Improvement in tensile strength of extruded Mg–5Bi alloy through addition of Sn and its underlying strengthening mechanisms
2022-12-30SngCheolJinJeWonChJeongHunLeeTekyungLeeSngHoHnSungHyukPrk
Sng-Cheol Jin,Je Won Ch,Jeong Hun Lee,Tekyung Lee,Sng-Ho Hn,Sung Hyuk Prk,∗
a School of Materials Science and Engineering,Kyungpook National University,Daegu 41566,Republic of Korea
b Ulsan Regional Division,Korea Institute of Industrial Technology,Ulsan 44413,Republic of Korea
c School of Mechanical Engineering,Pusan National University,Busan 46241,Republic of Korea
d Department of Materials Analysis,Korea Institute of Materials Science,Changwon 51508,Republic of Korea
Abstract Through an investigation of the microstructure and mechanical properties of extruded Mg–5Bi–xSn (BT5x,x = 0,2,4,and 6 wt%) alloys,this study demonstrates that the addition of Sn to an Mg–5Bi binary alloy significantl improves the tensile strength of the extruded alloy.All the extruded alloys exhibit a typical basal fibe texture and a partially dynamically recrystallized (DRXed) microstructure consisting of fin DRXed grains and coarse unDRXed grains.As the Sn content increases from 0 wt% to 6 wt%,the average size of the DRXed grains decreases from 4.2 to 2.8 μm owing to the increase in the amount of precipitates via their grain-boundary pinning effect.The extruded B5 and BT52 alloys contain numerous Mg3Bi2 precipitates,but their size and number density are smaller and higher,respectively,in the latter alloy.Numerous Mg2Sn precipitates as well as Mg3Bi2 precipitates are present in the extruded BT54 and BT56 alloys,and the number density of the Mg2Sn precipitates is higher in the latter alloy because of its higher Sn content.The addition of 2 wt% Sn to the B5 alloy significantl improves the yield strength (YS) and ultimate tensile strength (UTS) of the extruded alloy—by 76 and 57 MPa,respectively.This drastic improvement is the combined outcome of enhanced grain-boundary hardening,precipitation hardening,and solid-solution hardening effects induced by the refine DRXed grains,numerous precipitates,and Sn solute atoms,respectively.The further addition of 2 wt% or 4 wt% Sn to the BT52 alloy leads to moderate increments in the YS and UTS of the extruded alloy.Specificall ,each addition of 2 wt% Sn increases the YS and UTS by ˜26 and ˜20 MPa,respectively,which is attributed mainly to the additional precipitation hardening effect induced by the Mg2Sn precipitates.
Keywords: Mg–Bi–Sn alloy;Extrusion;Precipitation;Microstructure;Tensile property.
1.Introduction
As environmental and social demands for lightweight vehicles are increasing continuously,Mg alloys have been attracting considerable attention in the transportation industry owing to their low density and high specifi strength.Extruded Mg alloys with high alloy contents have been studied extensively in response to a rapid increase in the demand for high-strength lightweight metals.High-alloyed Mg–Al- and Mg–Zn-based alloys such as AZ80,AZ91,and ZK60 are used most commonly to fabricate high-strength extruded Mg materials.Numerous fin precipitates can form in these alloys during hot extrusion,and decrease the size of dynamically recrystallized (DRXed) grains through the grain-boundary pinning effect.Such a refine DRXed microstructure containing numerous precipitates consequently results in the high strength of the extruded alloy [1–3].However,both the Mg17Al12phase formed in Mg–Al alloys and the MgZn2phase formed in Mg–Zn alloys have much lower melting temperatures (437 and 416 °C,respectively) than the matrices of the respective alloys [4–8].Commercial Mg alloys with high Al or Zn contents are susceptible to hot cracking during extrusion because of the low thermal stability of these Mg17Al12and MgZn2phases [9–12].
New Mg–Bi-based alloys have recently been developed to overcome the drawbacks of commercial Mg–Al and Mg–Zn alloys.The maximum solubility of Bi in Mg is as high as 8.9 wt% and the Mg3Bi2phase formed in Mg–Bi alloys has a substantially high melting temperature of 823 °C;therefore,the Mg–Bi alloy system is suitable for high-speed extrusion.For example,a recently developed Mg–5Bi–3Al (wt%) alloy has been reported to be successfully extruded at a die-exit speed of 67 m/min without hot cracking [11],which is more than 10 times the maximum extrusion speeds of commercial high-alloyed Mg alloys such as AZ80 and ZK60 [9,11,12].Go et al.[13] reported that the addition of various amounts of Bi to pure Mg improves the tensile yield strength (YS) of the extruded alloy;specificall ,the YS increases from 88 to 129 MPa upon the addition of 6 wt% Bi.Several studies have been conducted on the addition of alloying elements (e.g.,Al,Ca,Si,and Zn[10,14–17])to Mg–Bi binary alloys to improve the mechanical properties of the extruded alloys or to develop high-performance extruded Mg–Bi-based alloys.However,to the best of the authors’ knowledge,the effects of Sn addition to Mg–Bi binary alloys on their mechanical properties have not been reported.Similar to the Mg3Bi2phase,the Mg2Sn phase formed by Sn addition to pure Mg or Mg alloys has a very high melting temperature of 773 °C [18,19].Therefore,a high-Sn-containing Mg–7Sn–1Al–1Zn(TAZ711,wt%)alloy exhibits high extrudability with the maximum extrusion speed of>27 m/min [9].Zeng et al.[20] demonstrated numerically and experimentally that Sn addition to pure Mg promotes the activation of pyramidal slip systems during metal forming,which,in turn,leads to an improvement in the workability of the alloy.It has also been reported that as the amount of Sn added to an Mg–8Al–2Zn (wt%) alloy increases from 0 wt% to 6 wt%,the YS of the extruded alloy gradually increases from 233 to 287 MPa,which is attributed mainly to the decrease in the DRXed grain size and the formation of numerous fin Mg2Sn precipitates [21].Based on the find ings of these studies,Sn is considered a promising alloying element for improving the mechanical properties of extruded Mg–Bi alloys.Therefore,this study investigates the effects of Sn addition to an Mg–Bi binary alloy on the microstructure and mechanical properties of the extruded alloy by adding different amounts of Sn (2,4,and 6 wt%) to a Mg–5Bi alloy and then performing hot extrusion of the alloy billets.The microstructural characteristics and tensile strengths of the extruded alloys are analyzed,focusing on the variations in the dynamic precipitation behaviors and the dominant hardening mechanisms with the Sn content.
2.Experimental procedure
Cast billets of Mg–5Bi (B5),Mg–5Bi–2Sn (BT52),Mg–5Bi–4Sn (BT54),and Mg–5Bi–6Sn (BT56) (all in wt%)alloys were prepared using the conventional mold casting method.This method involved melting of a pure Mg ingot,Bi granules,and Sn granules in an electric resistance furnace at 750 °C under an inert atmosphere containing a CO2–SF6gas mixture with subsequent pouring of the molten alloy into a steel mold preheated to 210 °C.The chemical compositions of the cast B5,BT52,BT54,and BT56 billets were confirme by inductively coupled plasma spectrometry to be Mg–4.97Bi,Mg–5.05Bi–2.18Sn,Mg–5.03Bi–4.41Sn,and Mg–5.04Bi–6.51Sn,respectively (all in wt%);these compositions were very close to their respective nominal values.The fabricated billets were homogenized at 480 °C for 10 h under an inert atmosphere containing an Ar–SF6gas mixture and then water-quenched.The homogenized billets were machined into a cylindrical shape (diameter: 68 mm,length:120 mm) for extrusion.The machined billets and a singlehole extrusion die were preheated at 300 °C for 1 h in a box furnace.Direct extrusion experiments were performed at an initial billet temperature of 300 °C,a ram speed of 1 mm/s,and an extrusion ratio of 10 using a horizontal hydraulic extrusion machine with a load capacity of 300 tons.The variation in the extrusion load generated during extrusion was measured using the load cell of the extrusion machine.The cross-sectional diameter of the extruded alloys was 22 mm,and the average strain and strain rate applied during extrusion were 2.30 and 0.21 s−1,respectively.
The microstructural characteristics of the extruded alloys were analyzed by optical microscopy,field-emissio scanning electron microscopy (FE-SEM),field-emissio transmission electron microscopy (FE-TEM),X-ray diffraction (XRD),electron probe microanalysis (EPMA),and electron backscatter diffraction (EBSD) analysis.The specimen for TEM observation was prepared using a focused ion beam (JIB-4601F) and TEM analysis was performed using a JEM-2100F equipped with an energy dispersive spectroscopy (EDS) system.All microstructural analyses except for TEM analysis were performed on longitudinal cross-sections in the central region of the extruded alloys.The specimens for the microstructural observations were ground sequentially with SiC abrasive papers(from#120 to#2000) and then polished using a 1 μm diamond paste slurry.Before OM and FE-SEM measurements,the polished specimens were etched with an aceticpicral solution composed of 10 ml acetic acid,3.0 g picric acid,10 ml distilled water,and 100 ml ethanol (99.5%).For the specimens for the EBSD measurements,additional polishing was conducted using a colloidal silica solution (0.04 μm)for 20 min,followed by cleaning in ethanol using an ultrasonic cleaner.The EBSD measurements were taken at an accelerating voltage of 20 kV with a working distance of 17 mm using an EBSD detector (Symmetry S2,Oxford Instruments) installed in a field-emissio scanning electron microscope (SU8230,Hitachi).The measured EBSD data,using only those indexed points with a mean angular deviation below 1.0°,were analyzed using AZtecCrystal software 2.0(Oxford Instruments).For tensile testing,dogbone-shaped specimens with gage dimensions of 6 mm (diameter) × 25 mm(length) were machined from the central region of each extruded alloy.The tensile tests were performed using a Shimadzu AGS-100kNX universal testing machine at room temperature (23 °C) and a strain rate of 1 × 10−3s−1;the loading axis was parallel to the extrusion direction (ED).

Fig.1.Variation in (a) extrusion load with ram displacement during direct extrusion and (b) maximum extrusion load with amount of Sn added to Mg–5Bi alloy.
3.Results and discussion
3.1.Variation in extrusion load generated during direct extrusion with Sn content
Fig.1a shows variation in the extrusion load generated during the direct extrusion of the BT5x(x= 0,2,4,and 6)alloys with the ram displacement.In the early stages of extrusion,the billet is compressed in the container,which causes a rapid increase in the extrusion load.When the extrusion load reaches its maximum value,the compressed billet begins to exit the extrusion die through the hole.Subsequently,the extrusion load gradually decreases as the extrusion proceeds.Friction is not generated between the billet and the container walls during the indirect extrusion process;in contrast,a considerable amount of friction is generated between the billet and the container walls during the direct extrusion process owing to the movement of the entire billet [22].Accordingly,during indirect extrusion,the extrusion load does not vary significantl after a material in the container exits the extrusion die.In contrast,during direct extrusion,the extrusion load decreases gradually after the material exits the die because the decrease in the length of the billet remaining in the container leads to a decrease in the amount of friction generated between the billet and the container walls.As the amount of Sn added to the B5 alloy increases from 0 wt%to 6 wt%,the maximum extrusion load generated during direct extrusion increases almost linearly from 170 to 246 tons(Fig.1b).The maximum extrusion pressure is calculated by dividing the maximum extrusion load by the cross-sectional area of the billet.Since the diameter of the billet used in this study is 68 mm (area: 3631 mm2),the calculated maximum extrusion pressures of the B5,BT52,BT54,and BT56 alloys are 459,548,607,and 664 MPa,respectively.Therefore,the addition of 1 wt% Sn to the B5 alloy increases the maximum extrusion pressure by ˜34.2 MPa because the dissolved Sn solutes increase the fl w stress of the alloy.The maximum extrusion pressure of the BT56 alloy is 1.45 times that of the B5 alloy,which suggests that Sn addition can significantl increase the resistance of Mg–Bi binary alloys to plastic deformation at elevated temperatures.The extrusion experiments cannot proceed when the stress required to cause the plastic fl w of a material becomes equal to the load capacity of the extrusion machine because the pressure limit is exceeded.Accordingly,the increase in extrusion pressure caused by Sn addition should be considered carefully when designing the hot extrusion process of Mg–Bi–Sn ternary alloys.
3.2.Variation in microstructural characteristics of extruded alloy with Sn content
Figs.2 and 3 show optical micrographs and EBSD inverse pole figur maps,respectively,of the extruded alloys.All the extruded alloys exhibit a partially DRXed microstructure,which consists of fin DRXed grains formed by DRX during extrusion and coarse unDRXed grains elongated along the ED via plastic deformation during extrusion.The occurrence of incomplete DRX during extrusion in all the alloys is because the adopted extrusion conditions are unfavorable for DRX.The mobility of dislocations and grain boundaries in a material increases with increasing deformation temperature.Hence,an increase in the extrusion temperature promotes the DRX behavior of a material during extrusion because the grain-boundary bulging and dislocation rearrangement at high temperatures cause the nucleation of DRXed grains [23–26].Kim et al.[27] investigated the effect of extrusion temperature on the DRX behavior of a commercial AZ80 alloy during hot extrusion.They reported that as the extrusion temperature increased from 250 to 350 °C,the area fraction of the DRXed grains (i.e.,DRX fraction) of the extruded alloy increased from 68.3% to 96.8%.Similarly,another study reported that as the extrusion temperature of a TAZ711 alloy increased from 250 to 450 °C,the DRX fraction of the extruded alloy increased from 65.4% to 91.9% [28].In the present study,all the extrusion experiments were performed at 300 °C with an extrusion ratio of 10.This extrusion ratio is identical to or larger than those used in the two abovementioned studies (8 and 10 for the AZ80 and TAZ711 alloys,respectively);that is,the amount of imposed strain in the present study is identical to or larger than those in the previous studies.Nevertheless,the DRX fractions of the BT5xalloys extruded at 300 °C (41%–59%) are lower than those of the AZ80 and TAZ711 alloys extruded at a lower temperature of 250 °C (68.3% and 65.4%,respectively).Such suppressed DRX behavior of the BT5xalloys is attributable primarily to the Zener pinning effect of the Mg3Bi2precipitates formed during extrusion.Go et al.[13] reported that in Mg–Bi binary alloys,numerous fin Mg3Bi2precipitates were formed throughout the material in the early stages of hot extrusion and that these precipitates,via their boundary pinning effect,inhibited the grain-boundary migration necessary for the nucleation of DRXed grains.This hindrance to DRX caused by fin Mg3Bi2precipitates formed during extrusion eventually leads to the formation of a bimodal grain structure containing many unDRXed grains in extruded Mg–Bi-based alloys.The DRX fractions of the Sn-containing BT5xalloys (41%–49%)are lower than that of the Sn-free B5 alloy (59%).Therefore,Sn addition to the B5 alloy is likely to suppress DRX during extrusion because of the enhanced boundary pinning effect from the increased number of fin precipitates induced by Sn addition.The variation in the precipitates formed during extrusion with the Sn addition is discussed in Section 3.3.

Fig.2.Optical micrographs of extruded (a) B5,(b) BT52,(c) BT54,and (d) BT56 alloys.ED denotes the extrusion direction.
The (0001) pole figure of the extruded alloys (insets of Fig.3) reveal that all the BT5xalloys have a basal fibe texture with basal poles oriented almost perpendicular to the ED.Such a basal fibe texture is typical of extruded Mg alloys devoid of Ca and rare-earth (RE) elements such as Gd and Ce,all of which can cause texture tilting or the formation of an RE texture [29–32].The maximum texture intensity in the (0001) pole figur of the extruded BT54 alloy (8.8)is higher than those of the other extruded alloys (5.5–6.1).The unDRXed grains of extruded Mg alloys have a considerably stronger basal texture than the DRXed grains because the former grains are deformed continuously during the entire extrusion process and no DRX occurs during this time[25,33,34].In the inverse pole figur maps,representing the crystallographic orientations of the indexed points in color,the DRXed grains are blue and green and most of the unDRXed grains are dark blue (Fig.3).This color pattern indicates that the unDRXed grains have a strong 〈10–10〉 basal texture.Accordingly,the higher texture intensity of the extruded BT54 alloy is attributed to the larger quantity of unDRXed grains.

Fig.3.Inverse pole f gure maps and (0001) pole f gures of extruded (a) B5,(b) BT52,(c) BT54,and (d) BT56 alloys.fDRX denotes the area fraction of dynamically recrystallized (DRXed) grains.
3.3.Variation in dynamic precipitation behavior during extrusion with Sn content
It can be seen from the SEM images of the extruded alloys (Fig.4) that all the alloys contain numerous fin precipitates (<500 nm in size).However,the precipitates in the Sn-containing BT5xalloys are smaller than those in the Snfree B5 alloy and their number density tends to increase with increasing Sn content.XRD measurements were performed to identify the phase types of the precipitates in the extruded alloys.Only second-phase peaks associated with the Mg3Bi2phase are observed in the XRD patterns of the extruded B5 and BT52 alloys (Fig.5a and b).In contrast,peaks associated with the Mg2Sn phase in addition to those associated with the Mg3Bi2phase are also observed in the XRD patterns of the extruded BT54 and BT56 alloys (Fig.5c and d).These XRD results suggest that when 2 wt% Sn is added to the B5 alloy,no Mg2Sn particles are formed during extrusion;however,the addition of ≥4 wt%Sn results in the formation of fin Mg2Sn particles via dynamic precipitation during extrusion.Although the XRD peaks associated with the Mg2Sn phase are not observed in the extruded BT52 alloy,a small amount of Mg2Sn phase can exist in this alloy if the concentration is below the detection limit of the XRD instrument.TEM observation of the extruded BT52 alloy reveals that short rod-shaped Mg2Sn precipitates (200–300 nm in length) are present in this alloy (see the blue dotted circles in Fig.6).The rod shape of the Mg2Sn precipitates is consistent with the reported find ings for Mg–Sn-based alloys[35–37].However,the amount of Mg2Sn precipitates is very small,and the majority of secondphase particles in the extruded BT52 alloy are the spherical Mg3Bi2precipitates.Thermodynamic calculations using Fact-Sage software also reveal that the phase fraction of Mg3Bi2precipitates (5.81 wt%) is significantl higher than that of Mg2Sn precipitates (0.11 wt%) (Table 1).As a result,the Sn content in theα-Mg matrix (2.11 wt%) is similar to that in the BT52 alloy (2.18 wt%),as shown in the TEM EDS spectrum at point B in Fig.6.This suggests that although some Mg2Sn precipitates are formed in the extruded BT52 alloy,most of the added Sn is dissolved in theα-Mg matrix as solute atoms.In contrast,abundant Mg2Sn precipitates are formed in the extruded BT54 and BT56 alloys,as shown in their XRD patterns.

Fig.4.SEM images showing fin precipitates in extruded (a) B5,(b) BT52,(c) BT54,and (d) BT56 alloys.
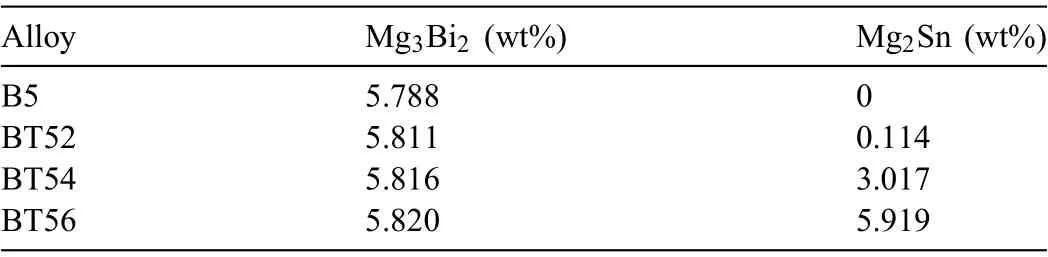
Table 1 Phase fractions of extruded alloys calculated using FactSage.
This difference in the formation of the Mg2Sn phase between the BT52 alloy and the BT54 and BT56 alloys can be explained using the equilibrium phase diagram of Mg–5Bi–xSn (x= 0–10 wt%) calculated using FactSage software(Fig.7).According to the phase diagram,in the case of the BT52 alloy,the equilibrium phases change fromα-Mg +Mg3Bi2toα-Mg + Mg3Bi2+Mg2Sn at a temperature of 304 °C.This equilibrium phase transition suggests that the Mg2Sn phase can form in the BT52 billet during heat treatment at temperatures below 304 °C.In the present study,although the extrusion experiments are performed at an initial billet temperature of 300 °C,the actual temperature in the deformation-concentrated zone near the extrusion die may be higher than the initial temperature because of the generation of heat by plastic deformation and friction [38].Therefore,the actual deformation temperature of the BT52 alloy during extrusion lies in the (α-Mg + Mg3Bi2) two-phase region.As a result,in this alloy,the Mg3Bi2phase precipitates dynamically from the supersaturatedα-Mg matrix during extrusion and precipitation of the Mg2Sn phase hardly occur.However,the size and number density of the Mg3Bi2precipitates in the extruded BT52 alloy are smaller and higher,respectively,than those in the extruded B5 alloy (Fig.4a and b).The addition of 2 wt%Sn to the B5 alloy increases the maximum extrusion pressure considerably(by 19.4%).Lee et al.[39]reported that the trace addition of 0.1 wt% Ti to an Al–Zn–Mg–Cu alloy could increase the dislocation density in the extruding alloy during hot extrusion by increasing the hot fl w stress of the alloy at the extrusion temperature.In this study,because all extrusion experiments are conducted using the same processing parameters (e.g.,billet temperature,extrusion ratio,ram speed,die geometry,and billet dimension),the difference in the maximum extrusion pressure of the B5 and BT52 alloys results from the difference in the fl w stress of the two alloys at the extrusion temperature.The fl w stress of the materials is proportional to the square root of the dislocation density[40];therefore,the amount of dislocations formed during extrusion is likely to be higher in the BT52 alloy than in the B5 alloy.Dislocations have a higher strain energy than the defect-free matrix regions because they cause local distortion of the crystal lattice from its ideal regularity;therefore,dislocations act as preferential nucleation sites for precipitation[41,42].Moreover,an increase in the dislocation density results in more numerous dislocation pipe diffusion paths for solutes dissolved in theα-Mg matrix [43–45].Hence,the addition of 2 wt% Sn increases the number of nucleation sites for Mg3Bi2precipitates and accelerates the diffusion of Bi solute atoms by inducing the formation of more dislocations during extrusion.Consequently,Sn addition promotes the dynamic precipitation of Mg3Bi2,and the extruded BT52 alloy,in turn,has more numerous and fine Mg3Bi2precipitates than the extruded B5 alloy.According to the phase diagram in Fig.7,the equilibrium phase transition fromα-Mg + Mg3Bi2toα-Mg + Mg3Bi2+ Mg2Sn occurs at 383 and 452 °C in the BT54 and BT56 alloys,respectively.Because these temperatures are considerably higher than the extrusion temperature of 300 °C,the deformation temperature during extrusion lies in the (α-Mg + Mg3Bi2+ Mg2Sn) three-phase region(Fig.7).Hence,in the BT54 and BT56 alloys,the Mg3Bi2and Mg2Sn phases precipitate simultaneously during extrusion.By applying the phase lever rule to the phase diagram,it can be estimated that the amount of Mg2Sn precipitates formed in the BT56 alloy will be higher than that in the BT54 alloy.

Fig.5.XRD patterns of extruded (a) B5,(b) BT52,(c) BT54,and (d) BT56 alloys.

Fig.6.TEM image and EDS spectra at positions A and B of extruded BT52 alloy.

Fig.7.Equilibrium phase diagram of Mg–5Bi–xSn (x = 0–10 wt%) calculated using FactSage software.Text. denotes the extrusion temperature adopted in this study (300 °C).
Fig.8 shows backscattered electron images and corresponding EPMA scanning maps of the Bi and Sn in the extruded alloys.The size and distribution of Bi and Sn elements in the EPMA scanning maps represent those of the Mg3Bi2and Mg2Sn precipitates,respectively.Few Mg2Sn precipitates are observed in the extruded BT52 alloy,whereas numerous Mg2Sn precipitates are present in the extruded BT54 and BT56 alloys.These results are consistent with the above-described XRD patterns of the extruded alloys.The Mg3Bi2precipitates in the extruded BT54 and BT56 alloys have nearly identical sizes of 100–300 nm,and their number and distribution are also similar in the two alloys.An analysis of the second-phase particles using FactSage software shows that the phase fraction of Mg3Bi2particles at the extrusion temperature of 300 °C is 5.82 wt% in both the BT54 and BT56 alloys.Therefore,there is no significan difference in the size and number density of Mg3Bi2precipitates between the extruded BT54 and BT56 alloys.The Mg2Sn precipitates in the extruded BT54 and BT56 alloys are similar in size at ˜200 nm.However,the phase fractions of Mg2Sn particles are calculated to be 3.02 and 5.92 wt%in the BT54 and BT56 alloys,respectively(Table 1);namely,the Mg2Sn phase fraction is 1.96 times higher in the BT56 alloy.Because the size of the Mg2Sn precipitates in the extruded BT54 and BT56 alloys are similar,their number density is also expected to be double in the BT56 alloy.Consequently,when 4 wt% or more of Sn is added to the B5 alloy,Mg2Sn precipitates are also formed during direct extrusion at 300 °C and their number density increase with increasing the amount of Sn added.Fine precipitates formed during extrusion can inhibit the growth of DRXed grains through their grain-boundary pinning effect.Such a decrease in DRXed grain size caused by fin precipitates has been reported in various extruded Mg alloys such as AZ80 [1],BA53 [11],and TAZ711 [46].In the present study,the total amount of precipitates formed in the extruded alloy increases with increasing Sn content.As shown from the inverse pole figur maps of the DRXed grains of the extruded alloys (Fig.9),the average size of the DRXed grains of the extruded alloy decreases gradually with increasing Sn content (4.2,3.8,3.1,and 2.8 μm for the B5,BT52,BT54,and BT56 alloys,respectively).Therefore,this gradual refinemen of the DRXed grains with increasing Sn content is attributed mainly to the increase in the amount of fin precipitates via their grain-boundary pinning effect.

Fig.8.(a) Backscattered electron images and corresponding EPMA scanning maps of (b) Bi and (c) Sn elements in extruded B5,BT52,BT54,and BT56 alloys.(For interpretation of the references to color in this figur legend,the reader is referred to the web version of this article.)

Fig.9.Inverse pole figur maps of DRXed grains of extruded (a) B5,(b) BT52,(c) BT54,and (d) BT56 alloys.dDRX denotes the average size of DRXed grains.

Table 2 Microstructural characteristics and tensile properties of extruded BT5x (x = 0,2,4,and 6 wt%) alloys.
3.4.Variation in tensile properties of extruded alloy with Sn content
Fig.10 and Table 2 present the tensile properties of the extruded alloys.As the Sn content increases from 0 wt%to 6 wt%,the YS of the extruded alloy increases from 136 to 264 MPa (94% increase),and its ultimate tensile strength(UTS) increases from 205 to 301 MPa (47% increase).These results suggest that the addition of Sn to an Mg–Bi binary alloy significantl improves the tensile strength of the extruded alloy.In particular,when 2 wt% Sn is added to the B5 alloy,the YS and UTS increase substantially by 76 and 57 MPa,respectively.These strength increments are even larger than those caused by the further addition of 4 wt% Sn to the BT52 alloy (52 and 39 MPa,respectively) (Fig.10b).All the extruded alloys have a bimodal grain structure.Although the extruded alloys have different maximum texture intensities,they have similar values of the average Schmid factor for(0002)<1120>basal slip under tension along the ED (0.10–0.13) because both DRXed grains and unDRXed grains are oriented unfavorably for basal slip.Consequently,the difference in the texture hardening effect during tension among the extruded alloys is insignificant As the Sn content increases,the average size of the DRXed grains decreases gradually and the total number density of the precipitates increases gradually.Therefore,both the grain-boundary hardening effect and the precipitation hardening effect gradually strengthen with increasing Sn content.However,the strength improvement caused by Sn addition is much greater in the extruded BT52 alloy than in the extruded BT54 and BT56 alloys,even though numerous Mg2Sn precipitates are formed only in the latter two alloys.Given the gradual strengthening of grain-boundary hardening and precipitation hardening effects with increasing Sn content,the drastic strength improvement in the former alloy is attributed mainly to the solid-solution hardening effect induced by the Sn solute atoms dissolved in theα-Mg matrix.

Fig.10.(a) Tensile properties of extruded alloys at room temperature and (b) increments in yield strength (YS) and ultimate tensile strength (UTS) caused by addition of 2 wt% Sn to B5,BT52,and BT54 alloys,as calculated from (a).
The breakaway stress required to pull dislocations away from a line of solute atoms is proportional to the cube of the atomic radius [22].Hence,the dissolution of Sn in Mg is expected to induce considerable solid-solution hardening because the atomic radius of Sn (0.217 nm) is larger than that of Mg (0.173 nm) [47].The extruded BT52 alloy has few Mg2Sn precipitates,indicating that almost all of the added 2 wt%Sn is present in the form of solute atoms in the alloy,and that all of it is used to cause solid-solution hardening during tension.At an extrusion temperature of 300 °C,the solubility limit of Sn in the B5 alloy is ˜2.0 wt% (Fig.7).Accordingly,the amount of Sn solute atoms dissolved in theα-Mg matrices of the extruded BT54 and BT56 alloys is the same as that in theα-Mg matrix of the extruded BT52 alloy (2.0 wt%);consequently,the extent of solid-solution hardening is the same in all three extruded alloys.When Sn is further added to the BT52 alloy,it is fully consumed in forming Mg2Sn precipitates and these precipitates induce precipitation hardening during tension.The increases in YS and UTS caused by the addition of 2 wt% Sn to the BT52 alloy (27 and 20 MPa,respectively) are similar to those caused by the addition of 2 wt% Sn to the BT54 alloy (25 and 19 MPa,respectively)(Fig.10b).These results suggest that the addition of 2 wt%Sn to the B5 binary alloy significantl improves the strength of the extruded alloy via a strong solid-solution hardening effect induced by Sn solute atoms and that the further addition of Sn to the BT52 alloy causes a relatively smaller improvement in the strength of the extruded alloy via a moderate precipitation hardening effect induced by Mg2Sn precipitates.
Precipitation hardening is generally known to be a stronger effect than solid-solution hardening [22,48] because precipitates—which are three-dimensional lattice defects—interfere more effectively with the movement of dislocations than do solute atoms—which are one-dimensional lattice defects.In the present study,however,the solid-solution hardening effect induced by Sn solute atoms is significantl stronger than the precipitation hardening effect induced by the Mg2Sn precipitates assuming that the same amount of Sn is dissolved completely in the matrix or entirely consumed in the formation of Mg2Sn precipitates.The reason for this unusual hardening effect (i.e.,more obvious solid-solution hardening than precipitation hardening) is not yet clear but there are some possible explanations for this behavior.According to the equilibrium phase diagram of the Mg–Bi binary system calculated using FactSage software,the solubility limit of Bi in pure Mg is very low at 300 °C,˜0.2 wt% [13].Therefore,most of the Bi contained in the B5 alloy(4.8 wt%)is consumed in the formation of the Mg3Bi2precipitates during extrusion at 300 °C and only 0.2 wt% Bi exists in the form of solute atoms in the extruded B5 alloy.The lattice distortion caused by Bi solute atoms in the B5 extrusion billet disappears almost completely after extrusion because of the precipitation of Mg3Bi2from the Bi-supersaturated matrix during extrusion.Therefore,almost no solid-solution hardening is expected to occur in the extruded B5 alloy.The addition of 2 wt% Sn to the B5 alloy leads to considerable lattice expansion of the matrix of the homogenized alloy billet because of the dissolution of Sn,whose atomic radius is larger than that of Mg.Because the precipitation of Mg2Sn barely occurs during extrusion,the Sn-induced lattice distortion of the matrix remains unchanged after extrusion,which,in turn,results in strong solid-solution hardening of the extruded BT52 alloy.
According to the Orowan particle hardening mechanism,the Orowan stress required to force a dislocation to glide between particles is inversely proportional to the average particle spacing [49,50].It is notable that the extruded BT52 alloy contains a tremendous amount of fin Mg3Bi2precipitates (Fig.4b).Accordingly,when Sn is additionally added to the BT52 alloy,the degree of the decrease in the average particle spacing caused by the formation of Mg2Sn precipitates can be insignifican because the particle spacing in the extruded BT52 alloy is already small because of the numerous Mg3Bi2precipitates.Accordingly,the degree of strengthening of the precipitation hardening effect caused by the formation of Mg2Sn precipitates can be smaller than expected.Consequently,the hardening effect induced by the Sn solute atoms can be stronger than that induced by the Mg2Sn precipitates.This is because of the differences in both the microstructural characteristics and the contribution of Sn to hardening between the extruded B5 and BT52 alloys (i.e.,Sn dissolution in a material in which almost no solid-solution hardening occurs (B5 alloy) and Mg2Sn precipitation in a material in which strong precipitation hardening occurs (BT52 alloy).It is notable that the YS of the extruded Mg–5Bi binary alloy approximately doubles (from 136 to 264 MPa) upon the addition of 6 wt% Sn.This drastic improvement is a combined outcome of the grain-boundary hardening,precipitation hardening,and solid-solution hardening effects induced by the refine DRXed grains,additionally formed precipitates,and Sn solute atoms in the extruded BT56 alloy,respectively.
Sn addition drastically improves the strength of the extruded alloy,but it does not affect the elongation of the alloy (Fig.10a).All the extruded alloys have relatively low elongations of 3.6%–5.0%.The tensile elongation of an extruded Mg alloy with a bimodal grain structure comprised of fin DRXed and coarse unDRXed grains increases gradually with increasing DRX fraction [14,46,51].Kim et al.[27] reported that when the DRX fraction of an extruded AZ80 alloy increased from 68.3% to 96.8%,its tensile elongation increased from 5.5% to 13.1%.Such a direct proportional relationship between the DRX fraction and the elongation was also reported for an extruded TAZ711 alloy;with an increase in the DRX fraction from 53% to 99%,the tensile elongation of the extruded alloy improved gradually from 10.4%to 15.2% [47].This strong correlation is because {10–11}contraction twining,{10–11}-{10–12} double twinning,and subsequent cracking along the formed twins occur more easily in coarse unDRXed grains during tension along the ED than in fin DRXed grains [52,53].In the present study,all the extruded alloys contain considerable amounts of coarse unDRXed grains (their area fractions are 41%–59%;see Fig.3),which eventually causes them to have low tensile ductility.Given that the extrusion temperature (300 °C) and extrusion ratio (10) adopted in this study are relatively low,the elongation of the extruded BT5xalloys will improve signifi cantly if the DRX fraction is increased through the implementation of an extrusion process at a higher temperature and/or larger extrusion ratio.Therefore,further systematic studies on the following aspects will be needed to increase the applicability of these Mg–Bi–Sn-based alloys as reliable structural components: (1) the influenc of the extrusion process parameters (e.g.,temperature,extrusion ratio,ram speed,die angle,and die-hole shape) on the DRX behavior during extrusion and on the resultant microstructure and (2) optimization of the process parameters to ensure a homogeneous microstructure and adequate ductility of the extruded products.
4.Conclusions
The effects of Sn addition to an Mg–Bi binary alloy on the microstructural characteristics and mechanical properties of the extruded alloy are investigated via the direct extrusion of BT5x(x= 0,2,4,and 6 wt%) alloy billets at 300 °C.As the Sn content increases from 0 wt% to 6 wt%,the maximum load generated during extrusion increases linearly from 170 to 246 tons.All the extruded alloys exhibit a bimodal grain structure comprised of fin DRXed grains and coarse unDRXed grains.The average size of the DRXed grains decreases gradually with increasing Sn content,which is attributed to the increase in the amount of fin precipitates via their grain-boundary pinning effect.Numerous Mg3Bi2precipitates and a few Mg2Sn precipitates are formed in the BT52 alloy during extrusion,and the BT52 alloy contains fine and more abundant Mg3Bi2precipitates than the B5 alloy because the former has a larger number of precipitate nucleation sites.In the BT54 and BT56 alloys,many Mg2Sn precipitates as well as Mg3Bi2precipitates are formed during extrusion and the amount of Mg2Sn precipitates is larger in the latter alloy because of its higher Sn content.The addition of 2 wt%Sn to the B5 alloy significantl improves the YS and UTS of the extruded alloy—by 76 and 57 MPa,respectively.This improvement is attributed mainly to the solid-solution hardening effect of the Sn solute atoms dissolved in the matrix.The refine DRXed grains and larger amount of precipitates also contribute to this drastic strength improvement.Further addition of 2 wt% or 4 wt% Sn to the BT52 alloy results in moderate improvements in the tensile strength of the extruded alloy.The YS and UTS increase by ˜26 and ˜20 MPa,respectively,with each addition of 2 wt% Sn,which is attributed mainly to the precipitation hardening effect induced by the additionally formed Mg2Sn precipitates.The tensile elongations of all the extruded alloys are relatively low,3.6%–5.0%,because they contain considerable amounts of coarse unDRXed grains,which are favorable for deformation twinning and subsequent cracking during tensile deformation.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science,ICT and Future Planning (MSIP,South Korea) (No.2019R1A2C1085272) and by the Materials and Components Technology Development Program of the Ministry of Trade,Industry and Energy (MOTIE,South Korea) (No.20011091).
杂志排行
Journal of Magnesium and Alloys的其它文章
- Effect of crystallization on purity of volatile metallic magnesium prepared from a one-step multi-region condensation process under vacuum condition
- Tribological behaviour of AZ31 magnesium alloy reinforced by bimodal size B4C after precipitation hardening
- Thermodynamic assessment of Mg−Ni−Y system focusing on long-period stacking ordered phases in the Mg-rich corner
- Primary Mg2Si phase and Mg2Si/α-Mg interface modifie by Sn and Sb elements in a Mg-5Sn-2Si-1.5Al-1Zn-0.8Sb alloy
- Quasi-in vivo corrosion behavior of AZ31B Mg alloy with hybrid MWCNTs-PEO/PCL based coatings
- The microstructure evolution and deformation mechanism in a casting AM80 magnesium alloy under ultra-high strain rate loading
