Stable chlorine isotopic signatures and fractionation mechanism of groundwater in Anyang,China
2022-12-21XiaoxiaTongRongGanShuqianGuXingleSunKaituoHuangXiaofengYan
Xiao-xia Tong,Rong Gan,Shu-qian Gu,Xing-le Sun,Kai-tuo Huang,Xiao-feng Yan
1 China geological Environmental Monitoring Institute,Beijing 100081,China.
2 School of Water Conservancy Engineering,Zhengzhou University,Zhengzhou 450001,China.
3 Henan ProvincialGeological Environment PlanningDesign Co.,ltd,Zhengzhou 450001,China.
4 Henan Provincial Institute of Geological Environment Exploration,Zhengzhou 450001,China.
5 Henan Baoyuan Exploration Technology Co.,Ltd,Zhengzhou 476341,China.
Abstract: The present work provides an online Bench II-IRMS technique for the measurement of stable chlorine isotope ratio,which is used to measure the δ37Cl of 38 groundwater samples from the Karst and Quaternary aquifers in Anyang area.The regional distribution and signature of δ37Cl value are characterized on the base of isotopic data.The results suggest that the δ37Cl value of Quaternary groundwater decreases with increasing Cl- concentration,and has no correlation with δ18O and δD values,but closely correlates with the depth to water table.The fractionation mechanism of the chlorine isotope is expounded according to the type of groundwater.The δ37Cl value of karst water is generally positive,which is relevant to the dissolution of evaporite (gypsum mine),and may be caused by the mixing of groundwater and precipitation.The groundwater of Quaternary unconfined aquifer is mainly recharged by precipitation,and the δ37Cl value of groundwater is generally negative.The δ37Cl value of groundwater in Quaternary confined aquifer is more negative with increasing the depth to water level and elevated Cl- concentration,which is possible to result from the isotope fractionation of ion filtration.The groundwater with inorganic pollutants in Quaternary unconfined aquifer has generally a positive δ37Cl value.
Keywords: North China Plain;Groundwater;Gas Bench II-IRMS;Chlorine isotope;Karst aquifer
Introduction
Chloride is often used as a conservative tracer in catchment hydrology studies.Chlorine has two stable isotopes,35Cl and37Cl,whose abundance are 0.755 3 and 0.254 7,respectively,and which have a mass difference of 53.7‰.
In recent decades,many studies were on the analytical procedure of chlorine isotope,fractionation mechanisms and geochemical applications.Nier and Hanson (1936) used electrons to bombard HCl gas,and measured the ratio of37Cl/35Cl through the current intensity of generated1H37Cl+,1H35Cl+ion,with a measurement accuracy of 10‰.Kaufmann (1984) redesigned the sample preparation process based on Taylor’s (1969),developed gas mass spectrometry based on CH3Cl+,and then improved the determination accuracy of chlorine to ±0.24‰.Long made great improvements on the measurement process in 1993,and improved the determination accuracy to ±0.09‰ (Long et al.1993).Shouaka-stash used continuous flow isotope ratio mass spectrometry (CF-IRMS) to determine inorganic chlorine isotopes.He combined gas chromatography (GC) and gas isotope ratio mass spectrometry (Shouakar-Stash et al.2005) and greatly reduced the sample dosage (1.4 μmol);the determination accuracy reached ±0.07‰.
The study work on chlorine isotopes in China started in 1990s.Xiao developed a method of positive thermal ionization mass spectrometry based on Cs2Cl+in 1992 (Xiao and Zhang,1992).Xiao et al(1995) improved the analyzed method to increase the accuracy to ±0.09‰,and successfully applied to the research on salt lakes in Qaidam and Qinghai Lake (Zhang and Xiao,1993;Xiao et al.1994;Liu et al.1996;Liu et al.1998).In recent years,with the advancement of stable chlorine isotope fractionation and test methods,chlorine isotopes have been widely used in the research of evaporite characteristics and groundwater evolution,seawater intrusion,and mineralization fluid effects in mineral deposit formation,fractionation mechanism on chlorinated organic solvents,and organic tracing in synthetic and natural degradatives(Kaufmann et al.1993;Xiao et al.1997;Jendrzejewski et al.2001;Sie and Frape,2002;Numata et al.2002;Edmunds et al.2003;Shouakar-Stash et al.2003).It is because of the physical and chemical characteristics of chlorine that the isotope composition will not change with the element and form transformation.However,the great relative quality difference between37Cl and35Cl may result in isotope fractionation in immixtures,evaporation,diffusion and ion filtration.
In view of hydrogeologic setting in Anyang,the isotope fractionation caused by diffusion and filtration should not be significant for the karst aquifer,while it may occur in the Quaternary aquifer.The purposes of present study is to (1) characterize the distribution of chlorine isotopes in the groundwater of study area,(2) clarify the source of chlorine in groundwater through the combined use of hydrogeology,stable isotopes of water and chlorine,and(3) expound the fractionation mechanism of chlorine stable isotopes.
1 Materials and methods
1.1 Study area
Anyang is located in northern Henan Province,in which the western region is the foothills of Taihang Mountains and eastern region is a part of the North China Plain.Geologically,the study area is located at the junction of the Taihang Mountains fault block.And the Taihang fault block,Tangy in down warpand Neihuang uplift are successively distributed from west to east.Sedimentary strata have from older to younger,Cambrian systems,Ordovician systems,Carboniferous systems,Permian systems,Neogene systems and loose deposition in Quaternary systems.Magmatic complex rocks are widely distributed and mainly comprised of diorite,granodiorite,hornblende diorite,and quartzdiorite.These rocks changes from alkaline rock in the east to intermediate and basic in the west.Hanxing iron ore is found in the contact zones of limestone and magmatic rock.The iron ore body in this area is distributed in the contact zones of the neutral intrusive complex in the Yanshan and carbonatite in the Middle Ordovician.According to the statistical data of medium sized and larger iron deposit,the ore body total ore quantity in the contact zones accounts for more than 95%,and the interlayer ore is found in the tectonic zone (interlayer shuttered zone and stripping zone) in the carbonatite in the Middle Ordovician near the contact zones,which accounts for 3.0%.The third ore body can be found in the residual body and xenoliths in the contact zones,accounting for less than 1%.The gypsum mine is distributed in the Ordovician limestone.
1.2 Methods
1.2.1 Sampling
Total 38 samples of groundwater (well water),spring water,and canal water were sampled during the field geologic and hydrogeologic surveys in the Anyang area.The sampling sites are shown in Fig.1,which is also shown the details geological information of the study area.Groundwater were sampled in 50 mL bottle of HDPE for oxygen and hydrogen stable isotope measurement,and 500 mL bottle of HDPE for chlorine isotope.All bottles were washed 3 times with the sample water before filling samples.The samples were kept in the refrigerator waiting for measurement.
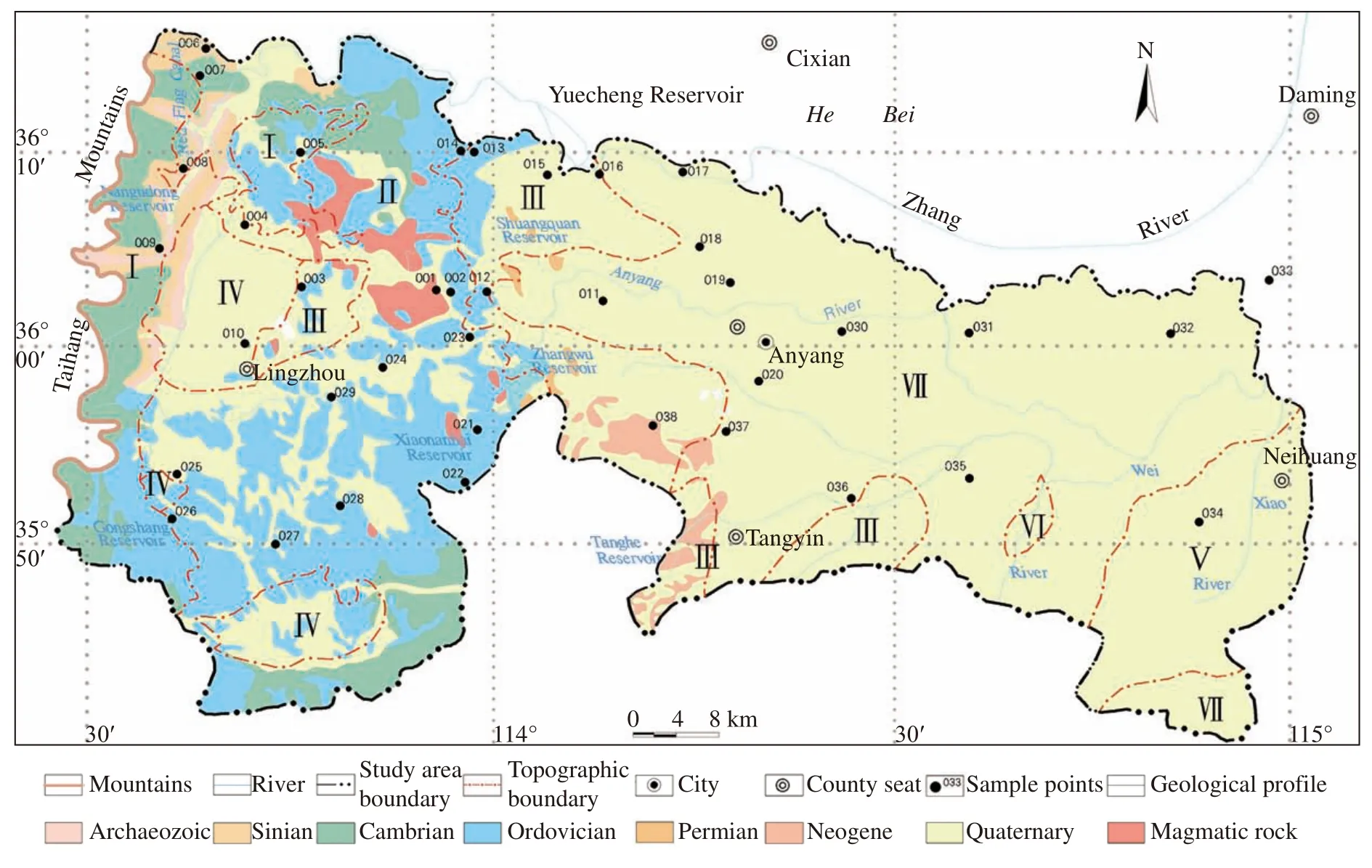
Fig.1 Geological map of the study area and distribution of sampling points
1.2.2 Measurement of oxygen and hydrogen stable isotope
The oxygen and hydrogen stable isotopes of groundwater were measured online using the method of heat transform element analysis isotope ratio mass spectrometry (TC/EA-IRMS) in the National Key Laboratory of Organisms and Environment Geology,China University of Geosciences (Wuhan).All water samples were filtered using a 0.45 μm PTFE Millipore filter before online analyzing.The instrument for sample measurement is stable gas isotope ratio mass spectrometry MAT253,and the determined accuracies of δ18O and δD were ±0.1‰and ±1.0‰,respectively.
1.2.3 Measurement of chlorine stable isotope
The analyses of chlorine isotopes include two processes: Preparation of samples and analysis on mass spectrometry.In the process of sample preparation,it is not only necessary to ensure the complete recovery of chloride ions in groundwater,but also to ensure that there is no fractionation of chlorine isotopes in the process of sample preparation.The different sample preparation methods depend on the different mass spectrometry;and this research use gas mass spectrometry based on CH3Cl+.Sample measurement was completed with Gas-Bench IIIRMS (Liu et al.2013) by the National Key Laboratory of Organisms Geology and Environment Geology in China University of Geosciences(Wuhan),the results showed in Table 1,and this method can realize online rapid analysis of samples,with less sample amount.
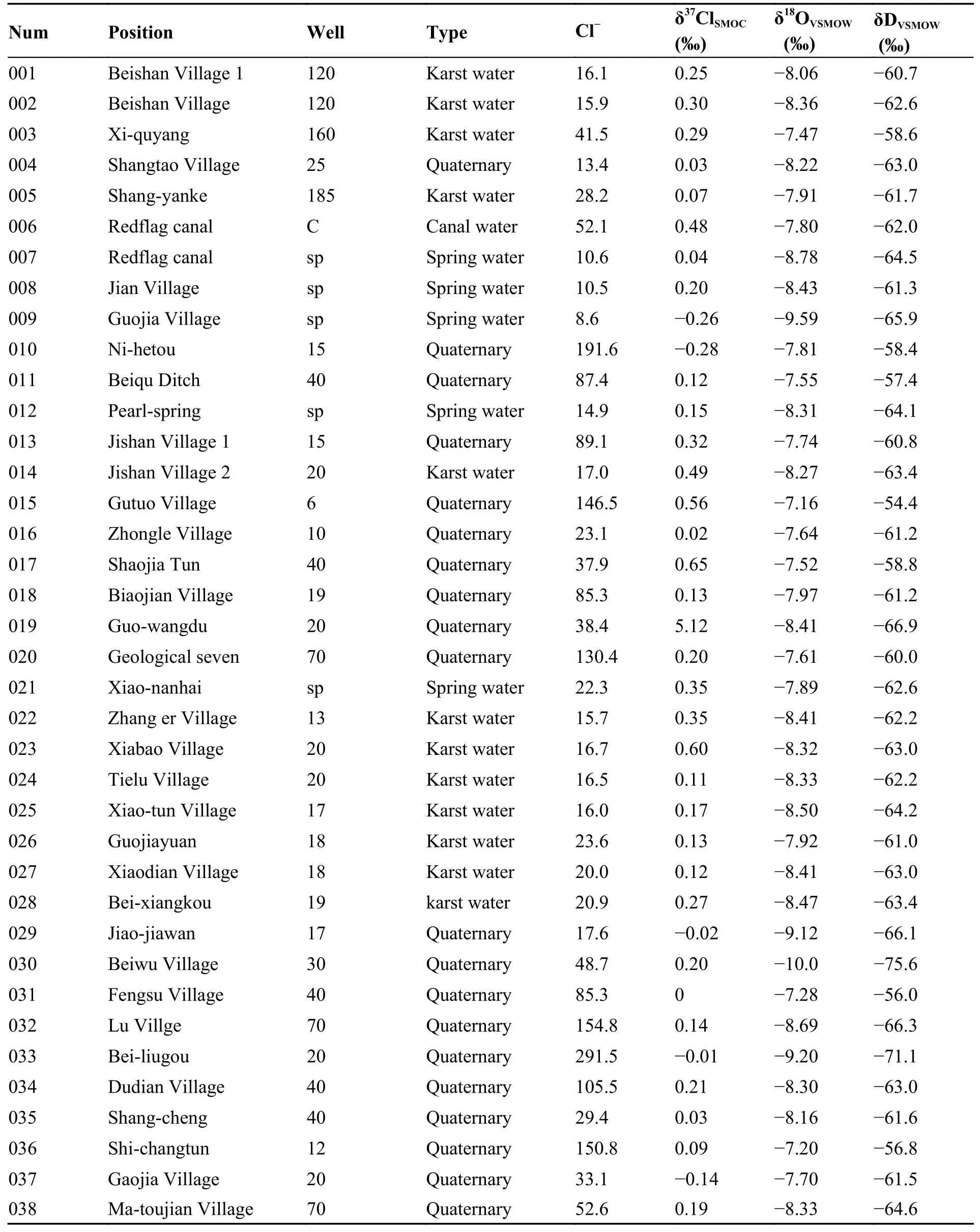
Table 1 Results of chemical compositions and isotope of groundwater in the Anyang area
1.2.3.1 Sample pretreatments
During sample pretreatments,the ideal water sample amount is related to the concentration of Cl-in water;generally,more than 3 mg of Cl-in water is needed.If the concentration is less than 3 mg/L,the water sample should be concentrated by evaporation under the condition of temperature less than 60°C.This process mainly includes the preparation of AgCl precipitation and the preparation of CH3Cl gas.
(1) The preparation of AgCl
a.Acidization of the water samples.Water samples were placed in a beaker,several drops of dense HNO3were added to make the pH<2,and the samples were heated slowly to slightly boiling for several minutes to expel the CO2in water.
b.Removal of SO42-.About 1mL of saturated solution of Ba(NO3)2was added and stirred with a glass rod,then slightly boiled and left to stand for 2 h.A drop of saturated solution of Ba(NO3)2was added to the supernatant for the complete precipitation of SO42-.Filtering was done after the BaSO4deposits,and the filtrate was kept.
c.Production of AgCl deposits.Excess AgNO3solution was slowly added to the water samples after pretreatment (the filtrate) to product AgCl deposit.The beaker was immediately encased with aluminum foil (tinfoil) to keep the AgCl from photolysis.The beaker was placed in the dark to stand for 2 h.After the solution was clarified,several drops of AgNO3solution were added and checked to confirm completely precipitation.This step was finished quickly to reduce the exposure time.
d.Purification and washing of the AgCl deposit.The AgCl deposit was washed with 0.001 mol/L diluted HNO3solution 3 times,and the supernatant was discharged.Excess dense NH4OH solution was added to the AgCl deposit to increase the pH to more than 10,and the solution was shaken slightly to make the deposit dissolve completely.The right amount of HNO3solution was slowly added to reduce the pH to less than 2,and the AgCl was precipitated again.The supernatant was discharged.This process was repeated 2-3 times,and AgCl deposits were continually purified.Finally,the deposit was washed with diluted HNO33 times.
e.Dry to reserve.The AgCl deposit was dried after purification at 40°C,stored in the dark and reserved.
(2) The preparation of CH3Cl gas
Under infrared lights,0.5 mg of AgCl was put into a thread mouth glass tube and then the super pure He was filled into the glass tube for 30 s to drive out the air.20 μL of CH3I was added into the tube immediately after stopping inlet He and the tube was sealed.
The glass tube was encased with aluminum foil(or tinfoil) and placed in an oven at 80°C for 48 h.The gas in the tube was the mixed gas producted by the reaction of CH3Cl and CH3I.The sample was removed,and a chlorine isotope measurement was performed in the mass spectrometer as soon as possible.If analyzing conditions were not acceptable,the sample was preserved in a refrigerator at 4°C to prevent isotope fractionation,which can affect the analysed result.PTFE/silica pads was used in the bottle cap to ensure tightness of the tube.
1.2.3.2 Principle of mass spectrometry method
Mass spectrometry analysis of chlorine isotopes was completed with stable gas isotope ratio mass spectrometry (MAT253) made in Germany by Finnigan.The principle of this method is that the mixed gas (CH3Cl and CH3I) is loaded into Gas-BenchⅡ,loaded into the GC pillar by switching the eightway-valves (VALCO),and then separated online.After separation,CH3Cl is dewatered and purified and is loaded into the ion source of isotope mass spectrometry through Open Split to receive ion current signals with two Faraday cups (m/z=50 and 52),which are converted to the37Cl/35Cl ratio.δ37Cl is conversed by comparing it to normal isotope37Cl/35Cl.The same sample is tested repeatedly,and δ37Cl is the average value.The international standard chlorine isotope material ISL-354 is taken as the analysis standard,and the accuracy of the chlorine isotope test method is ±0.08‰.
2 Results and discussions
2.1 Signatures and distribution of δ37Cl in waters
2.1.1 The chlorine isotopic signature of the Seawater
Seawater provides the largest and most uniform chlorine storage reservoir in the world and is the main source and sink of chlorine.Many researchers have confirmed that the chlorine isotope composition of global seawater is very consistent.Kanfmann et al.(1984) suggested using standard mean ocean chloride as the standard for stable chlorine isotope.However,the chlorine isotopic composition of seawater in different regions have minor differences.According to geographic location and atmospheric circulation,the chlorine isotope composition of the Bohai Sea,the Yellow Sea and the East China Sea may affect the chlorine isotope composition of atmospheric precipitation,surface water and groundwater in the Anyang area.Thus,the chlorine isotope composition of the Bohai Sea,the Yellow Sea and the East China Sea was measured in order to study the chlorine isotope composition of groundwater and trace the sources of chlorine in Anyang area.The measured values show close to 0.00‰and are listed in Table 2.

Table 2 The measured chlorine isotope values of the Bohai Sea and the Yellow Sea
2.1.2 Chlorine isotopic signature of precipitation
The precipitation is the main recharge source of groundwater and the carrier of chloride of sea water.Thus,it is vital to understand chlorine isotope signature and its variation of sea water.The largest source of Cl-in the atmosphere is marine aerosols,which enters the atmosphere through bubble breakage or tearing of the wave top (Duce,1998).In most cases,Cl-in the atmosphere may return to the Earth’s surface through wet and dry deposition.However,sea salt aerosols are changed by chemical reactions in the atmosphere,as proven by an increase in the Cl deficitWhere:Cl-volatilizes from NaCl to a gas containing Cl-.Most attention is given to HCl gas and potential Cl2、ClO-and ClNO2.The reactions of atmosphereinduced SOxor NOxand H2O product H2SO4or HNO3and HCl with NaCl in aerosols (Wagenbach et al.1998;Lightowlers and Cape,1988):
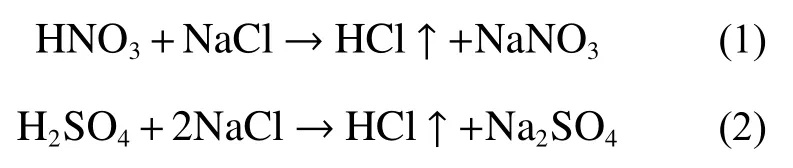
HCl gas dissolves into atmospheric precipitation due to its high solubility,and then fall onto the land surface.Thus,Cl is removed from the atmosphere after a short life of perhaps 1.5 days (Erickson et al.1999).Volpe and Spivack (1994) studied the Cl stable isotope composition of marine salt aerosol particles.The δ37Cl value of marine aerosol which was sampled from Bermuda showed to be positive with increasing Cl deficit in the samples.This suggested that the fractionation of Cl isotope in aerosols took place with volatilization,i.e.the salt aerosols are enrichment in37Cl (δ37Cl is positive),and HCl gas is enrichment in35Cl (δ35Cl is negative).Thus,in the volatilization experiment,the isotope fractionation is about -3‰ between HCl gasand saturated NaCl brine,which is similar to the value observed in sea aerosols.This indicates that acidification of sea salt aerosols and37Cl depletion resulting from the HCl production are most responsible for the fractionation.
The existing data indicate that the chlorine isotope signatures of coastal area or around salt lakes are positive values.For example,the δ37Cl value of rainwater is about +1.55% in the South China Sea,+1.61% in Xiamen,and +0.88% in Xining (Erickson et al.1999;Sun et al.2004;Xu and Sun,2001;Xiao et al.2001).However,this value is -4.07% (dry season) and -2.64% (wet season) (Lang et al.2008) in Guiyang city of Guizhou Province,where is far from the ocean.In Canada,the value is -3.5‰--1.2‰ in the Bonner Lake area,and -3.3‰-0.00‰ in the D-Espoir Gulf.The average value of these two places is about-3.5 ‰ (Koehler and Wassenaar,2010).These data indicate that δ37Cl of rainwater in the area far from the ocean is negative.Unfortunately,the δ37Cl value of rainwater in the study area was not measured,but δ37Cl value of groundwater is -0.26‰for the fissure spring in Guojiazhuang,Shibanyan town,and -0.28‰ for the Quaternary unconfined aquifer with the depth of water table about 10 m,respectively,which indicates that they are recharged by modern atmospheric rainfall and their δ37Cl value has beenaltered to be slightly positiveby the following processes (δ37Cl of modern atmospheric precipitation is approximately -4.67‰-+1.61‰ (Liu,2011)).
2.1.3 Chlorine isotopic signature of river water
In this study,one river water sample which was collected from the Qing-nian-dong of Hongqi Canal in 2010,has a value of δ37Cl = +0.48‰,and the other one which was sampled in the upper reaches of the Jia River in Yantai,Shandong Province in 1995,has a value of δ37Cl = +0.53‰.Xiao et al.(1997)reported the δ37Cl = +0.74‰ to +2.22‰ with a mean value of +1.35‰ for five rivers draining into Salt Lake in Qinghai,and +1.85‰ and+2.85‰ for two rivers in Qaidam Lake (Xiao et al.1994).Liu et al.(2008) measured the δ37Cl values of river water near Guiyang city and the results were +0.12‰ to +1.69‰ (during the dry period).Koehler and Wassenaar (2010) studied the relationship between Cl-and δ37Cl values in the Bow River and the Old Man River in Canada,and found the δ37Cl value increase with elevated Clconcentration (or decreasing 1/Cl-).They suggested that the increase in δ37Cl values was the result of mixing of earlier atmospheric rainfall (δ37Cl values of approximately -3‰) with more concentrated Cl-input sources later,or mixing of municipal wastewater (δ37Cl = 0.0‰ to +1.0‰).Volpe and Spivack (1994) suggested that the higher δ37Cl values of river may be caused by the atmospheric aerosol of high δ37Cl values.In summary,the δ37Cl values of river water are generally positive.The positive δ37Cl value of Hongqi canal water in this study area is thought to be caused by the mixing of atmospheric precipitation with a negative δ37Cl value and surface water or groundwater with a positive δ37Cl value.
2.1.4 Chlorine isotopic signature of groundwater
The δ37Cl value of groundwater in the Quaternary aquifers tends to be negative with elevated Cl-concentration along the flow path from west to east(Fig.1 and Fig.2).But there is no significant correlation between the δ37Cl values and Cl-concentration for the groundwater of karst aquifer.
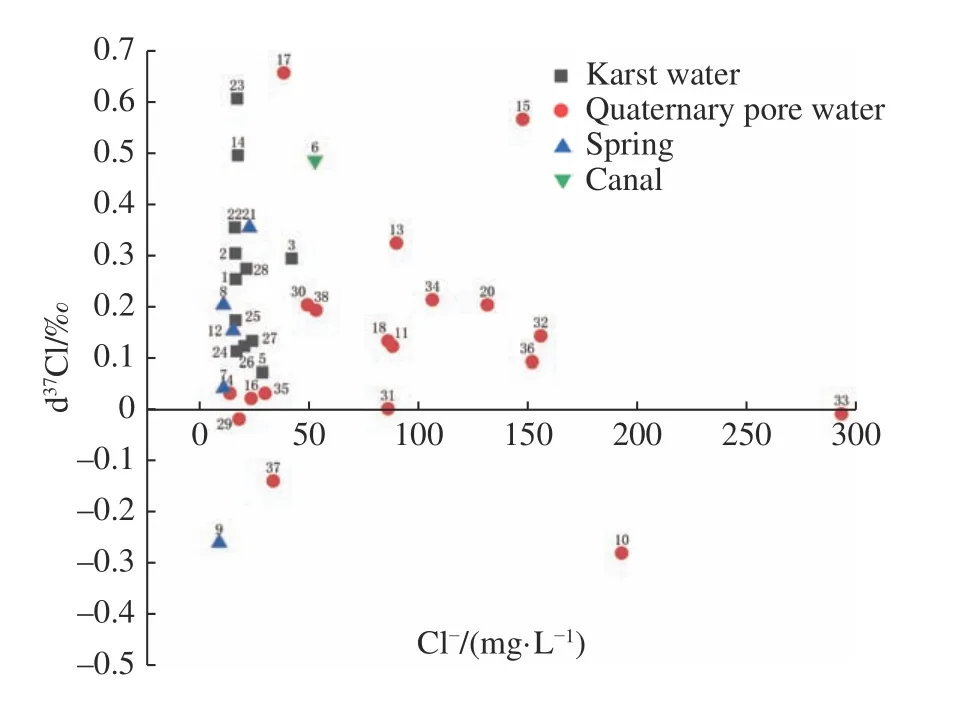
Fig.2 Correlation between groundwater Cl- concentration and δ37Cl values
The δ18O-δD plot of groundwater (Fig.3) shows that groundwater in the study area is recharged by precipitation and undergoes certain evaporation and concentration.However,the plot of δ37Cl-δ18OH2O(Fig.4) and δ37Cl-δDH2O(Fig.5) show that the δ37Cl values of groundwater do not correlate with the δ18OH2Oand δDH2Ovalues.This is mainly because the fractionation mechanism of δ18O and δD isotope is different from that of37Cl.Eastoe and Guilbert (1992) also found no significant relationship between δ37Cl and δ18O and δD values of water in their study of groundwater in the Gulf Basin,USA.
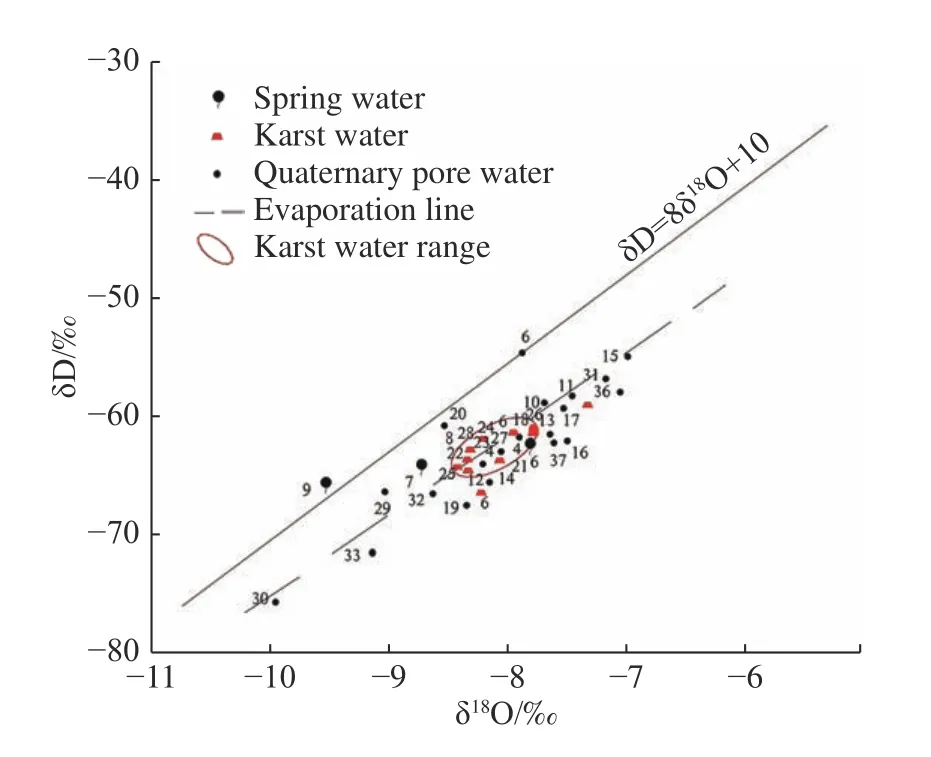
Fig.3 Relationship between δ18O and δD values of groundwater
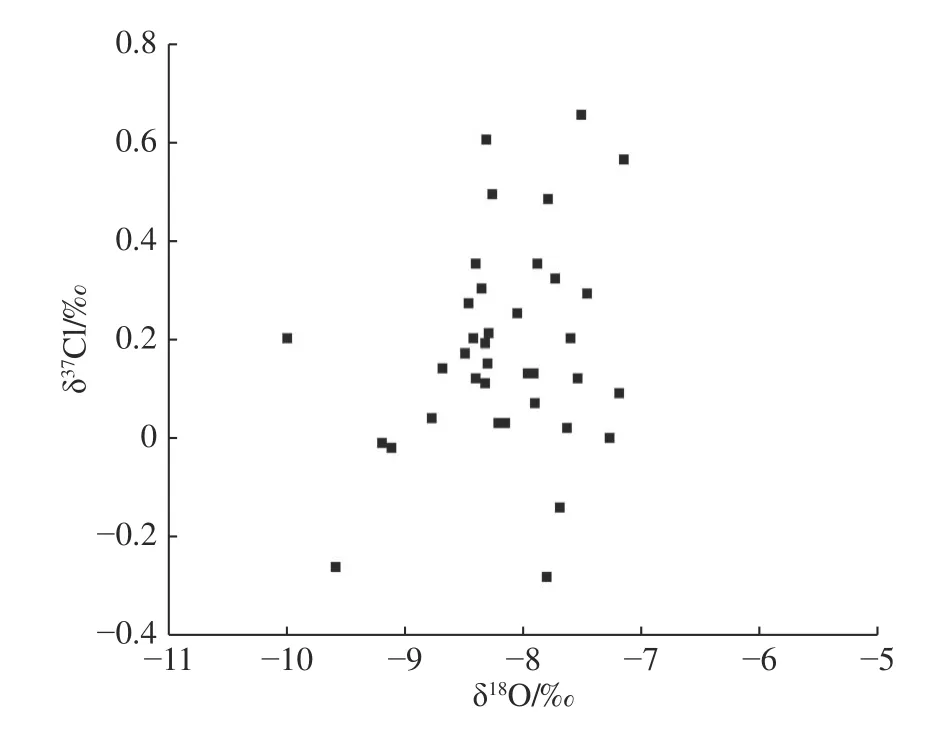
Fig.4 δ37Cl-δ18OH2O relationship
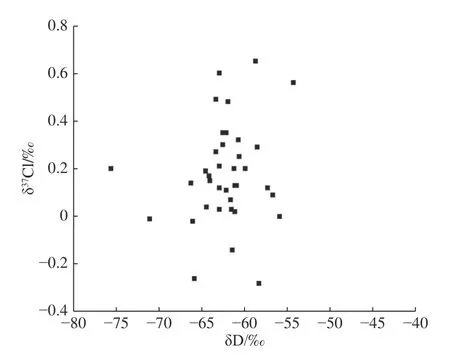
Fig.5 δ37Cl-δDH2O relationship
Karst water is mainly distributed in mountainous areas,and the burial depth is greater than 80 m.There is no correlation between the Cl-concentration and the burial depth of karst water,and the Clconcentration varies slightly with depth,from 10 mg/L to 30 mg/L (Fig.6 and Fig.7).The δ37Cl value of karst water is generally positive,but it varies greatly from +0.07‰ (ShangYanke) to+0.60‰ (XiaBao village).The δ37Cl values of karst water are also independent of depth.The springs recharged by karst water have low Cl-concentrations,14.9 mg/L for Pearl Spring and 22.3 mg/L for Xiaonanhai Spring,with δ37Cl values of+0.12‰ and +0.35‰,respectively,within the range of δ37Cl values of karst water.According to Liu et al.(2008),the δ37Cl values of karst water in the Guizhou area were positive in January 2002,from +0.17‰ and +1.34‰ during the dry season,and positive and negative in June 2002,from -1.46‰ to +0.29‰ during the wet season.The negative δ37Cl values of karst water in the Guiyang area may be related to the recharge of precipitation and the runoff conditions of karst development in wet season.In contrast,our study area is located in the northern China,where karst is not developed and groundwater runoff is relatively slow;thus,so far,no karst water has been found with negative δ37Cl values.The positive δ37Cl value of karst water in our study area is presumed to be caused by the mixing effect of precipitation and groundwater.The δ37Cl values and their distribution of groundwater in Quaternary aquifers show the following characteristics:

Fig.6 Relationship between groundwater Cl- ion concentration and burial depth
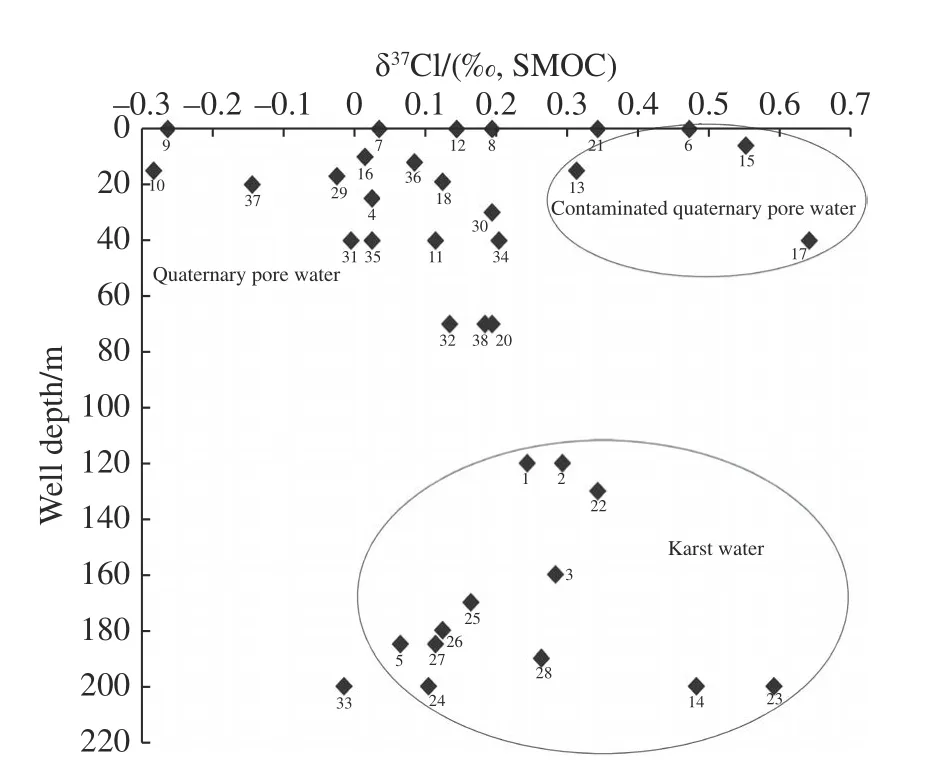
Fig.7 Relationship between groundwater δ37Cl value and burial depth
(1) The groundwater with buried depth less than 40 m is mostly from the unconfined aquifer which is recharged by precipitation,and has both positive and negative δ37Cl values.Generally,the value is negative in the western mountainous area;for example,groundwater in the site of sample 010,where the buried depth of groundwater is 15 m,has a δ37Cl=-0.28‰,and Cl-concentration of 191.6 mg/L,groundwater in Jiaojiawan,Hengshui,Linzhou (the site of sample 0029),where the buried depth of groundwater is 10 m,has a δ37Cl = -0.02‰,and Cl-concentration of 17.6 mg/L.Occasionally δ37Cl is close to 0.00‰,for example,in Shangtao village (sample site 040),the depth of groundwater is 10 m,and groundwater has a δ37Cl=+0.03‰ and the Cl-concentration of 13.4 mg/L.However,eastward to the plain area,the δ37Cl value of groundwater becomes positive,for example,in Beiqugou (site of sample 011),groundwater with buried depth of 40 m,has a δ37Cl = +0.12‰and Cl-concentration of 87.4 mg/L.In Beiwu village (site of sample 030),groundwater with buried depth of 30 m,has a δ37Cl = +0.20‰ and the Clconcentration of 48.7 mg/L.In Beijishan shallow well (sample 013),groundwater with buried depth of 15 m,has a δ37Cl = +0.32‰ and Cl-concentration of 89.1 mg/L.Theoetically,groundwater in Quaternary unconfined aquifer should have a negative δ37Cl valuethat is close to the value of precipitation,but most of these samples are positive,which may be the influence of atmospheric aerosols,pollution,diffusion or ion filtration.
(2) The contaminated groundwater in Quaternary aquifer has a more positive value of δ37Cl.Groundwater sample from shallow well with a depth 6 m (sample 015) in Gutuo Village,Lunzhang Town,has a Cl-concentration of 146.5 mg/L and δ37Cl = +0.56‰.Sample from well with a depth of 40 min Shaojiatun,An Feng Town (sample 017),is contaminated water with a Cr concentration of 33.3 ppb,and NO3-concentration of 34.087 mg/L,and has a δ37Cl = +0.65‰.Sample from a well with depth >100 m in Guo wangdu(sample 019) in Beijiao Township,is also contaminated with NO3-concentration of 19.93 mg/L,Cr concentration of 15.10 ppb,Zn concentration of 361 ppb,and has a δ37Cl = +5.12‰ and Cl-concentration of 22.34 mg/L,which is the most positive value of δ37Cl value in the study area.Therefore,the δ37Cl value of groundwater both in unconfined and confined aquifers is significantly affected by inorganic salt contaminants.
(3) The groundwater in Quaternary confined aquifer has a more negative value of δ37Cl.The Beiliugou well (site of sample 033) in Zhang Erzhuang Township,Weixian County,Hebei Province,is located in the easternmost part of the area,with a depth of 200 m,and penetrate the confined aquifer.Groundwater in this well has a δ37Cl = -0.01‰,δ18OH2O= -9.2‰,δD = -71.1‰ and Cl-concentration of 291.5 mg/L.The Lu Village well (site of sample 032),also located in the eastern part of this study area,is 70 m deep,and groundwater in this well has a δ37Cl = +0.14‰,δ18OH2O= -8.69‰,δD = -66.3‰ and Cl-concentration of 154.8 mg/L.The positive δ37Cl value is due to the elevated Cl-concentration of shallow burial depth.The sample from well (site of sample 020) with well depth of 70 m in Anyang city,is contaminated water with NO3-concentration of 52.6 mg/L,has δ37Cl = +0.20 ‰,δ18OH2O= -7.61 ‰,δD =-60.0‰ and Cl-concentration of 130.4 mg/L.From these data,it can be found that it can be found that the δ37Cl value of groundwater in the Quaternary aquifer tends to be more negative with increasing depth.Theoretically,this should be caused by the ion filtration.
2.2 Mechanism of chlorine isotope fractionation in groundwater
2.2.1 Chlorine isotope fractionation in karst water
Available data confirm that salt minerals such as halite and carnallite are preferentially enriched in37Cl during precipitation from seawater (Vengosh et al.1989).Xiao et al.(2000) pointed out that the enrichment of37Cl in different minerals is different.Vengosh et al.(1989) determined the δ37Cl value of +(24.7±2.9)‰ for a carnallite sample in the Qaidam Basin,Qinghai,while the δ37Cl value of the corresponding brine was -0.6±1.4‰.Eastoe et al.(2001) studied the enrichment of halite in the Gulf Coast.Palo,the Duro Basin and the evaporite of the Carpathian Mnt,those δ37Cl values ranged from 0.0‰ to + 1.0‰.In the present study area,the Ordovician limestone contains gypsum deposits,which also contains halite,and the dissolution of halite may cause the increases of δ37Cl value in karst water.The δ37Cl valueis also related to the size of the deposit.For example,the δ37Cl of Xiaonanhai Spring is+0.35‰ and the concentration of Cl-is 22.3 mg/L.The available information indicates that there are large mineable gypsum mines in the upstream of the spring.In contrast,the δ37Cl is+0.25‰ and the Cl-concentration is 16.1 mg/L in the Beishanzhuang karst water.The well in Zhang Erzhuang is located in granodiorite,because it is very close to the gypsum mine and is heavily contaminated by gypsum ore dissolution;therefore,the δ37Cl value also is up to +0.35‰ and the SO42-ion content reached 270.2 mg/L,which is an indicator for contamination from gypsum mine.Therefore,we believe that the positive δ37Cl value of karst water is due to the mixture of precipitation(δ37Cl value is negative) and groundwater (δ37Cl value is positive).
2.2.2 Chlorine isotope fractionation of groundwater in Quaternary aquifer
The Quaternary unconfined aquifer is generally in a shallow depth (<40 m),mainly recharged by precipitation,and easily polluted by anthropogenic activities.The δ37Cl value of groundwater in this aquifer ranges from positive to negative,or may also be close to 0.0‰.Thus,the factors influencing the variation on δ37Cl values in shallow groundwater is essentially a mixing process,since the groundwater which is mainly recharged by modern rainfall usually has a negative δ37Cl values or close to 0.0‰ of δ37Cl values.If shallow groundwater is contaminated with inorganic salts,its δ37Cl values are generally positive because inorganic salts have more positive δ37Cl values.
The δ37Cl value of groundwater in the Quaternary confined aquifer tends to be negative with increasing depth,while the Cl-concentration shows increase with depth (Fig.6,Fig.7,and Fig.8).This probably indicates the fractionation of chloride isotope caused by the ion filtration,which has been confirmed through the experiment by Campbell (1985).If NaCl water is a limited chlorine reservoir,the chlorine entering the porous medium (aquifer) will have a δ37Cl value which close to the value of chloride reservoir with time,and if the NaCl water is an unlimited chlorine reservoir,the chlorine isotope ratio in the porous medium (aquifer) will be greater than the value of this chlorine reservoir,and this ratio will always remain constant with time.Phillips et al.(1987)and Lavastre et al.(2005) further showed that chloride isotope fractionation in the process of ion filtration caused by the synergistic effect of the differential activity of35Cl and37Cl and negative ions of Cl-on filtration membrane (clay).Because35Cl migration is fast,it is more likely to be repelled by the negative ions on the filtration membrane (clay) and cannot enter the upper source layer (aquifer).Therefore,the seepage water is relatively enriched in37Cl and depleted in35Cl,i.e.the δ37Cl value in the seepage water is more positive.The phenomenon which the negative δ37Cl (35Cl-enrichment,37Cl-depletion) occurs in the deep part of the confined aquifer and the positive δ37Cl (37Cl-enrichment,35Cl-depletion) is present in the shallow part of the aquifer in the study area,should be resulted from the effect of ion filtration through clay layer.

Fig.8 (a) Relationship between Cl- and δ37Cl values in pore pressurized water of the Quaternary system (b)Conceptual illustration of ion permeation.034 (0.21,105.5) indicates point 034 (δ37Cl,Cl-) ,respectively
3 Conclusions
(1) The online technique for measuring37Cl isotopes by Gas-Bench II-IRMS used in this study is a new technique.It is characterized by low water consumption,fast analysis speed,online continuous analysis and accuracy of ±0.08%.
(2) The δ37Cl value of groundwater (karst water and Quaternary pore diving) in Anyang area has no correlation with the δ18O and δD of groundwater.This is mainly due to their different fractionation mechanisms.
(3) The karst water in Anyang area,is mainly distributed in mountainous areas,with a buried depth of more than 80 m.It is mainly recharged by precipitation,and the Cl-concentration of karst water has no correlation with the buried depth of groundwater.The measured data suggests that the changes of δ37Cl value is closely related to the gypsum mine.The positive δ37Cl value of karst water is caused by the mixture of precipitation (recharge water,δ37Cl is negative) and groundwater with dissolution of halite in the stratum (δ37Cl value is positive).
(4) The distribution of the δ37Cl value in Quaternary aquifers is closely related to the hydrodynamic conditions and the fractionation mechanism of37Cl isotope (mixing,ion filtration and diffusion).The δ37Cl value of groundwater in unconfined aquifer ranges from negative to positive.And the main process which affect the δ37Cl values is mixing of waters and contaminated with inorganic salts.The δ37Cl value of groundwater in the Quaternary confined aquifer is negative and tends to be more negative with increasing depth.The mechanism is the chlorine isotope fractionation cause by ion filtration through clay layer during water downward movement.
杂志排行
地下水科学与工程(英文版)的其它文章
- Tracing runoff components in the headwater area of Heihe River by isotopes and hydrochemistry
- Spatial distribution characteristics and main controlling factors of germanium in soil of northern Dabie Mountains,China
- Identification of groundwater potential in hard rock aquifer systems using Remote Sensing,GIS and Magnetic Survey in Veppanthattai,Perambalur,Tamilnadu
- Effect of groundwater on the ecological water environment of typical inland lakes in the Inner Mongolian Plateau
- Geoelectrical survey over perched aquifers in the northern part of Upper Sakarya River Basin,Türkiye
- Evolution of the freeze-thaw cycles in the source region of the Yellow River under the influence of climate change and its hydrological effects
