybrid coating to 7.51 × 105Ωcm2.In contrast,the Rctfor the HA/TiO2composite coating was reduced to 3.36 × 102.After 14 days onwards,the PCL/HA/TiO2hybrid coated sample shows a continuous increase in Rp with an increase in immersion time up to 28 days.The parallel increase in Rctshows that with increased immersion time,the corrosion product’s deposition and stability were increased.Alternatively,the HA/TiO2composite coating shows a continuous decrease in resistance values after 14 days due to delamination and unstable corrosion product deposition.A similar drop for A/P and A/M samples indicates severe corrosion upto 28 days of SBF immersion.
3.3.Degradation behavior
After 28 days of prolonged SBF immersion,the surface morphologies of the PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P,and A/M samples are shown in Fig.8.The PCL/HA/TiO2hybrid sample did not show any sign of degradation up to 28 days,except a little sign of debonding at the sharp edges of sample immersed for 28 days.The hydrogen evolution did not occur at the coating surface and consequently blister formation was not observed.However,a lean white layer of apatite deposited on the sample.In contrast,the delamination of the HA/TiO2composite coating initiated on the seventh day of immersion that continued with more rigor up to 28 days.A/P and A/M samples exhibited active degradation starting from the firs day of immersion and continued aggressively up to 28 days.
The surface topographies of PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P,and A/M samples are given in S4.Even after the SBF immersion for 28 days,neither the cracks nor the pits appear on the PCL/HA/TiO2hybrid coating,rather the existing micro porosities got fille up with the apatite as became evident from the decrease in the surface roughness Ra and Rz values in 28 days from 1.83 μm and 11.81 μm to 1.17 μm and 8.72 μm,respectively.In contrast,a severe deterioration of the surface in HA/TiO2composite coated sample was evident from a significan increase in Ra and Rz in 28 days of immersion from 0.95 μm and 7.11 μm to 5.52 μm and 29.24 μm,respectively.The degradation of A/P and A/M samples was rather severe as well as localized,as is obvious from their far higher values of Ra and Rz values at 6.84 μm and 31.04 μm and10.47 μm and 54.17 μm,respectively.
As shown in Fig.9(a,b) the weight loss due to degradation after 28 days of immersion transpired,following a ratio of 1:6:10:11 for PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P,and A/M samples,respectively.The PCL/HA/TiO2hybrid coated sample display a very low degradation rate with 0.055 ± 0.009,0.037 ± 0.018,0.032 ± 0.012,0.033 ± 0.010 mg /cm2/day,in comparison with HA/TiO2composite coated sample 0.994 ± 0.161,0.748 ± 0.20,0.975 ± 0.157,and 1.904 ± 0.222 mg/cm2/day on the 7th,14th,21st and 28th day,respectively.The coating defects in the later seem to be primarily responsible for the higher degradation rate.A/P and A/M samples displayed significantl higher degradation rates on 28th day of immersion at 3.110±0.253 and 3.847±0.383 mg/cm2/day,respectively suggesting nearly 100 times of that of PCL/HA/TiO2hybrid coated sample.

Fig.8.Optical images for PCL/HA/TiO2 hybrid coated,HA/TiO2 composite coated,A/P and A/M ZM21 samples after 7,14,21,and 28 days of immersion in SBF.
Fig.9(c) display the pH change of SBF during immersion of samples in SBF for 28 days.Initially,the pH was at 7.40,it dropped a little to 0.21 ± 0.03 in initial 72 h of immersion probably due to the acidic release by the degradation of PCL layer of PCL/HA/TiO2hybrid coated sample [41],but it neutralized again after 72 h due to the apatite formation.Even after 28 days of immersion,there was only little change in pH attaining a value up to 7.49 ± 0.03.In the case of HA/TiO2composite coated,A/P and A/M samples,the pH of immersion media changes with an alternate increase and decrease up to 28 days.Fig.9(d) shows hydrogen evolution rate (HER) during immersion of all samples in SBF for 28 days.The hydrogen evolution during the degradation of magnesium,occurred according to the chemical Eqs.(7) and(8) [42]:

Fig.9.(a) Relative weight loss percentage,(b) degradation rate,(c) pH change,and (d) hydrogen evolution rate of PCL/HA/TiO2 hybrid coated,HA/TiO2 composite coated,A/P,and A/M ZM21 samples after immersion in SBF.
The HER is proportional to the rate of anodic dissolution of Mg [6].The HER declined from 0.09 mL/cm2/day (on 7th day) to 0.06 mL/cm2/day (on 28th day) for PCL/HA/TiO2hybrid coated sample.This suggests that the PCL micro pores fille with apatite effectively shield the penetration of the aggressive ions from immersion media to reach the Mg substrate and hence reduce the rate of hydrogen evolution.While the hydrogen evolution starts immediately within an hour of immersion of HA/TiO2composite coated sample,it became so aggressive on the seventh day that HER at 1.506 ± 0.300 mL/cm2/day was beyond the acceptable hydrogen amount in the human body and continued evolving till the fourteenth day at the similar level of HER at 1.385 ± 0.143 mL/cm2/day [2].After 28 days of SBF immersion,the HA/TiO2composite coated sample allowed HER at 3.106 ± 0.411 mL/cm2/day,which was nearly 65 times greater than HER of PCL/HA/TiO2hybrid coated samples.The HER in A/P at 3.518 ± 0.332 mL/cm2/day and A/M at 3.982 ± 0.411 mL/cm2/day samples were obviously beyond the acceptable levels.The combined corrosion rates(mm/year) calculated from hydrogen evolution rate,weight loss study and polarization tests are shown in S5 and summarized in Table 4.The overall results show the decreasing order of corrosion rate as A/M>A/P>HA/TiO2composite coated>PCL/HA/TiO2hybrid coated sample up to 28 days of SBF immersion.
3.4.Mechanical integrity loss and fractographic analysis
Bending stress-strain curves for pristine ZM21 alloy,PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P and A/M samples with prolonged SBF immersion up to 28 days are given in S6.The ultimate bending strength (σUBS),bending yield strength (σ0.2BYS),and elongation (%El) computed from the bending stress-strain curves are plotted in S7.The loss in bending properties such asσUBS,σ0.2BYS,and%El during immersion of samples in SBF are provided in Table 5.

Table 4 Calculated corrosion rates (P) for PCL/HA/TiO2hybrid coated,HA/TiO2 composite coated,A/P,and A/M ZM21 Mg samples based on hydrogen evolution rate,weight loss rate,and Icorr.
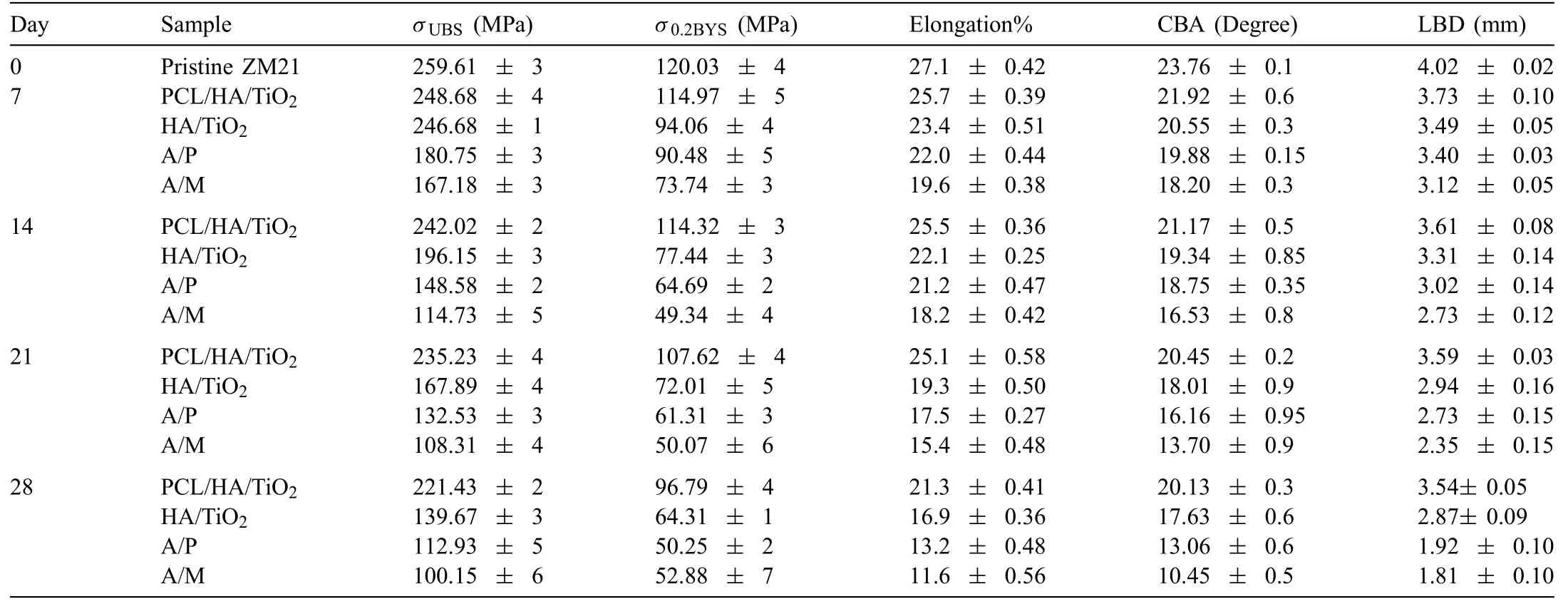
Table 5 Bending properties of PCL/HA/TiO2hybrid coated,HA/TiO2 composite coated,A/P and A/M ZM21 Mg samples during 28 days of SBF immersion.
The averageσUBS,σ0.2BYS,and %El of the pristine ZM21 are 259.61 MPa,120.03 MPa and 27.1%,respectively.TheσUBS,σ0.2BYS,and %El after SBF immersion for seven days reduced by 4.2%,4.1%,and 5.1%for PCL/HA/TiO2hybrid coated sample and by 5.0%,21% and 13.6% for the HA/TiO2composite coating,respectively.The decline inσUBS,σ0.2BYS,and %El of both of the coated samples continued up to 28 days.However,theσUBS,σ0.2BYSand %El reduced only by 14.7%,19.3% and 21.4% for PCL/HA/TiO2hybrid coated sample even after SBF immersion for 28 days,whereas,the loss inσUBS,σ0.2BYS,and%El was much higher in other samples,being 46.2%,46.4% and 37.6% for HA/TiO2composite coating,56.7%,58.1%,51.2% for A/P and 61.4%,55.9%,57.19% for A/M samples,respectively.On 28th day of immersion,theσUBShad reduced to 112.93 MPa for A/P and 100.15 MPa for A/M samples which are even lower than theσUBSof pristine ZM21 (120.03 MPa).It is suggested that there was a continuous rise in HER throughout the immersion period in both,A/M as well as A/P samples which might lead to hydrogen embrittlement as well as crack formation.
The stereozoom micrographs (SMs) of all the failed samples from the bending test after 28 days of SBF immersion are displayed in Fig.10(a–e).The values were measured as: CA-42.34°,40.28°,13.51°,12.77°,9.98°,CBA- 23.76°,20.13°,17.63°,13.06° and 10.45° and LBD- 4.02 mm,3.54 mm,2.87 mm,1.92 mm and 1.81 mm for Pristine,PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P,and A/M samples,respectively.Thus,it was only the PCL/HA/TiO2hybrid coated sample that maintained the structural integrity till 28 days of immersion but the other samples suffered substantial damage in structure.The SEM and BSE fractographs of the bend test samples are shown in Fig.10(f–o).The fractographs revealed cleavage steps,tear ridges and few dimpled areas signifying mostly a brittle fracture in PCL/HA/TiO2hybrid coated sample.In contrast,there were many corrosion cavities in HA/TiO2composite coated sample,which are believed to accommodate large volume of hydrogen evolved during degradation and result in hydrogen embrittlement [43].Whereas the fracture surface of A/P and A/M samples display appearance of many cavities,pits,and cracks which become the stress raisers leading to their premature failure.

Fig.10.(a–e) SMs,(f–j) SEM and (k–o) BSE images of bending fractures for Pristine ZM21 Mg,PCL/HA/TiO2 hybrid,HA/TiO2 composite,A/P,and A/M ZM21 samples after 28 days of immersion in SBF.
The tensile stress-strain curves pristine ZM21 alloy,PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P and A/M samples up to 28 days of SBF immersion are given in S8.The ultimate tensile strength(σUTS),tensile yield strength (σ0.2TYS),and elongation (%El) calculated from the tensile stress-strain curves are plotted in S9.The loss in tensile properties such asσUTS,σ0.2TYS,and %El during immersion of samples in SBF are compared in Table 6.The averageσUTS,σ0.2TYS,and %El are 231.67 MPa,121.62 MPa and 9.70%,respectively for the pristine ZM21.TheσUTS,σ0.2TYS,and%El after seven days of SBF immersion reduced by 3.0%,4.6%,and 0.2% for PCL/HA/TiO2hybrid coated sample and by 6.7%,12.9% and 12.5% for the HA/TiO2composite coating,respectively.The decreases inσUTS,σ0.2TYS,and %El continued up to 28 days in both the coated samples.However,theσUTS,σ0.2TYS,and %El for PCL/HA/TiO2hybrid coated samples reduced by 10.9%,14.4%and 19.8%whereas,the total loss inσUTS,σ0.2TYS,and %El was 38.1%,33.9%and 54.5% for HA/TiO2composite coating,54.1%,52.8%,61.5% for A/P and 64.4%,74%,68.1% for A/M samples,respectively,after SBF immersion for 28 days.The stereozoom micrographs of fractured surfaces obtained from the tensile test of all samples after immersion of 28 days in SBF are shown in Fig.11(a–e).
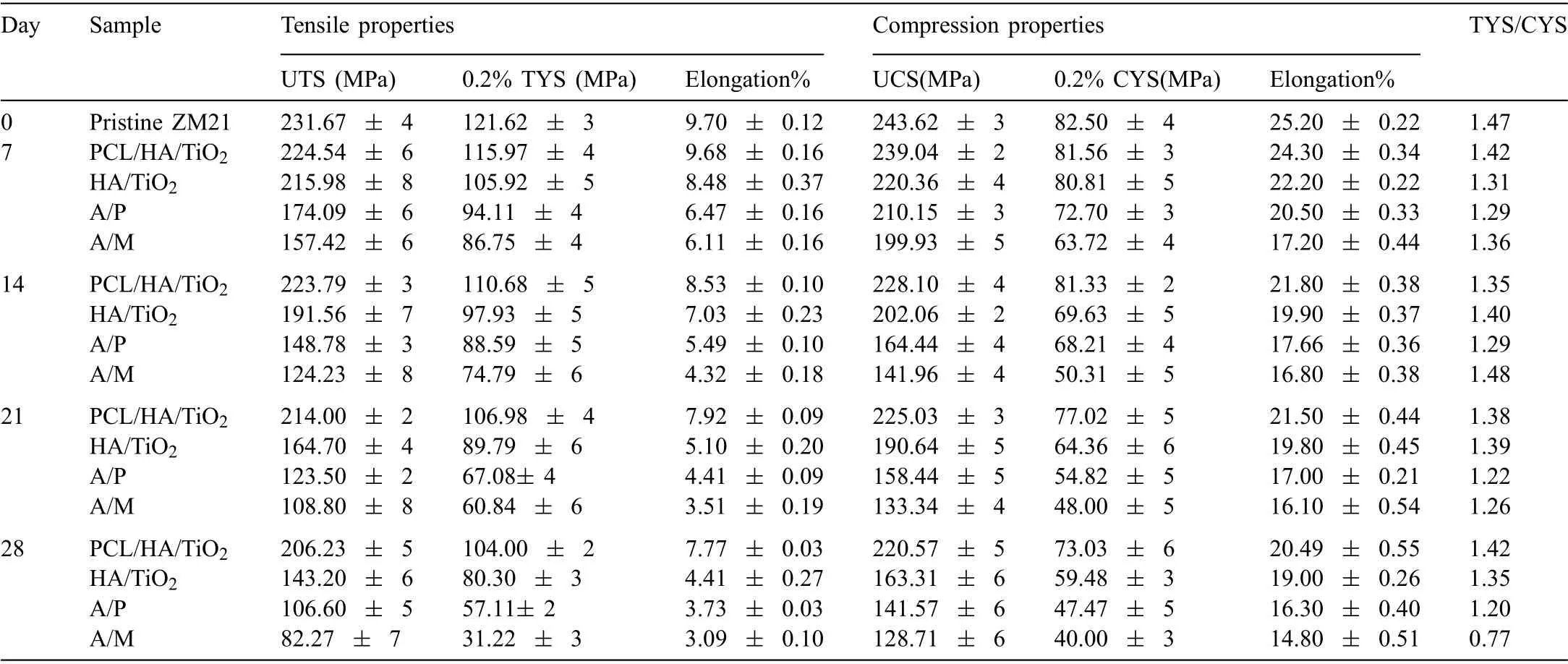
Table 6 Tensile and compression properties of PCL/HA/TiO2 hybrid coated,HA/TiO2 composite coated,A/P,and A/M samples during 28 days of SBF immersion.
The SEM and BSE fractographs obtained from the tensile test of PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P,and A/M samples,after 28 days of SBF immersion are shown in Fig.11(f–o).The fractographs of Pristine ZM21 and PCL/HA/TiO2hybrid coated samples exhibit cleavage-dominated surface consisting of dimples and tear ridges.Besides these features,some tubular voids and flute could also be observed.The tubular voids have possibly formed as a result of elongation of the dimple structure during plastic deformation.Due to continued elongation under tensile loading,a planer intersecting slip mechanism cause a few tubular voids to rupture into two halves forming the flute[44].The fracture surface of HA/TiO2composite coating exhibited many localized cavities which form the stress concentration sites and cause premature failure.The fractographs of the A/P and A/M samples exhibited a quasi-cleavage surface consisting of a high density of localized corrosion cracks,cavities and pits resulting in irregular or missing of cleavage facets.
The compressive stress-strain curves for pristine ZM21 alloy,PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P and A/M samples up to 28 days of SBF immersion are given in S10.The ultimate compressive strength(σUCS),compressive yield strength (σ0.2CYS),and elongation (%El) calculated from the compressive stress-strain curves are plotted in S11.The loss in compressive properties such asσUCS,σ0.2CYS,and %El during immersion of samples in SBF are provided in Table 6.The averageσUCS,σ0.2CYS,and %El are 243.62 MPa,82.50 MPa and 25.20%,respectively for the pristine ZM21 sample.TheσUCS,σ0.2CYS,and %El after seven days of SBF immersion was decreased by 1.8%,1.3%,and 3.5% for PCL/HA/TiO2hybrid coated sample and by 9.5%,2.0% and 11.9% for the HA/TiO2composite coating,respectively.After 28 days of immersion,theσUCS,σ0.2CYS,and%El for PCL/HA/TiO2hybrid coated samples was reduced by 9.4%,11.4%,18.6%,Whereas,the total loss inσUCS,σ0.2CYS,and %El was 32.9%,27.9%,24.6% for HA/TiO2composite coating,41.8%,42.4%,35.3% for A/P and 47.1%,51.5%,41.2% for A/M samples,respectively.The stereozoom micrographs of fractured surfaces obtained from the compression test of all samples after immersion of 28 days in SBF are shown in Fig.12(a–e).
The SEM and BSE fractographs obtained from the compression test of PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P,and A/M samples,after immersion of 28 days in SBF are shown in Fig.12(f–o).The fractographs of Pristine ZM21 and PCL/HA/TiO2hybrid coated samples comprises cleavage and dimple features represents brittle fracture.Despite of nearly same area under fracture,HA/TiO2composite coated sample exhibited features of more brittle fracture like cracked cleavage facets with the absence of dimple features [45].The fractographs of the A/P and A/M samples show subsequent drop in fractured area.Moreover,the fractured surface show highly discontinuous fracture features,limiting the load-bearing capacity.
The extent of plastic deformation depends on the multiple factors like: magnitude of shearing stress,crystal structure geometry and active slip plane’s orientation with respect to shearing stress.Slipping phenomena started,when the shearing stress reaches threshold value called the critical resolved shear stress(CRSS).The slip direction is determined from the plane on the fracture surface which show maximum amount of slip deformation as shown in Fig.11 and Fig.12 for tensile and compressive test,respectively.
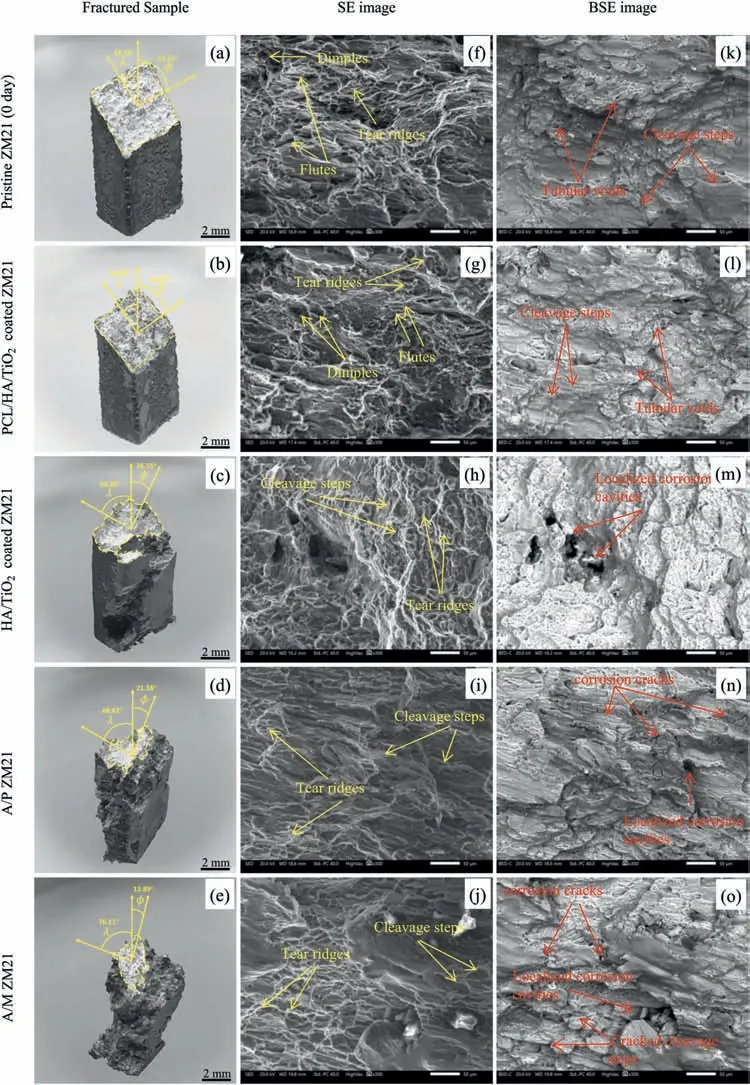
Fig.11.(a–e) SMs,(f–j) SEM and (k–o) BSE images of tensile fractures for Pristine ZM21 Mg,PCL/HA/TiO2 hybrid,HA/TiO2 composite,A/P,and A/M samples after 28 days of immersion in SBF.
After 28 days of SBF immersion,the CRSS under tensile testing conditions for Pristine ZM21 Mg alloy,PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P,A/M samples were at 60.57 MPa,51.79 MPa,32.04 MPa,19.36 MPa,7.27 MPa and Schmid’s factor,m at 0.49,0.49,0.39,0.33 and 0.23,respectively that suggests the trend of their declining resistance to slip as shown in S12(a).The magnitude of CRSS depends on the interaction of dislocations with other dislocations and other structural defects,when the density of defects increases,the CRSS decreases.PCL/HA/TiO2hybrid coating provides the best corrosion protection to the substrate as a result there is only a minimal decline in CRSS and Schmid’s factor remains unchanged.According to Eq.(5),the CRSS is a function ofσYand m.The same value of m for pristine and PCL/HA/TiO2hybrid coated samples suggestsσYto be responsible for the change in CRSS.Due to the severe degradation of the HA/TiO2composite coated,A/P and A/M samples in the presence of SBF,the increasing density of defects such as cracks,pits,etc.reduced the CRSS,proportionately.Under compression testing,the CRSS of Pristine ZM21 Mg alloy,PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P,A/M samples was 38.03 MPa,33.59 MPa,19.03,11.16 MPa,5.6 MPa and Schmid’s factor,m was 0.46,0.46,0.32,0.24 and 0.14,respectively that makes the declining order of resistance to slip obvious as shown in S12(b).The CRSS as well as‘m’values under tensile loading are higher than those under compressive loading for all the samples.
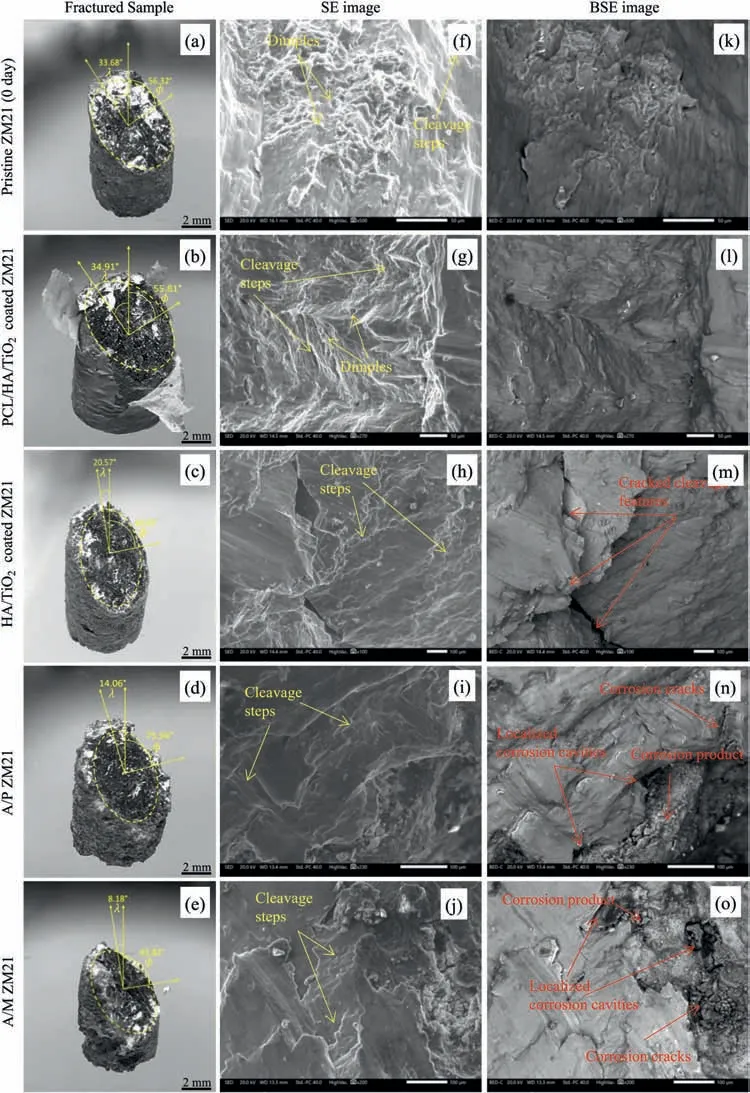
Fig.12.(a–e) SMs,(f–j) SEM and (k–o) BSE images of compressive fractures for Pristine ZM21 Mg,PCL/HA/TiO2 hybrid,HA/TiO2 composite,A/P,and A/M samples after 28 days of immersion in SBF.
The tensile-compression yield asymmetry is identifie from the ratio of tensile yield strength (TYS) to compression yield strength (CYS) as given in Table 6.As shown in S12(c),the yield anisotropy of pristine ZM21 Mg alloy,PCL/HA/TiO2hybrid coated,TiO2/HA coated A/P,A/M sample were in the ratio of 1.47:1.42:1.35:1.20:0.77 after 28 days of SBF immersion.Natural human bones have a yield anisotropy from 1.33 to 1.37 for the femur cortical and 1.62 to 1.87 for the cancellous bone,respectively and 2.17–2.34 for spine vertebral endplates in the age group between 21 and 94 years [46].The difference in bone composition and bone mass density(BMD)is responsible for such a variation in the tensile-compression yield anisotropy.Thus,PCL/HA/TiO2hybrid coated sample seems to be structurally promising,whose mechanical performance able to mimic the natural bone during the bone healing period.However,the TiO2/HA coated,A/P and A/M samples,due to lack in anisotropy,will exhibit shear shielding and consequently the implant failure under multi-dimensional dynamic loading conditions.
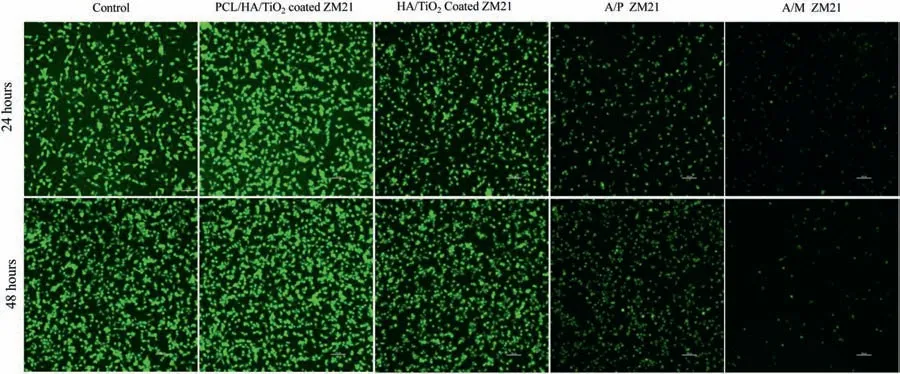
Fig.13.Fluorescent images of L929 cells for Control,PCL/HA/TiO2 hybrid coated,HA/TiO2 composite coated,A/P,and A/M samples after culturing for 24 and 48 h.
3.5.Cell viability and antibacterial response
Biocompatibility is one of the primary concerns for the development of a biodegradable implant.Therefore,cellular response of PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P,and A/M samples was examined using fibroblas L929 cell-line by employing fluorescenc microscopy.When compared with control sample,PCL/HA/TiO2hybrid coated sample exhibited healthy cellular growth with spindle-like morphologies as shown in Fig.13.The optical density data is given in Fig.14.The cellular viability PCL/HA/TiO2hybrid coated sample is significantl higher as compared to control sample by 31.53%and 50.47%after 24 and 48 h,respectively because the top micro-porous PCL layer provided the preferential site for cell adhesion and growth [47].The effective corrosion resistance restricted the hydrogen evolution and pH rise,which otherwise could negatively affect the cellular proliferation.On the other hand,HA/TiO2composite coated sample showed only marginal improvement by 3.18%and 15.47%over the control sample after 24 and 48 h.Whereas,TiO2is bio-inert and does not interfere with cell adhesion and growth,but HA is a bioactive ceramic still,coating results in limited cellular growth due to the presence of spatial defects which activate the hydrogen evolution and alkalization.The A/P and A/M samples exhibit the poor cell viability because the localized degradation in these samples result in a higher Mg2+concentration leading to significan rise in pH of SBF immersion media and also vigorous hydrogen evolution.Even,the corrosion product like Mg(OH)2is not able to support the cell proliferation [48].
During the antibacterial test,the pH of immersion media was recorded before co-culturing with bacterial suspension.The pH value of immersion media after 24 h and 48 h for PCL/HA/TiO2hybrid coated ZM21 – 7.35,7.31;HA/TiO2composite coated ZM21 – 7.54,7.81;A/P ZM21 – 7.66,8.05;and A/M ZM21 – 7.98,8.72 respectively.S13 and 14 displays the antibacterial response of PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P,and A/M ZM21 Mg samples againstS.aureusandE.colibacterial culture.As shown in Fig.15,the PCL/HA/TiO2hybrid coated sample allowedS.aureusgrowth but limits theE.coligrowth by 9.66% and 21.08% after 24 h,but suppressed growth of both bacteria by 57.15% and 62.35% after 48 h,respectively.The PCL/HA/TiO2hybrid coating maintained neutral pH conditions,with minor change in buffered pH value (i.e.7.40) of immersion media to 7.35,and 7.31 after 24 h and 48 h,respectively,which assisted the bacterial growth.Nevertheless,a good antibacterial response was observed after 48 h.During this period,the SBF was penetrated through the hybrid coating.Consequently,the reactive oxygen species (ROS) from polarized TiO2molecules present in HA/TiO2layer of hybrid coating were transported to the immersion media.These ROS were primarily responsible for the anti-bacterial response of PCL/HA/TiO2hybrid coating.The detailed mechanism of antibacterial response has been explained later in discussion part..However,the immediate release of ROS from HA/TiO2composite coated sample reducedS.aureusandE.colibacterial growth by 55.82% and 48.17%,respectively after 24 h,which reduced further by 78.36% and 97.93% after 48 h,due to increase in alkalinity of immersion media.For the A/P samples,theS.aureusandE.colibacterial growth was reduced by 67.82% and 69.16% respectively,after 24 h and 82.64% and 99.42% respectively after 48 h,due to abrupt pH rise.The bactericidal effect was most prominent in the A/M sample due to strongest alkalization of immersion media by leached out Mg ions and higher hydrogen evolution due to the severe corrosion of Mg alloy.
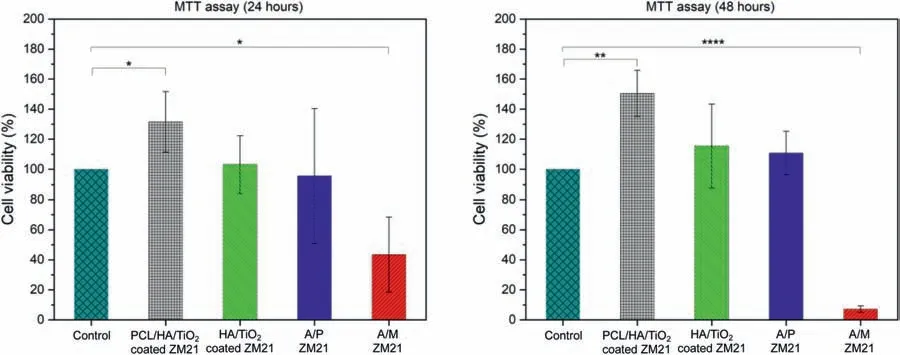
Fig.14.Cell viability of L929 Cells for Control,PCL/HA/TiO2 hybrid,HA/TiO2 composite coated,A/P,and A/M samples after culturing 24 and 48 h in MTT assay.The statistical analysis was performed via Student’s t-test;the difference for ∗p < 0.05 was considered statistically significant

Fig.15.S.aureus and E.coli viabilities in XTT assay for Control,PCL/HA/TiO2 hybrid coated,HA/TiO2 composite coated,A/P,and A/M ZM21 Mg samples after 24 and 48 h of incubation
4.Discussion
The investigated results in terms of corrosion resistance,mechanical integrity loss and biological response shows PCL/HA/TiO2hybrid coated ZM21 Mg alloy has superiority as compared to HA/TiO2composite coated,A/P,and A/M ZM21 samples.The key aspects responsible for such an outstanding accomplishment by hybrid coating are discussed below.
4.1.In vitro corrosion performance
PCL/HA/TiO2hybrid coating was produced on ZM21 Mg by sol-gel dip coating technique.The top PCLNIPSlayer has provided good corrosion protection that maintained least degradation rate up to 28 days of SBF immersion.
The PCL layer reduced the Icorrof underlying HA/TiO2composite layer for 3 orders,A/P and A/M ZM21 samples by 4 orders of magnitude directly.Even after 28 days of immersion,PCL/HA/TiO2hybrid coating maintained lower Icorrby two order of magnitude as compared to TiO2-HA,A/P and A/M ZM21 samples.Recently,Chen et al.fabricated a MAO/PLGA double layered coating on Mg-4Zn-0.6Zr-0.4 Sr alloy by dip coating method.The prepared coating initially show corrosion resistance by reducing the Icorrfrom 1.95 × 10−1A/cm2to 1.39 × 10−4A/cm2.But after 2 weeks of immersion in SBF,the damaged caused by H2blisters,give significan rise in Icorr[10].Li et al.prepared a multilayered HA/CaTiO3/TiO2coating on AZ31 Mg alloy by dip coating method.Initially,the multilayered physical barriers significantl enhanced the Ecorrto -0.430 V and reduced Icorrto 8.91 × 10−10A/cm2,which is quite similar to this work.Nevertheless,after 3 days of immersion in Hanks solution the coating start delaminating,thus resulted into high Icorr,onwards [49].Thus,it is inferred that maintaining low Icorrwith prolonged immersion is a major challenge for existing multilayered hybrid coatings,which has been addressed in present work.
A biodegradable metallic implant in orthopaedic application should possess corrosion rate less than 0.5 mm/year forin-vitroconditions [50].As seen from the results presented in Table 4,PCL/HA/TiO2hybrid coating meets this requirement up to 28 days while HA/TiO2composite coated,A/P and A/M samples fail to do so even up to 7 days of SBF immersion.In order to analyze the degradation phenomena evolving with immersion time,the surface morphology of the PCL/HA/TiO2hybrid coated samples immersed in SBF were examined using SEM-EDX as shown in Fig.16(a,b) The degradation phenomenon is explained with the help of a schematic representation given in Fig.16(c).On the seventh day,only a small amount of apatite is formed on the microporous PCL top layer,but there was no other coating defect such as blister formation or delamination.This suggests that the hybrid coating facilitated the escape of hydrogen gas,even though the amount of hydrogen evolved would also be low at this time,because only few ions reached the Mg substrate through multiple levels of barriers.On the fourteenth day of immersion in SBF (Fig.16(a.ii)),a loosely packed one dimensional apatite consisting of mud like cracks was observed.The presence of cracks limits its shielding efficien y.Whereas,on the twenty-firs day,apatite growth took up the fibe-like shape with much less crack intensity.The apatite growth became dense and exhibited 3-dimensional micro-fl wer shape after 28 days of immersion (Fig.16(a.iv)).Multiple factors may be responsible for the 3d fl wer-like HA growth.The polar functional groups like -COOH or -C=O of PCL molecule induce a dipole-dipole interaction,thereby regulating the assembly and orientation of apatite mineralization by Vander wall forces [51].The PCL micropores contract due to water uptake from SBF,which significantl decrease the distance between polar functional groups on the opposite sides of a micropore and the strong dipole interaction induced between them enhances the Vander wall forces,facilitating the formation of 3d fl wer-like HA crystals [52].Moreover,after 21 days,the apatite growth took place from the environment of stable pH and ionic concentration.In such a stable environment,the dipole interactions between similarly charged ligands of HA and PCL molecules increases the surface energy,thus Vander wall forces come into play to reduce total system energy by the formation of 3d fl wer-like morphology of HA crystals [53].Thus formed HA shape imitates the structure of natural bone.This type of the apatite layer offered increased resistance to the penetration of aggressive ions to reach the Mg substrate and hence resulted in lowering the corrosion rate with increase in immersion time up to 28 days.
According to the EDX results the Ca/P atomic ratio of apatite was 0.92 on the seventh day.Ideally,hydroxyapatite is considered as the most stable form of apatite,it has an ideal Ca/P ratio of 1.67 and a solubility index of 3.7 × 10−58[54].The Mg2+ions released from the Mg substrate travel up to the apatite layer and there it results in the formation of MgPO43−compound and few ions substitute Ca ions in apatite lattice.The Mg/Ca ratio in apatite was 7.71 on the seventh day.The MgPO43−compounds have a relatively higher solubility index of 1.04 × 10−24[55].Consequently,one can expect the solubility index to rise and crystallinity to decline of the apatite layer.With an increase in immersion time to 14,21,and 28 days,the Mg/Ca ratio declined to 1.38,0.211 and 0.155 while the Ca/P ratio increased to 1.21,1.32,and 1.60,respectively,indicating diminished Mg2+ions transfer from the substrate.The decline in the Mg/Ca ratio and the Ca/P ratio approaching towards the value of 1.67 suggests decreased solubility index and increased structural stability of deposited apatite at longer immersion times,both responsible for providing an effective corrosion resistance against aggressive ions in SBF[56].
Rahman et al.developed a fla e-like Dicalcium phosphate dihydrate (DCPD) and rod-like HA coating on WE43 Mg alloy with Ca/P ratio 0.99 and 1.67,respectively.HA coated sample significantl suppress the Icorrby nearly six times as compared to DCPD [57].Maurya et al.fabricated 3d fl werlike HA coating on Mg-Li alloy with Ca/p ratio of 1.9,which positively shifts the Ecorr from -1.49 V to -0.59 V by suppressing the Icorrby 22 folds.Thus,the synergistic effect of improved Ca/P ratio and closely packed crystals positively influenc the structural stability of apatite [58].Despite of such chemically and structurally stable characteristics,major issues like poor adhesion and lattice distortion caused by Mg substitution in HA compromise HA tendency to protect Mg alloys with prolonged SBF immersion.The PCL/HA/TiO2hybrid coating fabricated in the present study overcome existing issues as: (i) HA growth occurred in compact PCL micropores,which provide structural stability against erosion of deposited HA from coating surface.Additionally,assist the formation of closely packed 3d fl wer-like shape of grown HA,(ii) the effective shielding provided by PCL/HA/TiO2hybrid coating greatly limits the Mg dissolution,thus Mg substitution is highly suppressed which attributes chemically stable HA growth.
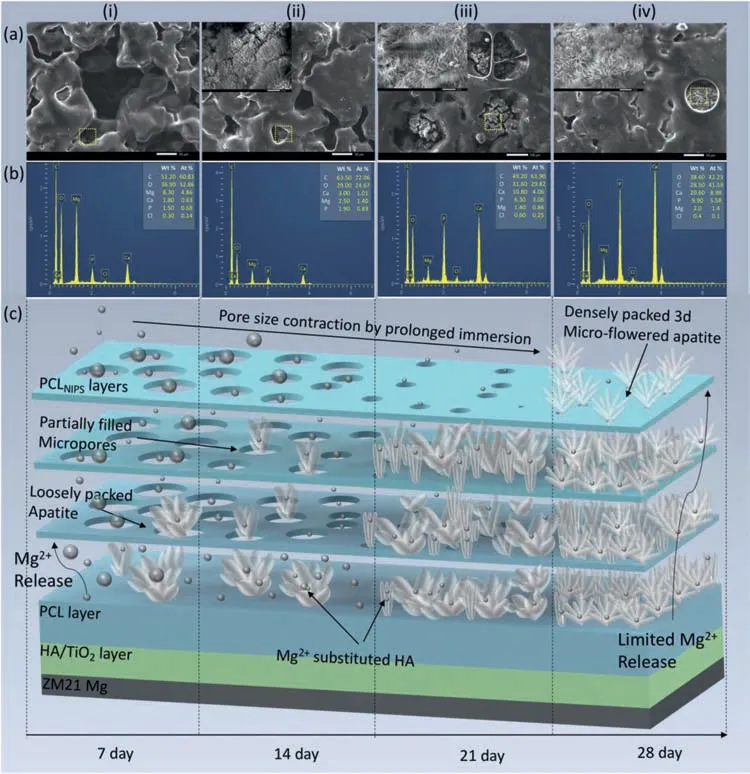
Fig.16.(a,b)SEM-EDS analysis and(c)schematic representation of PCL/HA/TiO2 hybrid coated sample degradation after 7,14,21,and 28 days of immersion in SBF.
4.2.Post immersion mechanical integrity loss
The mechanical properties of samples of three-point bending,compression,and tensile testing,after immersion in SBF,deteriorated significantl.It is observed that the samples encountered two types of corrosion during immersion -uniform corrosion and localized corrosion.Whereas,the uniform corrosion resulted in the overall reduction in the cross-sectional area,the localized corrosion led to the formation of pits,cracks and cavities and both are responsible for deterioration of mechanical properties.
EBSD analysis of tensile and compression zones of Threepoint bend test samples elucidates the mechanism of mechanical deformation.The Pristine ZM21,PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,and uncoated A/M samples were examined after 28 of SBF immersion.Mg alloys having HCP structure possess insufficien slip systems,thus twinning commonly plays an important role during plastic deformation at room temperature [59,60],in which three twinning modes are involved: {102}<1011>tension twin(TTWs),{101}<1012>contraction twins(CTWs)and{102}-{101} double twins (DTWs) which are orientated at 86°,56°and 38°from the matrix,respectively,as shown in inverse pole figur (IPF) maps in Fig.17(a).Apparently,TTWs and CTWs dominate in compression and tension zones,respectively.In the tension zone,the strain being parallel to the c-axis of RD and TD direction (Fig.10),causes the reduction of thickness as well as width and the crystallographic orientation activates CTWs [61].Although,{101} CTWs do not accommodate plastic deformation but generate shear strain in the direction of the load application,and thus promote premature failure [62].The localized stress in {101} of the DTWs tend to propagate{102}twin,but the latter is not sufficientl activated in the tensile region,thereby resulting in catastrophic failure even at smaller load at initial stage of loading [63].In the compression zone,negative strain along a-axis cause positive radial strain along the c-axis,resulting in the formation of {102} TTWs.It is the activation of {102} TTWs with fully coherent low-energy twin boundary,which accommodated large plastic deformation,thereby,producing 86° lattice reorientation and alter the basal texture.Moreover,the activation of {102} TTWs weakened the basal texture and further improved the plastic deformation.The sample exhibited better mechanical integrity as the fraction of {102}TTWs was significantl higher in compression zone than in the tensile zone.
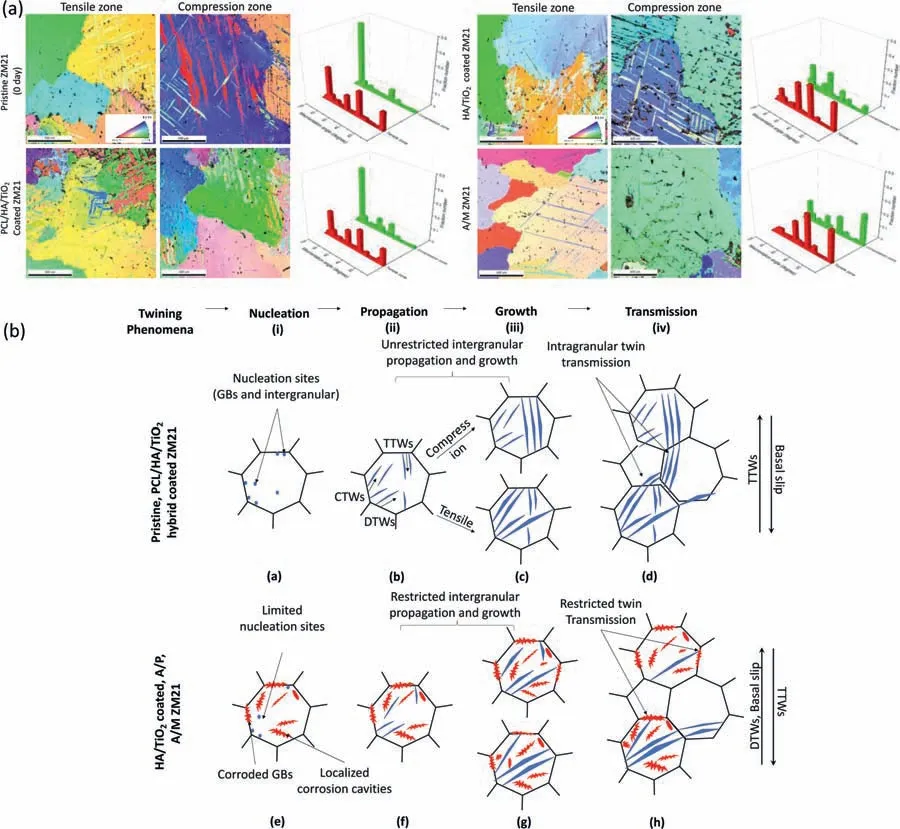
Fig.17.(a) IPF maps and misorientation graphs,(b) schematic representation of twinning phenomena under compression and tensile loading for Pristine,PCL/HA/TiO2 hybrid coated,HA/TiO2 composite coated,A/P,and A/M samples after 28 days of immersion in SBF.
The basal texture as well as TTWs distribution were identical in both tensile and compression zones in the pristine ZM21 Mg sample before the immersion as well as in the PCL/HA/TiO2hybrid coated sample after 28 days of SBF immersion indicating no localized corrosion in the later.After 28 days of SBF immersion,the HA/TiO2composite coated sample displayed a relatively stronger basal texture in the tensile zone and weaker prismatic texture in the compression zone than the PCL/HA/TiO2hybrid coated sample.Besides,fewer number of {102} TTWs in the compression zone but higher number of {101} CTWs and {102}-{101} DTWs in the tension zone when compared to PCL/HA/TiO2hybrid coated sample.The localized corrosion resulting in cavities and decreased fractured area promotes activation of CTWs.The activation of CTWs decreases the grain misorientation promoting the low angle grain boundaries(LAGBs).Thus the lower misorientation from the basal texture in this case as well as in uncoated A/M samples is responsible for severe degradation and their premature failure after 28 days of SBF immersion as schematically shown in Fig.17(b).Though,severity of corrosion cavities suppresses {102} TTWs in both tensile and compression zones but activates {101} CTWs,{102}-{101} DTWs and increases LAGBs which strengthen the basal texture and thus promote the catastrophic failures at appreciably lower load.
A biodegradable Mg implant should have adequate mechanical strength at the time of implantation and provide adequate support till the bone reunion and healing period.A cladogram shown in Fig.18.provides the loss in mechanical integrity with time immersion in SBF for different samples PCL/HA/TiO2hybrid coated,HA/TiO2composite coated,A/P,and A/M ZM21 and comparison with various human cortical bones [64].Multiple factors like bone mass density(BMD),bone anisotropy,viscoelasticity,and fracture nature,whether transverse or spiral,determine the bone healing time.Literature reported for common arm fractures associated with humerus,ulna,and radius bones,the healing time can be achieved within 4–6 weeks.In comparison,the fracture reunion time for femur,tibia,and fi ula extended up to 8–12 weeks.Furthermore,if the fracture type is transverse,it may take 24 weeks to regain mechanical strength [65].
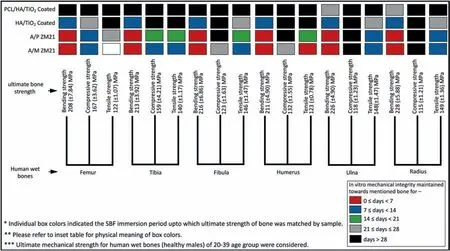
Fig.18.A Cladogram chart representing bending,compression,and tensile strength for various human cortical bones along with a period up to which the required mechanical strength criteria of these bones were fulfille by PCL/HA/TiO2 hybrid coated,HA/TiO2 composite coated,A/P,and A/M samples during 28 days of immersion in SBF.
The results shown by PCL/HA/TiO2hybrid coated ZM21 Mg sample from three-point bending,compression,and tensile testing seems to be promising for applications like femur,tibia,fi ula,humerus,ulna,and radius bones even after 28 days ofin vitroimmersion.However,the ultimate bending strength requirement for ulna and radius bone was compromised after 21 days;no sudden sharp decline was observed during the test duration.Moreover,various studies reported that the corrosion rate for Mg alloys underin-vivoconditions are 5–10 times lower thanin-vitroconditions [66].As proteins and amino acids present in actual physiological media made complex interactions with Mg sample to suppress corrosion.Therefore,it makes rational sense to believe that the PCL/HA/TiO2hybrid coated ZM21 Mg sample will own a similar mechanical integrity response for a much higher time underin-vivoconditions.In contrast,the HA/TiO2composite coated sample lost bending and compression strength within 2,3 weeks of immersion,indicating their insufficien mechanical support during prolonged immersion.The A/P and A/M samples show excessive loss of mechanical integrity within the firs week of immersion due to very high corrosion rate.Additionally,the presence of localized corrosion sites promotes the diffusion of hydrogen that evolved duringin-vitrodegradation.Thus,hydrogen embrittlement and severe degradation combined appear responsible for their catastrophic failure prematurely.
4.3.Biological evaluation
A biodegradable metallic implant must possess adequate biocompatibility and necessary bactericidal response to the injured bone.ISO 10993 – 5 regulating biosafety criteria mentioned that a biodegradable metallic implant demonstrating cell viability 70% or more than the control group (media only) should be considered as non-toxic.PCL/HA/TiO2hybrid coated samples show distinguished cellular response as compared to the HA/TiO2composite coated,A/P and A/M samples.Numerous factors like high corrosion resistance,microporous surface topology,and stable apatite growth contributed to such a response.As shown in Eqs.(7) and (8),Mg alloy degradation in physiological media resulted in the release of Mg2+,OH−ions,and H2gas.A slow degradation as possessed by a PCL/HA/TiO2hybrid coated sample suppresses the release of Mg2+ions to low concentrations.Pan et al.reported that low concentration release of Mg2+ions facilitates cell metabolism,resulting in enhanced cell viability and promoted osteoconductivity [67].In contrast,at higher concentrations,Mg2+ions have a damaging effect on cellular response [68].Similarly,slow degradation exhibit a limited release of OH−ions,which resulted in a constant pH value (7.49 ± 0.03) that promotes better cellular proliferation.Abundant hydrogen release and bubble formation have a severe effect on cellular growth and attachment.Several studies reported local cell injuries caused by the abrupt release of hydrogen gas during immersion [69–71].PCL/HA/TiO2hybrid coated samples show a low hydrogen evolution rate (0.06–0.09 mL/cm2/day),which attributes stable cellular growth.Surface topography is a crucial factor in determining cytocompatibility.Generally,large PCL micropores allow sufficien nutrient supply,metabolic waste removal,and gas diffusion.Such a pore size promotes the growth of chondrocytes (bone regeneration) and osteoblasts.Small and medium pore size provides sufficien high surface area for efficien anchoring and intracellular signaling,promoting neovascularization,fibroblas ingrowth and binding [47].A PCL/HA/TiO2hybrid coated sample with a wide range of pore sizes (2–35 μm) demonstrates the cellular characteristics of multiple pore sizes,which resulted in significantl high cell viability.Additionally,the formation of needle type HA crystals promotes localized adhesion of cells with the‘tip of needle-like’ morphology,thereby,enhancing the cellanchoring sites.On the other hand,despite the presence of HA on a HA/TiO2composite coated surface,cellular activities are relatively lower than hybrid coated sample.The ineffective shielding arise from coating defects like pores and cracks leads to localized degradation,resulting in a relatively higher pH and hydrogen release rate.Additionally,the leaching of Mg2+ions during degradation resulted in lower cell viability.Similar behavior has been observed for A/P sample;the formation of Mg(OH)2protective layer provides partial shielding during initial immersion hour and gradually depleted under the influenc of Cl−ions.The highest alkalized environment and hydrogen evolution rate of 3.98 mL/cm2/day endow severe cytotoxicity to A/M samples.
In a PCL/HA/TiO2hybrid coated sample,TiO2plays a significan role in combating bacterial growth as PCL and HA have inert biocidal nature [72].In PCL/HA/TiO2hybrid coating,TiO2presence in a sandwiched HA/TiO2composite layer provides strategic importance.As the composite layer is directly coated over the Mg substrate,Ruzaina et al.mentioned that alkaline metals like Mg could quickly polarize TiO2molecule,as shown in Eq.(9).The h+pair reacted with H2O present in media and form hydroxyl radicals as shown in Eq.(10).Similarly,e−interacted with O2and form superoxide ion Eq.(11) [73,74]:
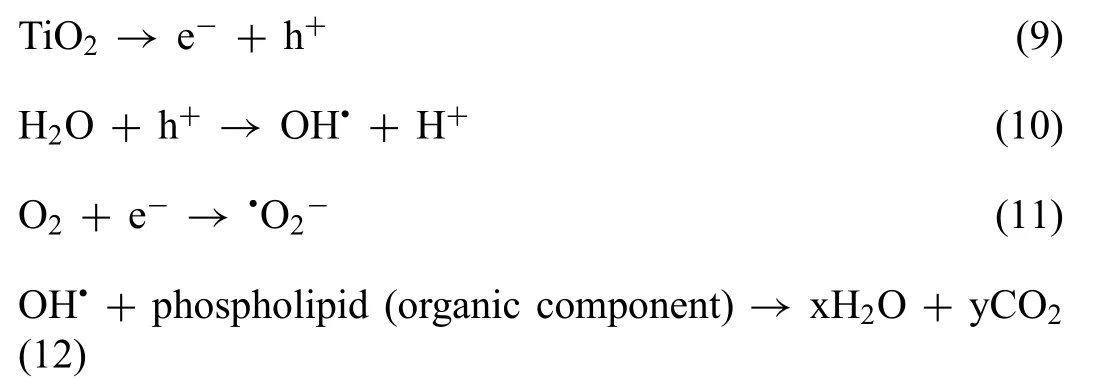
Thus formed reactive oxygen species (ROS) at the HA/TiO2composite layer can quickly diffuse through the amorphous polymeric matric of the PCL layer present at top.Afterward,these ROS,especially hydroxyl radicals,oxidize the unsaturated phospholipid component of the cell membrane of bacteria and decompose them to water and carbon dioxide,as shown in Eq.(12).An amorphous PCL layer in PCL/HA/TiO2hybrid coating hurdles ROS transportation to reach the surface;therefore,its bactericidal response is delayed.It was observed that the antibacterial response againstE.coliwas more pronounced than S.aureus.This was becauseS.aureushas a thicker peptidoglycan cell membrane compared toE.coli[75].In HA/TiO2composite coated samples,the immediate contact between ROS and microbes and higher degradation rate provide a complex environment for bacterial growth.The A/P and A/M samples exhibit highest antibacterial response due to abundant release of Mg2+ions,H2release,and severe alkalization.
Therefore,It is worth noting that PCL/HA/TiO2hybrid coated samples respond towards bacteria and L929 cells are different in this study.The viability of bacteria is suppressed while for L929 cells is enhanced with respect to control,respectively.The individual role of coating ingredients like bactericidal yet biosafe response by TiO2,enhanced biocompatibility provided by HA and PCL,was responsible for such a significan response.Moreover,the difference between structure,size and metabolic activities of cells and bacteria also contributed towards distinguished individual response.
5.Conclusions
In present work,thein-vitrodegradation behaviour of PCL/HA/TiO2multi-layer hybrid coating has been investigated and compared with HA/TiO2composite coating,A/M and A/P ZM21 Mg-alloy.The key finding of the present work can be concluded as:
•The PCL/HA/TiO2hybrid coating showed excellent corrosion resistance for ZM21 alloy as compared to HA/TiO2composite coated,A/P and A/M ZM21 Mg-alloys.The PCL/HA/TiO2hybrid coating remained intact and defectfree by escaping evolved H2through top microporous PCLNIPSlayer.The PCL/HA/TiO2hybrid coating showed H2evolution,weight loss and corrosion rate as 0.062±0.036(mL/cm2/day),0.033±0.010(mg/cm2/day)and 0.069 ± 0.006 (mm/year),respectively up to 28 days of SBF immersion,which are within acceptable safe limits in human body.
•A dense and stable 3d micro-fl wer shaped HA(Ca/P:1.60)grown in the hybrid coating provided effective shielding against aggressive ions up to 28 days of SBF immersion.The contraction of PCL micropores due to water uptake in a stable pH environment facilitates its formation by means of Vander-wall forces.The corrosion inhibition efficien y demonstrated by the PCL/HA/TiO2hybrid coating is 99.2% even after 28 days of SBF immersion.
•The mechanical integrity loss in PCL/HA/TiO2hybrid coated samples after 28 days of SBF immersion was only 14.7% of UBS,10.9% of UTS and 9.4% of UCS as compared to the significan loss observed in HA/TiO2composite coated,A/P and A/M samples.
•Fractography revealed that the hybrid coated samples even after 28 days of SBF immersion,shows identical fraction of tension twins (TTWs) as of pristine ZM21 sample.The activation of TTWs allowed the plastic deformation of hybrid coated ZM21 sample up to a higher extent.But in case of HA/TiO2coated,A/P and A/M samples,localized corrosion pits and cavities restricted the plastic deformation through twinning.Thus,catastrophic failure occurred at lower load values.
•The PCL/HA/TiO2hybrid coating has enhanced the viability for L929 cell line remarkably whereas;the viability ofE.coliandS.aureusbacteria were suppressed with reference to the controls.
•The developed PCL/HA/TiO2hybrid coating is biocompatible and able to delay the degradation of Mg alloy signifi cantly,proving its potential for biodegradable orthopaedic implants applications.Therefore,future research can be explored forin-vivoanalysis of multi-layer hybrid coated Mg-alloys.
Declaration of Competing Interest
The authors declare that they have no conflic of interest.
Acknowledgment
The authors are thankful to CSIR-IMTECH laboratory for providing the technical support in biocompatibility testing.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2021.10.004.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Effect of crystallization on purity of volatile metallic magnesium prepared from a one-step multi-region condensation process under vacuum condition
- Tribological behaviour of AZ31 magnesium alloy reinforced by bimodal size B4C after precipitation hardening
- Thermodynamic assessment of Mg−Ni−Y system focusing on long-period stacking ordered phases in the Mg-rich corner
- Primary Mg2Si phase and Mg2Si/α-Mg interface modifie by Sn and Sb elements in a Mg-5Sn-2Si-1.5Al-1Zn-0.8Sb alloy
- Quasi-in vivo corrosion behavior of AZ31B Mg alloy with hybrid MWCNTs-PEO/PCL based coatings
- The microstructure evolution and deformation mechanism in a casting AM80 magnesium alloy under ultra-high strain rate loading
