Siphon-Specific Expression of an Actin Encoding Gene Is Regulated by Six1/2 in Ciona savignyi
2022-12-27YUEWenxuanQIAOJinghanYANGXiuxiaandDONGBo
YUE Wenxuan, QIAO Jinghan, YANG Xiuxia, , and DONG Bo,
1)Sars-Fang Center, MOE Key Laboratory of Marine Genetics and Breeding, College of Marine Life Sciences,Ocean University of China, Qingdao 266003, China
2)Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
3)Institute of Evolution and Marine Biodiversity, Ocean University of China, Qingdao 266003, China
Abstract Actin is a ubiquitous protein and plays essential roles on cellular structure maintenance and cellular motility in both muscle and non-muscle tissues. Multiple genes encoding muscle actin have been identified from the ascidians, including those expressed in the larval tail muscle, the adult body-wall muscle, and adult heart muscle. In this study, a novel striated non-tail muscle actin gene was identified from the RNA-seq data of Ciona savignyi embryos. Phylogenetic analysis, alignment of the N-terminal amino acid sequences and comparation of diagnostic residues provided evidence that it had high similarity with vertebrate cardiac and skeletal muscle actin. In situ hybridization and promoter-driven GFP reporter assay revealed that it was specifically expressed in the primordia of the oral and atrial siphon. We hereby defined it as siphon-specific muscle actin coding gene (Cs-SMA). A 201 bp (-1350 bp to -1150 bp)sequence containing T-box and Six1/2 binding motif within the upstream region of Cs-SMA confined the expression of GFP in the siphons of electroporated embryos. Six1/2 binding motif was experimentally confirmed to play indispensable role in controlling the siphon-specific expression of Cs-SMA. The tissue-specific expression of Cs-SMA in the siphon primordia indicated its potential crucial roles in Ciona embryogenesis and organogenesis.
Key words Ciona savignyi; actin encoding gene; siphon; Six1/2; expression regulation
1 Introduction
Actin, the principal component of the microfilament, is one of the most highly conserved proteins. The discovery of actin structural homologs in bacteria and archaea indicates that actin-like polymers are used by cells across the tree of life (Gunninget al., 2015). Bacterial actin-like proteins are characterized with one filament one function, and many of them are not readily identified from sequencebased homology searches (Doiet al., 1988; Addinall and Lutkenhaus, 1996; Jensen and Gerdes, 1997). The actin family may undergo rampant expansion during eukaryotic evolution with members extremely well conserved in sequence (Gunninget al., 2015). For example, there are two cytoplasmic actins (β and γ)and four muscle actins (α-cardiac, α-skeletal, α-smooth and γ-smooth)in humans. The actin family contains not only conventional actin but also actin-related proteins (ARPs)that have high similarity with the conventional ones (Machesky and May, 2001). The ARPs have well characterized roles in actin polymerization(ARP2/3), dynein motor activity (ARP1 and ARP 10), and chromatin remodeling (ARP4, ARP5, ARP6, ARP8), while the functions of some ‘orphan’ ARPs are still unknown (Machesky and May, 2001; Goodson and Hawse, 2002).
Muscle linage is specified by the expression of muscle structure gene such as muscle actin (Carmenet al., 2015).The expression of muscle actin depends on the myogenic regulatory factors (MRF)includingMyf5,MyoD,Mrf4andMyogeninin vertebrates by binding to the E-box motif (Tapscott, 2005). In vertebrates,Myf5(Braunet al., 1989)andMyoD(Sassoonet al., 1989)play redundant roles in myoblast specification in the dorsal or ventral dermomyotome respectively; andMrf4(Braunet al., 1990)andMyogenin(Edmondson and Olson, 1989)instruct myoblasts for terminal differentiation. The sine oculis related homeobox group(Six)are required in the formation and development of cardiac muscle progenitors in a combinatorial fashion (Grifoneet al., 2005). In mammals,Six1,Six2,Six4,andSix5have a similar binding specificity to consensus sequence AACCTGA (Ohtoet al., 1999)and are able to activate the target genes such asPax3,MyoD,Mrf4, andMyogeninin myogenesis (Grifoneet al., 2005). In addition,Tbx6-related gene is expressed in cells of the muscle lineage in ascidians and is involved in the maintenance of transcriptional activity of muscle-specific structural genes (Takadaet al.,2002).Tbx6belongs to T-box gene family encoding transcription factors which includesBrachyury,Tbx1,Tbx2/3/4/5,Tbx6andTbr/Eomes/TBX21, that play critical roles in various processes of development, particularly, mesoderm formation in chordate embryos (Takatoriet al., 2004).
There are at least three distinct muscle actin isoforms expressed in the larval tail muscle, adult body-wall muscle and adult heart muscle in ascidians (Chibaet al., 2003).InStyela clava, at least four different muscle actin genes encoding the same actin isoforms are expressed in the larval tail muscle cells (Beach and Jeffery, 1992). Little is known about the expression of muscle actin genes in adult heart muscle, although they seem to be different from those which are expressed in the larval tail muscle (Kusakabe,1997).A maternally localized zinc-finger type transcription factor Macho-1 activated the expression ofTbx6(Kobayashiet al., 2003), which in turn regulates several downstream muscle-specific genes in bothHalocynthiaandCionaembryos (Sawadaet al., 2005; Yagiet al., 2005). Ascidians express a single MRF memberMrfthat is equally related by sequence to the four vertebrate MRFs (Meedelet al., 2007). InCionaspp, knockdown ofMrfresults in the loss of tail muscle myofibrils at early stages(Meedelet al., 2007); however, some unknown factors other than Mrf may regulate the expression of tail muscle actin gene inHalocynthia roretzi(Satou and Satoh, 1996).
In this study, we identified three actin genes from the transcriptome data ofC. savignyi, of which two coded for muscle actin isoforms and one for cytoplasmic actin. We focused on a novel non-tail muscle actin gene,Cs-SMA,to explore its temporal and spatial expression and to identify the factors that regulate its expression.
2 Materials and Methods
2.1 Experimental Animals
C. savignyiadults were collected from the coast of Qingdao and Rongcheng, Shandong Province, China. The animals were maintained under constant light to induce oocyte maturation in the laboratory. Eggs and sperm were obtained surgically from several adult gonad ducts and mixed for fertilization. Larvae were collected at 22, 26, and 66 hours post fertilization (hpf)forin situhybridization.
2.2 Phylogenetic Analysis
The sequences of actin and ARPs ofC. savignyiwere obtained from previous RNA-seq data (Weiet al., 2017).Those from additional six species (Homo sapiens,Xenopus laevis,Danio rerio,Ciona intestinalis,Branchiostoma belcheri,Drosophila melanogaster,Caenorhabditis elegansandArabidopsis thaliana)were obtained from the NCBI database (http://www.ncbi.nih.gov). The phylogenetic tree was constructed by MEGAX using the neighbor-joining(NJ)method. The resulting tree was graphed by the program in MEGAX, and was prepared for presentation by Adobe Illustrator 2018.
2.3 Whole-Mount in situ Hybridization
TheCs-SMAprobe sequence was from coding region(86 bp – 691 bp)and was amplified with primersCs-SMAF 5’ CCGTCTTTCCATCCATCG 3’ andCs-SMA-R 5’ CA GCGGTGCTCATTTCTT 3’. Digoxigenin-11-UTP (Roche,Germany)-labeled probes were synthesized by Sp6 or T7 RNA polymerase following the general protocol of the DIG RNA labeling kit (SP6/T7)(Roche, Germany). Probes were treated with DNase, extracted by ethanol precipitation, and used to examine gene expression in ascidian larval samples.The larvae were fixed in 4% paraformaldehyde (PFA)in 0.5 mol L-1NaCl-MOPS (pH 7.0)buffer overnight at 4℃and then permeabilized by digested with 12 μg mL-1Proteinase K at 37℃ for 30 min. Subsequently,in situhybridization was performed at 56℃ with 1 ng μL-1antisense or sense Digoxigenin-11-UTP labeled probes. After hybridization, samples were washed in gradient saline-sodium citrate at the hybridization temperature. Finally, samples were incubated with anti-DIG-Alkaline Phosphatase antibody at a 1:2000 dilution and developed with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (BCIP/NBT)(Roche, Germany).
2.4 Generating GFP Reporter Constructs and Transfering Genes with Electroporation
PCR-amplified DNA fragments from 5’ flanking sequence of geneCs-SMAwere cloned into pEGFP-C/N vectors using the One Step Cloning Kit (Vazyme, China)to produce reporter plasmidsCs-SMA(-3000 – -1 bp)> EGFP,Cs-SMA(-2000 – -1 bp)> EGFP,Cs-SMA(-1000 – -1 bp)> EGFP,Cs-SMA(-2000 – -1000 bp)> EGFP,Cs-SMA(-1350 –-1150 bp)> EGFP, respectively.Cs-SMA(ΔSix)> EGFP were generated by internal deletion of Six1/2 binding motif on the base ofCs-SMA(-2000 – -1000 bp)> EGFP. PCRamplified DNA fragments from 5’ flanking sequence of geneSix1/2with specific primers were cloned into pEGFP-C/N vectors using the One Step Cloning Kit (Vazyme, China)to produce plasmidsCs-Six1/2(-3000 – -1 bp)> EGFP. Then tdtomato replaced EGFP to generate theCs-Six1/2(-3000– -1 bp)> tdtomato. PlasmidsCs-Mesp> tdtomato was constructed using the same way as above. All the primers used were listed in Table 1.
Eggs and sperm were obtained surgically from several adult gonad ducts and mixed for 5 min. Then the fertilized eggs were dechorionated in seawater with 1% sodium thioglycolate (Sigma, America)and 0.5% proteinase E (Sigma,America)and reared at 18℃ in Millipore-filtered seawater in agar-coated dishes for further development. For electroporation, 300 μL eggs were mixed with 500 μL mixture of 0.77 mol L-1mannitol (Sigma, America)and 30 μg DNA in 0.4 cm electroporation cuvette. After electroporation at 2000 μF capacitance, 50 V voltage with Gene Pulser XcellTM(Bio-Rad Laboratories), the eggs were immediately resuspended in fresh seawater and cultured for development.
3 Results
3.1 Identification of Actin Encoding Genes in C. savignyi
Based on the previously constructed cDNA libraries and the sequenced transcriptome fromC. savignyi, we identi-fied three conventional actin isoforms and nine ARPs by sequence blast. Phylogenetic analysis was performed to define evolutionary relationships of these identified actin isoforms and ARPs amongC. savignyiand other fully sequenced organisms, includingH. sapiens,X. laevis,D. rerio,D. melanogaster,C. intestinalis,B. belcheri,C. elegansandA.thaliana. The results showed that the actin superfamily contained at least nine subfamilies including the conventional actin subfamily and ARP subfamilies (Fig.1). At least one protein was characterized inC. savignyifor most of subfamilies, but no transcript were identified for Arp5 and Arp8 subfamily. Six ARPs encoding genes were grouped into Arp1, Arp2, Arp3, Arp4, Arp6 and Arp10 subfamilies,respectively. Three additional ‘orphan’ ARPs were identified,which were not grouped into any subfamilies. Two conventional actin encoding genes were grouped into chordate muscle actin, and one was grouped with cytoplasmic actin. The molecular phylogenetic analyses showed that the muscle actin isoforms from vertebrates were more closely related to each other than to their counterparts from the ascidians.
3.2 Characterization of C. savignyi Muscle Actin Genes
The actin family is an ancient and highly conserved group of proteins (Goodson and Hawse, 2002). Typing actin mostly relies on the length and highly variable sequence of the N-terminal regions among actin isoforms and in different species, and comparison of the diagnostic amino acids (Vandekerckhove and Weber, 1978c, 1978a, 1978b, 1981, 1984).We aligned the predicted amino acid sequence of two ascidian muscle actin isoforms against actins from other metazoans. The alignment suggested thatthey were more closely related to zebrafish and human cardiac and skeletal muscle actin with more than 96% identity. The alignment of N-terminal amino acid sequences of muscle actin from ascidians and other chordates reveals thatCionamuscle actin isoforms contain the characteristics of cardiac and skeletal muscle actin. These twoCionamuscle actin isoforms represented a cluster of more than four acidic amino acids(Glu)following the first two amino acids in the very end of N-terminus, similar to their counterparts in other species.However, they were distinct from the cytoplasmic actin characterized by no more than three successive acidic amino acids next to the first Met residue. In addition, muscle actin isoforms are different from smooth muscle actin by adopting Leu and Val at positions 19 and 20 (Fig.2), rather than Leu andc Cys in smooth muscle actin. The actin isoform II was similar to muscle actin expressed in the tail region of the ascidians based on the identical diagnostic residues (Table 2). In contrast, the isoform I distinguished it from tail muscle actin by different amino acids at position 103 and 176. It showed high similarity withC. intestinalismuscle actin 2 which is highly expressed in the adult heart and also in the body-wall muscle (Chibaet al., 2003). Therefore, we mainly focused on the muscle actin isoform I and aimed to uncover its expression pattern.
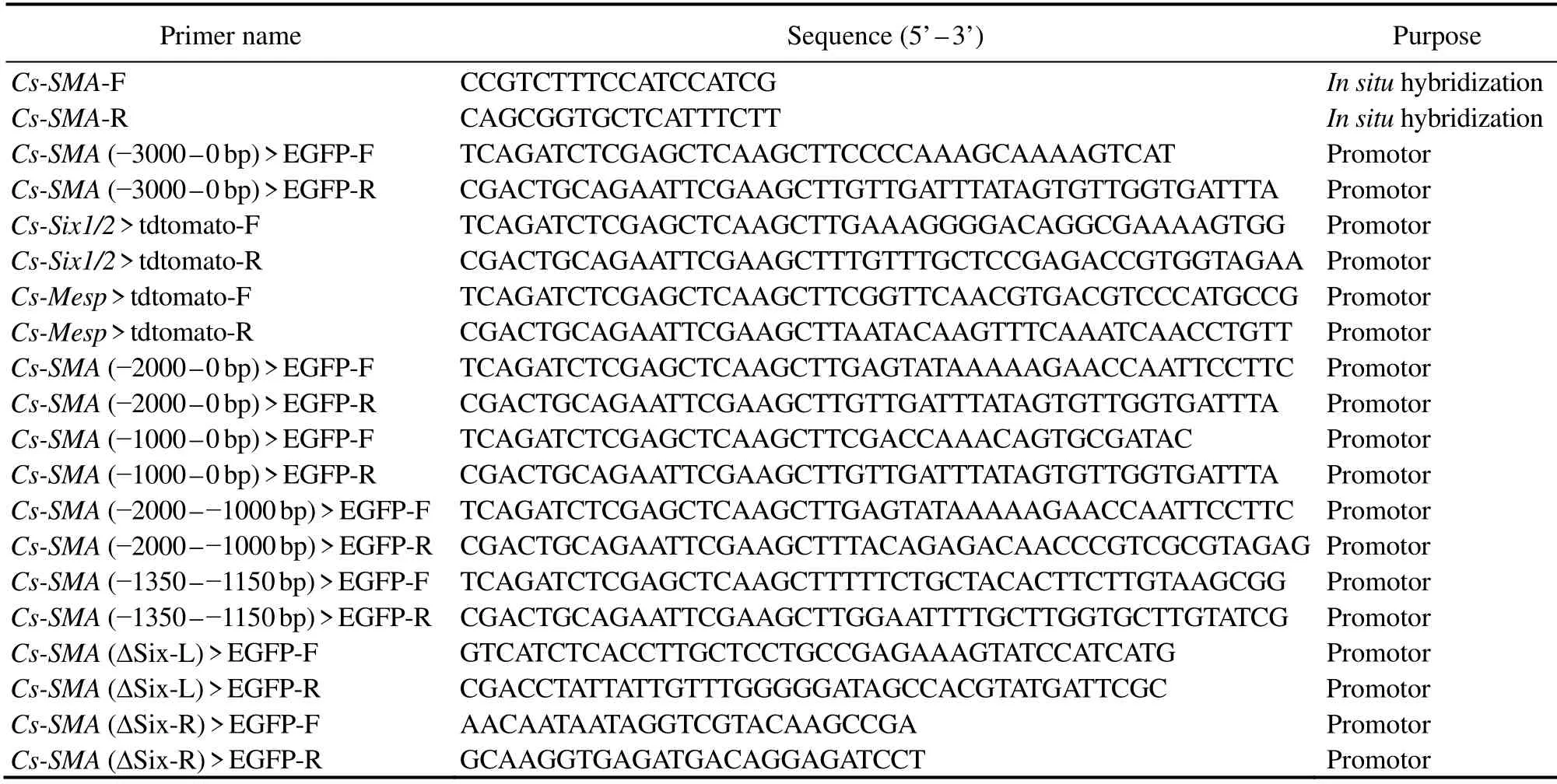
Table 1 Primers used in this study
3.3 Expression of C. savignyi Muscle Actin Isoform I Was Confined at the Oral and Atrial Siphon
To examine the temporal and spatial expression ofC.savignyimuscle actin isoform I, we performedin situhybridization to detect its expression pattern in theCionalarva. The expression ofC. savignyimuscle actin isoform I appeared as early as 22 hpf at the anterior and posterior trunk inCionalarvae in accordance with the location of oral and atrial siphon primordium, respectively (Fig.3A). At 26 hpf, muscle actin isoform I was clearly detected to express at the primordia of one oral siphon and two contralateral atrial siphons of the trunk when the siphon placodes began to invaginate (Fig.3B). After metamorphosis, the larvae tails were retracted and the siphon primordium formed tubular structure for feeding, whenC. savignyimuscle actin isoform I was detected at the oral and two converging atrial siphons (Fig.3C). Based on thein situhybridization data,we concluded that the muscle actin isoform 1 expressed at the oral and atrial siphon primordia and we hereby defined it as siphon-specific muscle actin coding gene (Cs-SMA).
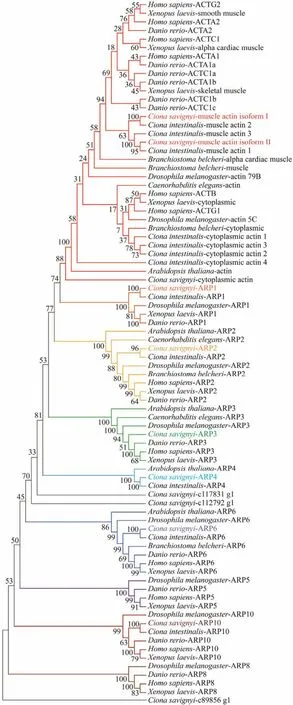
Fig.1 Phylogenetic analysis of actin proteins and actin related proteins from vertebrates and invertebrates. Full lengths of actin protein sequences were aligned using the ClustalW program. The bootstrap test of phylogeny was performed with 1000 replicates to construct phylogenetic tree with neighbor-joining (NJ)method of MEGAX. The actin proteins and actin related proteins identified from C. savignyi are shown in different color.
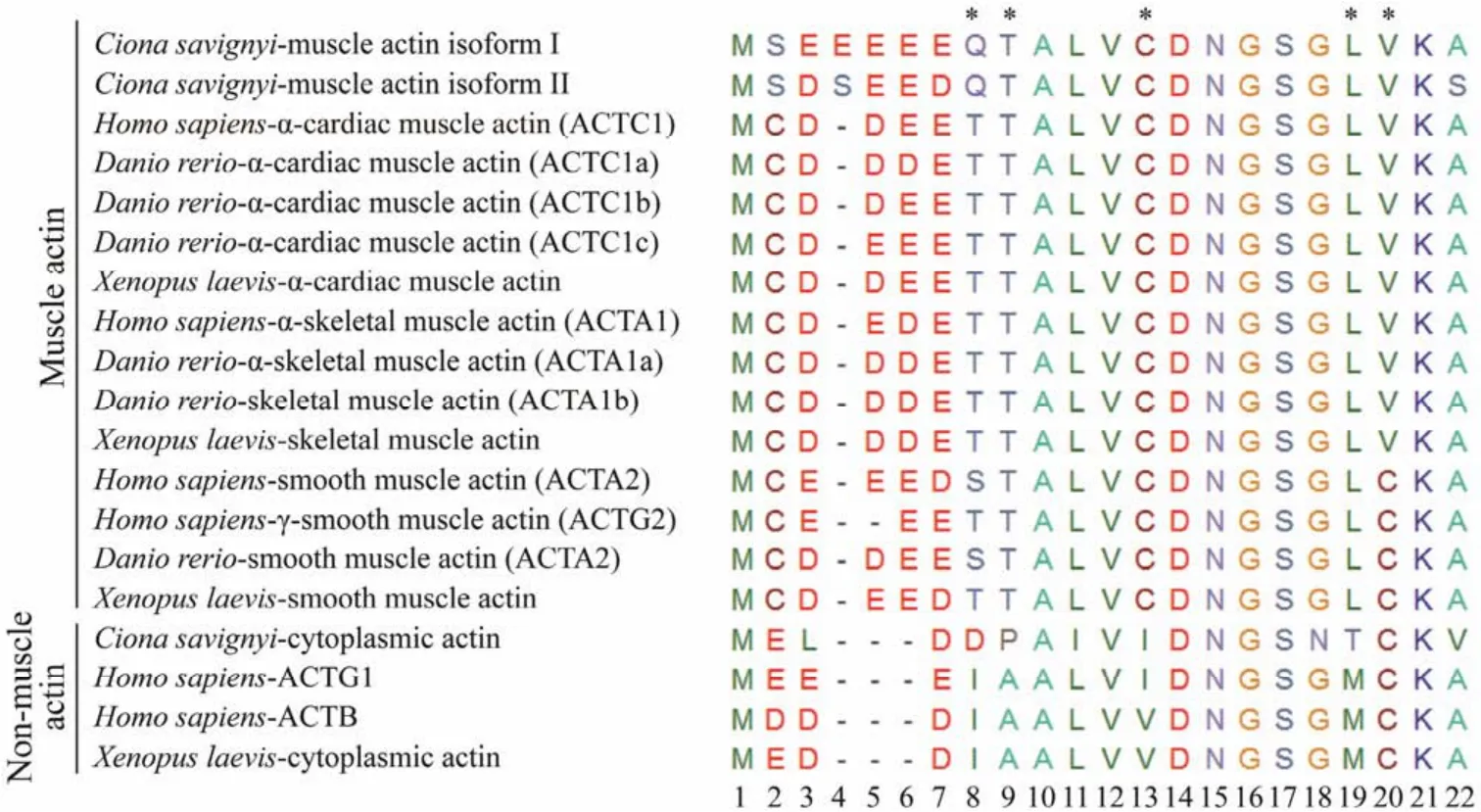
Fig.2 N-terminal sequence alignment of multiple isoforms of actin from different species. Three types of actin are from C.Savignyi (muscle actin isoform I, muscle actin isoform II, and cytoplasmic actin); six from H. sapiens (ACTC1, ACTA1,ACTA2, ACTG2, ACTG1, ACTB); six from D. rerio (ACTC1a, ACTA1a, ACTC1b, ACTA1b, ACTA2, ACTC1c); and four from X. laevis (alpha cardiac muscle actin, smooth muscle actin, skeletal muscle actin, cytoplasmic actin). Amino acids are represented by a single letter code. Dashes represent gaps introduced into the sequence to optimize alignment. Diagnostic amino acids that distinguish the muscle actin from cytoplasmic actin are indicated by asterisks. The order numbers indicate the positions of amino acids from the beginning.
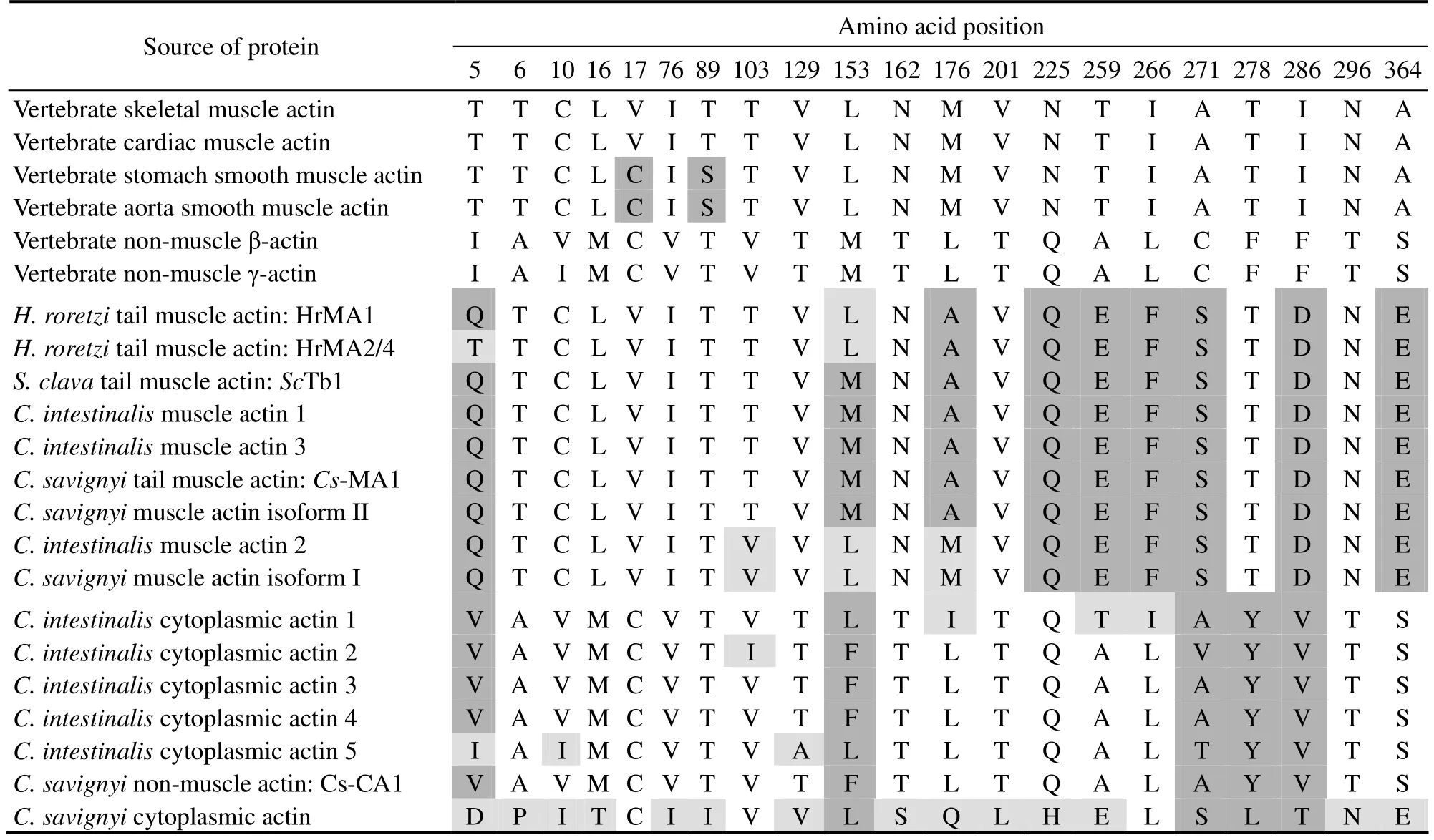
Table 2 Comparison of diagnostic amino acid residues in ascidian and vertebrate actins
To further confirm the expression ofCs-SMAinCionalarvae, we carried out the promoter driven GFP fusion constructs assay. The proximal upstream flanking regions of theCs-SMA, including -3000 – -1 bp and -5000 – -1 bp, were fused to GFP and transferred into the fertilized eggs electroporation. We found that -3000 – -1 bp fragment was efficient to convey specific expression. In order to further confirm the expression pattern ofCs-SMA, we co-electroporatedCs-SMApromoterreporter with two known transcription factorsSix1/2andMesppromoterreporter, which express at the oral siphon primordium and atrial siphon precursor cells that share the common cellular origin with cardiac primordium, respectively (Mazetet al., 2005; Abituaet al., 2015; Liu and Satou, 2019). The expression domains overlapped betweenCs-SMAand the transcription factorsSix1/2andMesp(Figs.4A, B).Taken together, we concluded thatCs-SMAis expressed at the primordia of oral and atrial siphon.
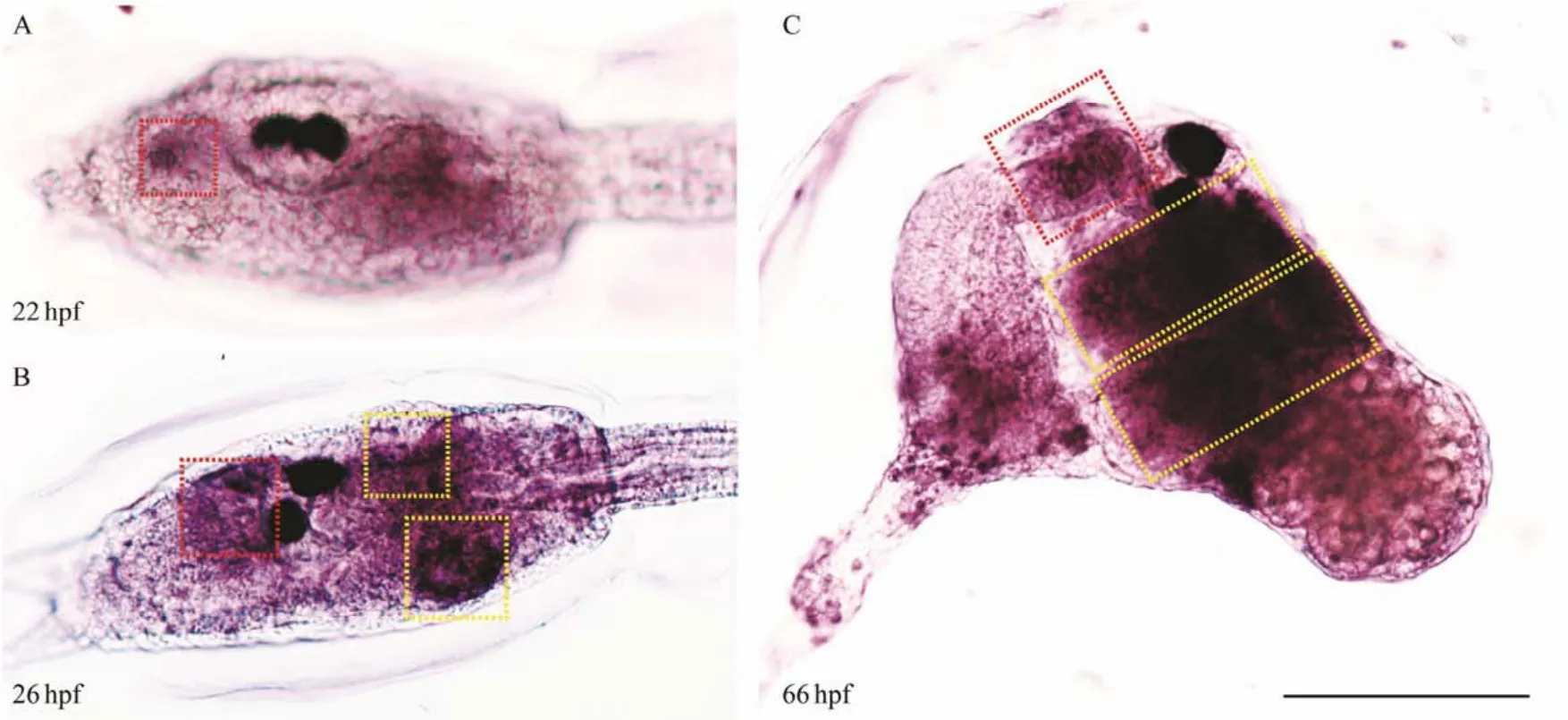
Fig.3 Expression patterns of Cs-SMA in Ciona larvae and juveniles. The expression of Cs-SMA possibly appears in the oral siphon at 22 hpf (A). The expression of Cs-SMA shows in the invaginating oral and atrial siphon at 26 hpf (B). The expression of Cs-SMA shows in the tubular oral and atrial siphon at 66 hpf (C). Red box indicates the oral siphon and yellow box indicates the atrial siphon. Scale bar represented 100 μm.
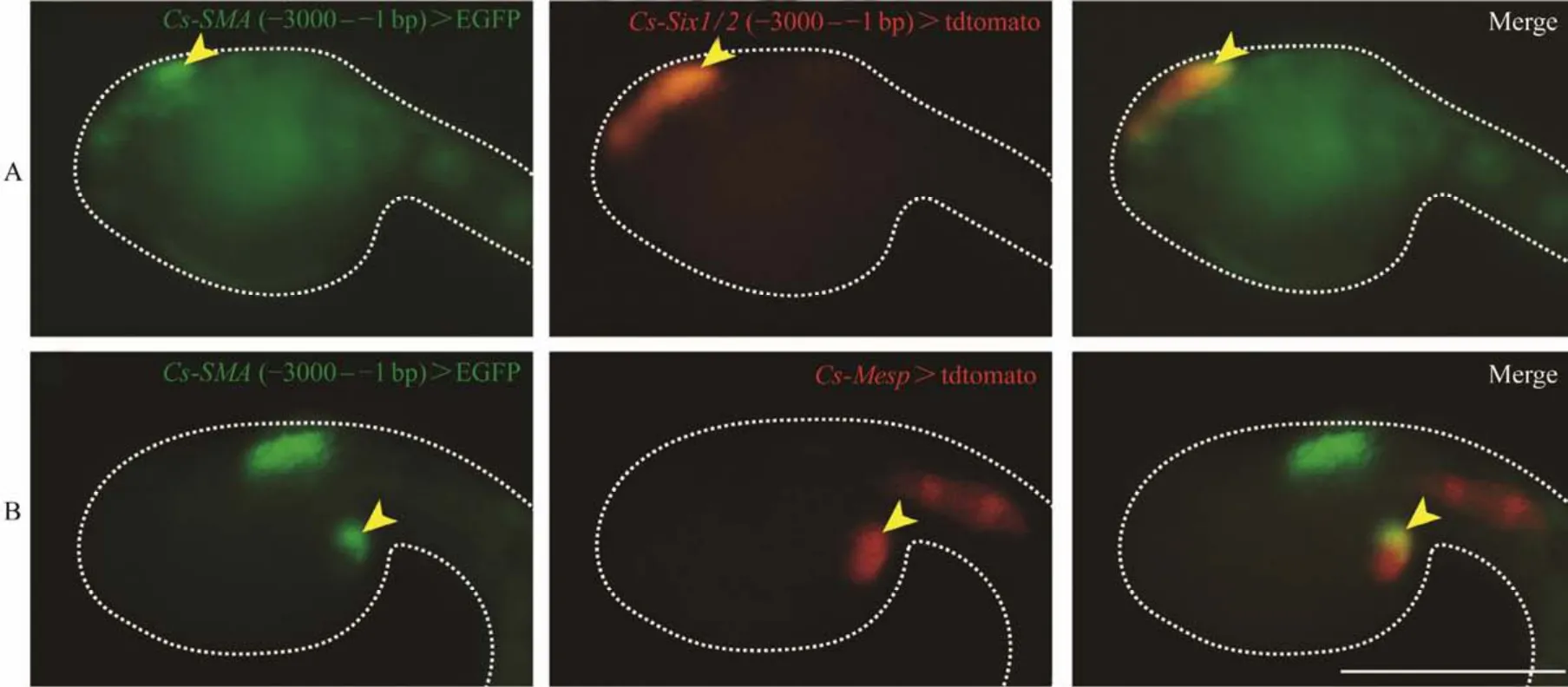
Fig.4 Expression of Cs-SMA overlapped with Six1/2 and Mesp. (A)Both of the Cs-SMA and Six1/2 expressed at the oral siphon detected by GFP reporter, when Cs-SMA (-3000 – -1 bp)> EGFP and Cs-Six1/2 (-3000 – -1 bp)> tdtomato co-electroporated into the embryos. (B)The expression of Cs-SMA and Mesp overlapped at cardiac primordium which generates atrial siphon. when Cs-SMA (-3000 – -1 bp)> EGFP and Cs-Mesp > tdtomato co-electroporated into the embryos. The different expression pattern of Cs-SMA driven by promoter sequence -3000 – -1 bp are caused by mosaic development of ascidians. Figs.A and B represent two typical expression patterns, i.e., signal at the oral siphon or at the primordium of atrial siphon. Scale bar represents 100 μm.
3.4 Siphon-Specific Expression of Cs-SMA Is Under the Control of Six1/2
Computational prediction with JASPAR database of the effective transcription factor binding profiles from 0 to-3000 bp upstream ofCs-SMAshowed that it contained duplicate muscle related cis-regulatory elements such as E-box, T-box and Six1/2 binding motif. To analyze the core cis-regulatory region, we constructed a series of truncated promoters by progressively deleting 1000 bp. It was worth noticing that we kept at least one copy of the above cis-regulatory element in the truncated promoters. The -2000 –-1 bp sequence drove the expression of GFP reporter gene at the oral or atrial siphon primordia as the same level as the -3000 – -1 bp sequence but was higher than the -1000– -1 bp sequence (Figs.5B – D). Sequence -2000 – -1000 bp was confirmed to play fundamental roles in driving the expression ofCs-SMA(Fig.5E). Three conserved cis-regulatory elements, the classical muscle determinants E-box,T-box, and the Six1/2 binding motif were present in the sequence -2000 – -1000 bp. A truncated 201 bp sequence containing Six1/2 motif and T-box could drive the GFP expression at oral or atrial siphon at relatively low level (Fig.5F).In contrast, an internal deletion of Six1/2 motif from the promoter region -2000 – -1000 bp caused a specific loss of expression at siphons (Fig.5G). These results indicated that Six1/2 was necessary in controlling the siphon-specific expression ofCs-SMA.
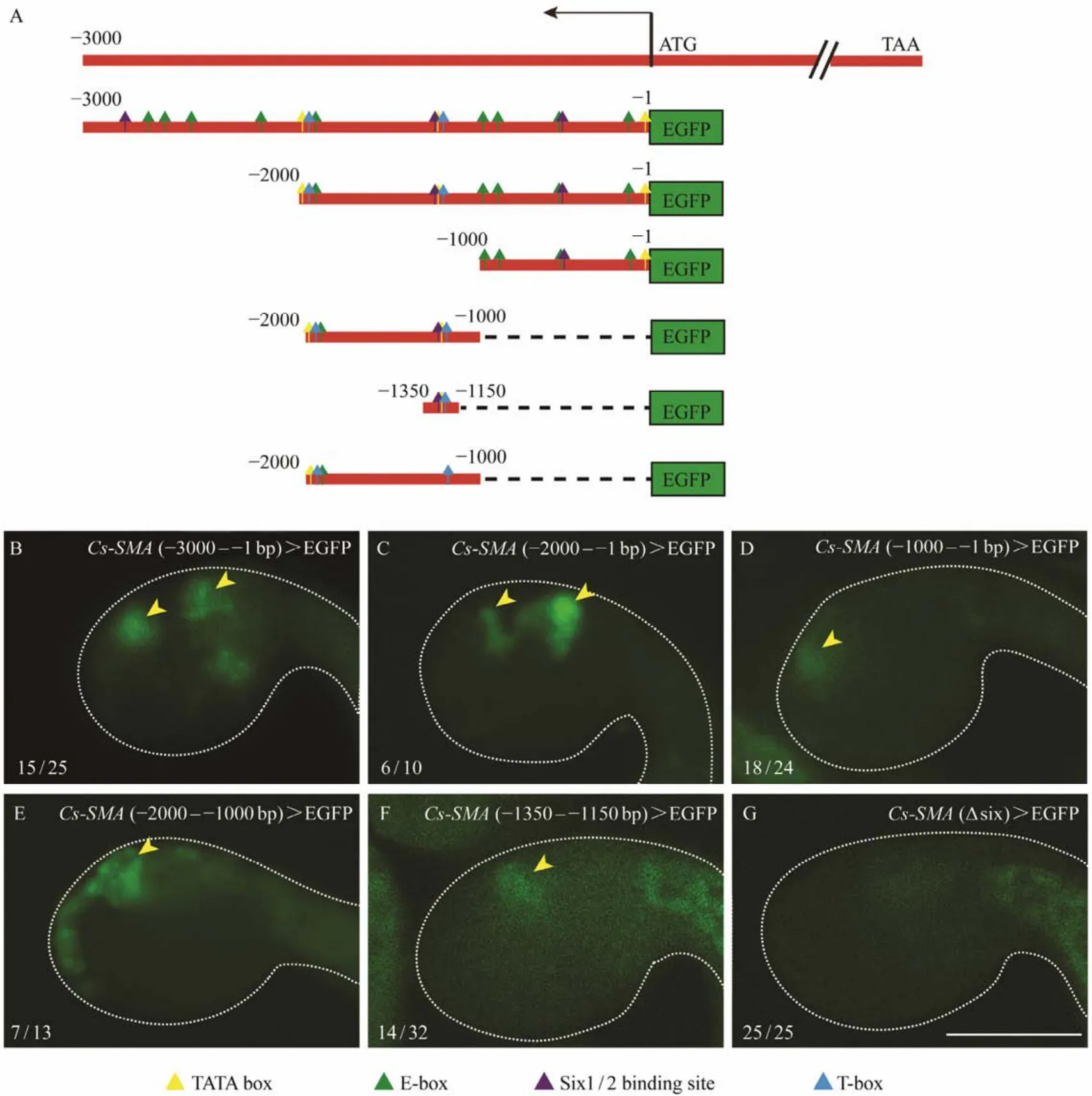
Fig.5 Expression patterns of Cs-SMA driven by different promoter truncations. (A)Schematic diagrams of truncated upstream sequences containing duplicate cis-elements. (B)GFP expression driven by promoter -3000 – -1 bp. 15/25 indicates 15 embryos represent GFP signal out of 25 embryos counted. (C)GFP expression driven by promoter -2000 – -1 bp, 6 out of 10 embryos show GFP signal. (D)GFP expression driven by promoter -1000 – -1 bp, 18 out of 24 embryos show GFP signal. (E)GFP expression driven by promoter -2000 – -1000 bp, 7 out of 13 embryos show GFP signal. (F)GFP expression driven by promoter -1350 – -1150 bp, 14 out of 32 embryos show GFP signal. (G)GFP expression driven by promoter-1350 – -1150 bp with internal deletion of Six1/2 binding site. No GFP expression in the trunk of the embryo out of 25 embryos counted. Arrowhead indicates the location of siphons. The GFP signals in the tail of embryos (F and G)are autofluorescence from tail muscle. Scale bar represent 100 μm.
4 Discussion
4.1 Evolution and Typing of Actin Genes Within a Multigene Family
Actin, like many contractile protein genes, is encoded by a multigene family. It was postulated that an ancestral actin gene duplicated and gave rise to a cytoplasmic isoform and a muscle isoform (Koviluret al., 1993; Kusakabeet al.,1999; Chibaet al., 2003). The muscle isoform underwent a second duplication resulting in striated muscle actin (skeletal and cardiac)and smooth muscle actin, respectively(Miwaet al., 1991). Our evolutionary analysis for actin subfamily based on the construction of phylogenetic tree, as well as the comparison of N-terminal sequences and diagnostic residues of the encoded proteins, suggested that the muscle and non-muscle subfamily arose by divergence prior to the vertebrate origin from a single common ancestral gene. The muscle actin isoforms from subphylum urochordate grouped together and were distinct from vertebrates,and so did muscle actins from cephalochordate. These suggested that vertebrate muscle actin gene family was established after the divergence of the vertebrate and urochordate and cephalochordate lineages.
Ascidians present three muscle actin types and the expression of each isoform characterizes a specific developmental stage or tissue during their life cycle: striated muscle actin in the tail, striated muscle actin in the adult heart,and non-striated muscle (commonly called smooth muscle)actin in the adult body-wall (Shinohara and Konishi,1982; Nevitt and Gilly, 1986; Terakado and Obinata, 1987;Crowther and Whittaker, 1996; Chibaet al., 2003). The deduced three actin isoforms ofC. savignyifall to the striated muscle actin group and cytoplasmic actin group, respectively. The striated muscle actin isoform II was suspected to express in the tail region based on its high expression level (700 times more thanCs-SMA)and the composition of diagnostic amino acids (Table 1). Given its location, locomotory function, and ultrastructure, ascidian larval tail muscle actin is clearly homologous to vertebrate skeletal muscle actin (Meedel, 1997). Cs-SMA distinguished it clearly from cytoplasmic actin by the number of acidic amino acids and amino acid composition in N-terminal sequence(Fig.2). It presents high similarity withC. intestinalismuscle actin 2 which is almost exclusively expressed in the adult heart and also in adult body-wall muscle based on the comparison of diagnostic amino acids (Table 1)(Chibaet al.,2003). The muscle actin genes expressed in larval or adult heart muscle seem to be different from those expressed in the larval tail muscle (Kusakabe, 1997). Our results showed that Cs-SMA was clearly distinct from the tail muscle actin based on the comparison of diagnostic residues and the expression pattern. We did not identify any smooth muscle actin, perhaps because the transcriptome database ofC.savignyiwas from embryos before 42 hpf when they did not develop any adult muscle actin. The study of the different musculatures during the complete cycle of ascidians can be of interest from developmental and evolutionary perspectives, especially considering that the tunicates are the closest group to vertebrates (Delsucet al., 2006).
ARPs, together with conventional actin, belong to the ancient and divergent actin superfamily (Frankel and Mooseker, 1996). All proteins in this superfamily form four conserved actin-like subdomains and contain a five-stranded beta sheet of identical topology, suggesting that the molecules may have evolved by gene duplication (Kabsch and Holmes, 1995). ARPs are clearly divergent from each other though the subfamilies of ARPs are conserved and can be recognizable among all eukaryotes (Mulleret al., 2005).In this study, seven ARPs encoding genes fromC. savignyilarvae were grouped into Arp1, Arp2, Arp3, Arp4, Arp6 and Arp10 subfamilies but no gene was identified for Arp5 and Arp8 subfamily. Previous studies have revealed that Arp5 and Arp8 are essential for chromatin remodeling including DNA binding, nucleosome mobilization and ATPase activity (Shenet al., 2003), so whether such activities were undertaken by other members of ARP subfamily needs further experiments to study. Three additional ‘orphan’ ARPs did not belong to any of these subfamilies, which suggested that there might be some unclassified ARPs whose features and functions have not yet been explored (Schafer and Schroer, 1999; Machesky and May, 2001).
4.2 Confined Expression of Cs-SMA in C. savignyi
Analysis of the effective promotor region from 0 to -3000 bp upstream ofCs-SMAshowed that it contains duplicate muscle determinant cis-regulatory elements such as TATAbox, E-box, T-box and six1/2 binding motif, which may explain why sequence -2000 – -1000 bp was sufficient to direct the expression ofCs-SMA. It is conceivable that a basic helix-loop-helix (bHLH)regulatory factor, such as MyoD family, recognizes the E-box motif to induct the muscle transcriptional program. InCionaspp.,Mrfis the sole member of the MyoD family and is required for larval tail muscle development (Meedelet al., 2007). In the current study, a 201 bp sequence without Mrf binding motif E-box did not hamper the expression pattern ofCs-SMA,indicating that E-box is dispensable in controlling siphonspecific muscle actin expression in the early development embryos. This is consistent with the expression pattern ofMrf, whose transcripts are restricted to larval tail muscle precursors but not detectable in the cardiac lineage prior to settlement (Razy-Krajkaet al., 2014). The lower level expression driven by the 201 bp promoter (-1350 – -1000 bp)may be the missing of fragment (-2000 – -1351 bp)compared with expression level driven by the promoter-2000 – -1000 bp. A further validation was needed to find out the functional elements.
Six family consists ofSix1/2,Six3/6andSix4/5subfamilies. In deuterostomes, both Six1/2 and Six3/4 members and their cofactor Eya are involved in the formation of the myogenic lineage (Carmenet al., 2015). The Six-Eya transcription complex works in parallel with Pax3 and Pax7 factors which are expressed in muscle satellite cells and share with vertebrates a conserved role in muscle formation (Carmenet al., 2015). InC. intestinalis, the expression ofSix1/2was confined to oral siphon progenitor cells in mid-tailbud embryos, while its expression was activated in endoderm and in several lateral ectodermal domains in late-tailbud embryos. In larvae, the expression ofSix1/2could be detected in the invaginated oral siphon and atrial siphon primordia (Mazetet al., 2005). The confined expression ofCs-SMAat the oral and atrial siphon under the 201 bp promoter mostly depends on the existence of Six1/2 binding motif.As Six1/2 showed the same expression pattern ofCs-SMA,we propose that Six1/2 is the main trans-acting factor controllingCs-SMAexpression.
5 Conclusions
As shown in the present study, a siphon-specific muscle actin coding gene (Cs-SMA)was identified from the RNA-seq data ofCiona savignyiembryos. It had high similarity with vertebrate cardiac and skeletal muscle actin.Cs-SMAis expressed at the primordia of oral and atrial siphon. And Six1/2 was necessary in controlling the siphonspecific expression ofCs-SMA.
Acknowledgements
This research was funded by the National Key Research and Development Program of China (Nos. 2019YFE019 0900, 2018YFD0900705).
杂志排行
Journal of Ocean University of China的其它文章
- Comparison of Flavor Substances in Dried Shrimp Products Processed by Litopenaeus Vannamei from Two Aquaculture Patterns
- Identifying Summer/Autumn Habitat Hotspots of Jumbo Flying Squid (Dosidicus gigas)off Chile
- Allelopathic Interactions Between the Tropical Macrophyte Enhalus acoroides and Epibenthic HAB Dinoflagellate Prorocentrum concavum
- Removal of Arsenic from Chlamys farreri with Different Methods
- Optimal Culture Capacity of White Shrimp (Litopenaeus vannamei)and Razor Clam (Sinonovacula constricta)in a New Series-Connection Culture Model
- Identification of Non-Coding RNAs Based on Alignment-Free Features in Crassostrea gigas (Pacific Oyster)Transcriptome
