Neopestalotiopsis eucalypti,a causal agent of grapevine shoot rot in cutting nurseries in China
2022-12-01MAXuanyanJIAOWeiqiLIHengZHANGWeiRENWeichaoWUYanZHANGZhichangLIBaohuaZHOUShanyue
MA Xuan-yan ,JIAO Wei-qi ,LI Heng ,ZHANG Wei ,REN Wei-chao ,WU Yan ,ZHANG Zhi-chang,LI Bao-hua,ZHOU Shan-yue
1 College of Plant Health and Medicine,Qingdao Agricultural University/Engineering Research Center for Precision Pest Management for Fruits and Vegetables of Qingdao,Qingdao 266109,P.R.China
2 Beijing Key Laboratory of Environment Friendly Management on Fruit Diseases and Pests in North China,Institute of Plant and Environment Protection,Beijing Academy of Agriculture and Forestry Sciences,Beijing 100097,P.R.China
3 Shandong Zhichang Agricultural Science and Technology Development Co.,Ltd.,Rizhao 276500,P.R.China
Abstract Grapevine (Vitis vinifera L.) is an economically important fruit crop in the world,and China ranks first in the production of grapes with approximately 15% of the world’s total yield. However,diseases that cause the death of grapevine shoots pose a severe threat to the production of grapes. In this study,the fungus Neopestalotiopsis eucalypti was identified as a causal pathogen of grapevine shoot rot based on the morphology of conidia and a phylogenetic analysis. The phylogenetic analysis was performed with three isolates based on the combined sequence of internal transcribed spacer (ITS) region of ribosomal DNA,part of the translation elongation factor 1-alpha (Tef) and the β-tubulin (Tub2) genes. The three isolates were all identified as N.eucalypti. Pathogenicity tests of the three fungal isolates were conducted on grapevines shoots in vitro and in vivo. The results showed that all three fungal isolates caused severe rot lesions on the inoculated grapevine shoots,and N.eucalypti was re-isolated from the inoculated grapevine shoots. Therefore,N.eucalypti was confirmed as a causal agent of the grapevine shoot rot. This is the first report of N.eucalypti causing grapevine shoot disease in China.
Keywords: Neopestalotiopsis eucalypti,grapevine shoot rot,multigene phylogenetic analysis,pathogenicity
1.Introduction
Grapevine (VitisviniferaL.) is an economically important fruit crop in the world. It offers not only nutritious table grapes but also fruit for wine production. Statistically,China ranks first in the production of grapes in the world with a yield of more than 11 million tons,which represented approximately 15% of the world’s total production (Chethanaet al.2017).However,most of the grapevine cultivars planted in China are susceptible to fungal pathogens,which considerably limit grape production. Moreover,the grape diseases are getting worse owing to the increases in climate change in recent years.
It is well known that some serious diseases occur on grape leaves. Examples include grape downy mildew caused byPlasmoparaviticolaand grape powdery mildew caused byErysiphenecator. Other diseases can infect fruit,such as grape anthracnose caused byElsinoeampelina.Trunk diseases can cause the death of the grape shoots,branches,and even adult plants. However,few effective measures are available for the control of diseases in production fields (Mugnaiet al.1999;Van Niekerket al.2006;Fussleret al.2008;Bertschet al.2013;Gramajeet al.2016). Therefore,grapevine trunk diseases are considered to be the most severe threat to the grape production. Some destructive trunk diseases have been reported,such as dieback caused byBotryosphaeriaceaesp.(Shafi 2016) andEutypaspp.(Rolshausenet al.2006),and grapevine decline caused byDiplodiaseriata(Augeret al.2005;Martín and Cobos 2007). Grapevine shoot rot is also a severe threat to the grapevine. It has been shown that the shoot rot caused bySclerotiniasclerotiorumleads to the death of grapevine stems and branches (Latorre and Guerrero 2001;Parket al.2009). Recently,Pestalotiopsis-like fungi were proven to be pathogens of grapevine shoot rot as well. Among these fungi,Neopestalotiopsisare more virulent than isolates ofPestalotiopsis(Jayawardenaet al.2015).
The members ofPestalotiopsis-like taxa are divided into three genera,includingNeopestalotiopsis,PestalotiopsisandPseudopestalotiopsis,based on the morphological features of conidia and the phylogenetic analysis of the nuclear ribosomal RNA gene (Maharachchikumburaet al.2014). Typically,the members whose conidia with three light concolorous median cells are members of the genusPseudopestalotiopsis,while those with dark concolorous median cells are members ofPestalotiopsis;those with versicolored median cells are members ofNeopestalotiopsis(Maharachchikumburaet al.2014). Further identification of the species was successfully performed with the phylogenetic analysis on the combined sequence of internal transcribed spacer (ITS),β-tubulin (Tub2) and translation elongation factor 1-alpha (Tef) (Maharachchikumburaet al.2014;Sunet al.2021).
SomePestalotiopsis-like fungi have been characterized as endophytes that produce numerous compounds used widely in agriculture,industry,and medicine (Alyet al.2010;Watanabeet al.2010;Xuet al.2010,2014;Debbabet al.2013). However,an increasing amount of evidence has revealed thatPestalotiopsis-like fungi are important phytopathogens that infect plant leaves,fruit,roots,and shoots to cause severe plant diseases (Zhanget al.2012,2013;Ismailet al.2013;Maharachchikumburaet al.2013a,b;Maharachchikumburaet al.2016;Sunet al.2021). For example,N.vitiscauses grapevine leaf spot (Jayawardenaet al.2016),andPestalotiopsisspp.cause a postharvest rot of grape (Denget al.2013).
In this study,we aimed to identify the causal pathogen that caused grapevine shoot rot in cutting nurseries in Rizhao City,Shandong Province,China. The causal pathogen was identified based on the morphology and molecular characteristics. Moreover,the pathogenicity of the fungus was verified based on Koch’s postulates. To our knowledge,this is the first report ofNeopestalotiopsiseucalypticausing grapevine shoot rot in China.
2.Materials and methods
2.1.Isolation of the fungus
To isolate the pathogens that cause grapevine shoot rot,diseased grapevine shoots were collected form cutting nurseries in Rizhao City. The tissues from the edge of the rot lesions on each sample were cut into small pieces,surface-disinfected with 70% ethanol for 30 s,rinsed three times with sterile water,and then transferred to potato dextrose agar (PDA) medium and cultured at 25°C. As the colonies developed,the mycelial margins were cut off and sub-cultured on new PDA plates. The single spore isolates were obtained as described by Sunet al.(2021). In brief,the conidia were washed off the colonies with sterile water,and an optimal concentration of the conidial suspension was placed on water agar plates. After incubation at 25°C for 12 h,a single spore was removed with a sterile syringe needle and transferred onto PDA.
2.2.Morphological analysis
The isolates were incubated on PDA at 25°C for morphological identification. After incubation for 7 days,the colonies were observed,and the hyphal growth rates were calculated.Three repetitions were performed for each isolate. The morphological features of the conidia were observed and photographed with an Axiocam 506 Color Carl Zeiss Microscope (Carl Zeiss,Jena,Germany). In addition,the size of the conidia (length and width) were measured. In this assay,50 conidia were observed for each isolate.
2.3.PCR amplification and sequencing
The ITS,Tub2,andTefof the fungus were amplified with the genomic DNA extracted from the hyphae for the molecular identification. The specific primers ITS5/ITS4(Whiteet al.1990),BT2A/BT2B (Glass and Donaldson 1995;O’Donnell and Cigelnik 1997) and EF1-728F/EF2(O’Donnellet al.1998;Carbone and Kohn 1999) were used for DNA amplification. The PCR was performed in a 50-μL solution,including 50 ng of genomic DNA as template,2 μL of each primer (10 μmol),2 μL dNTP mixture (ATP,CTP,GTP and TTP,each 2.5 μmol),5 μL of 10× PCR buffer,DNA polymerase 1 U,and ddH2O was added to adjust the volume to 50 μL. The PCR procedures were as follows: an initial denaturation at 95°C for 5 min,followed by 30 cycles of 95°C for 15 s,55°C for 15 s,and 72°C for 1 min,and a final extension at 72°C for 5 min. The DNA was sequenced at Beijing Tsingke Biological Technology Co.,Ltd.(Beijing,China). The resulting sequences were all submitted to GenBank (https://www.ncbi.nlm.nih.gov/genba nk/).
2.4.Phylogenetic analysis
The phylogenetic analysis was performed with the ITS,Tub2andTefsequences of the three isolates QN5071,QN5072 and QN5073 obtained in this study and otherNeopestalotiopsisstrains that were downloaded from GenBank (Table 1),aligned using MAFFT (v.7) (https://mafft.cbrc.jp/alignment/server) (Katoh and Standley 2013),and edited with BioEdit (v.7.0.5.2).
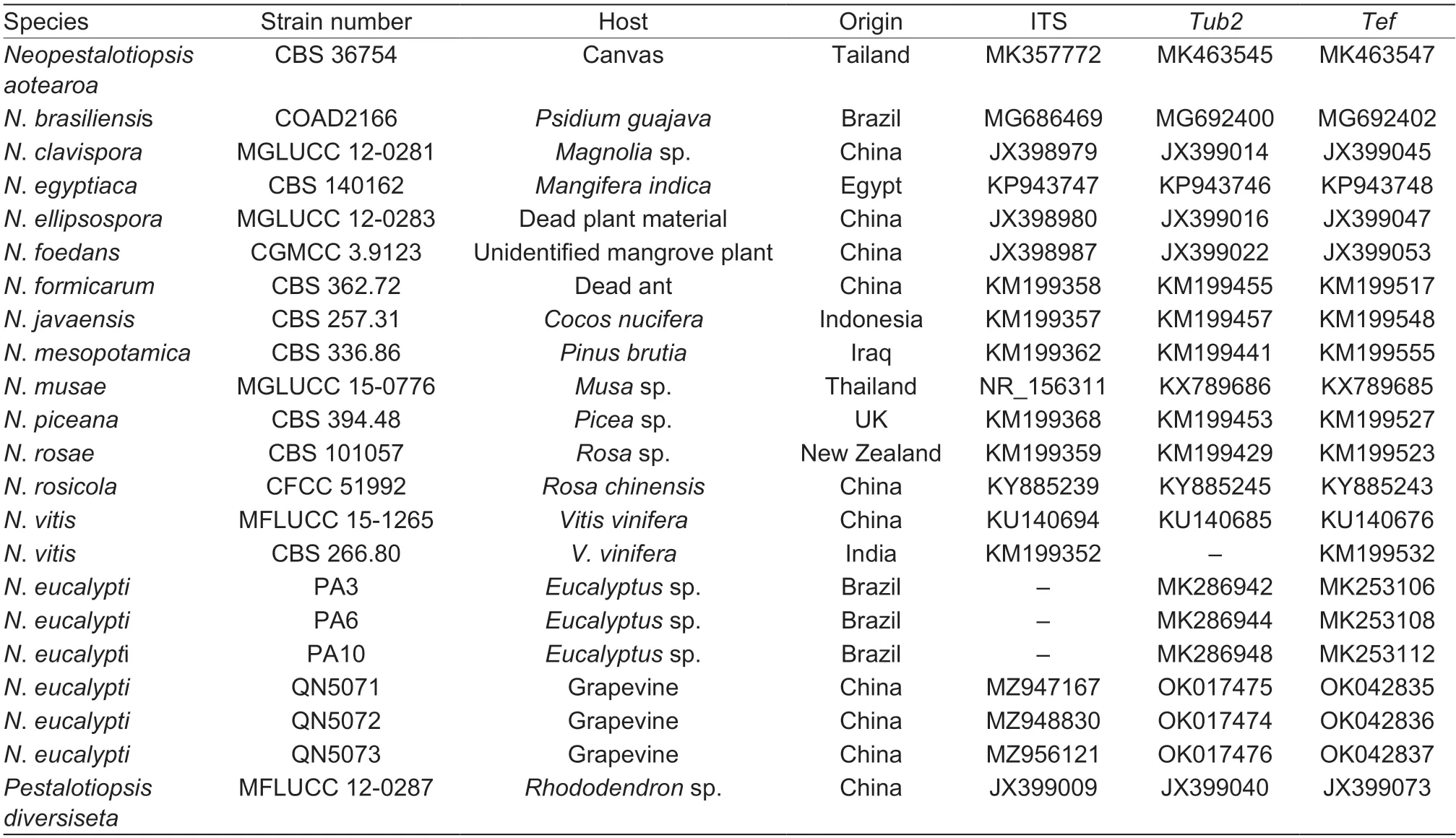
Table 1 Sequence used for the phylogenetic analysis with GenBank accession numbers1)
For the phylogenetic tree,a maximum likelihood (ML)analysis was performed using RAxML-HPC2 on XSEDE(v.8.2.8) through the CIPRES Science Gateway (https://www.phylo.or g/portal2) (Milleret al.2010). A total of 1 000 bootstrap replicates were run using the GTRGAMMA model. ML bootstrap values ≥50% are shown on each node in the resulting phylogenetic tree.Pestalotiopsisdiversiseta(MFLUCC 12-0287T) was used as an outgroup for the phylogenetic tree.
2.5.Pathogenicity tests
The isolates were incubated on PDA at 25°C. After incubation for 3 days,mycelial plugs (5 mm in diameter)were taken from the margin of the colonies. For the pathogenicity assayinvitro,shoots of the grapevine cultivar Manicure Finger that had not been exposed to pathogens were cut off from the vines. The detached grapevine shoots were then surface-disinfected with 75% ethanol for 30 s and washed three times with sterile water. The grapevine shoots were wounded with sterile needles,and the mycelial plugs were covered on the wounds. Moreover,the mycelial plugs were covered on the unwounded grapevine shoots as well. Subsequently,the grapevine shoots were incubated in sealed plastic boxes maintained at 25°C and 100% relative humidity. The disease symptoms were examined after inoculation for 48 h. For the pathogenicity assayinvivo,the mycelial plugs of the fungus were covered on the needlewounded shoots and unwounded shoots of the grapevine trees that were planted in a greenhouse maintained at 100% relative humidity. Symptoms of the disease were examined 48 h later. Sterile PDA plugs covered on the wounded grapevine shoots served as controls for both theinvitroandinvivopathogenicity assays. In each assay,three repetitions were performed,and five shoots were used for inoculation in each repetition.
In addition,the conidia of the fungus were used for the inoculations on grapevine shoots. In brief,a suspension that contained 2×105conidia mL-1was used to replace the mycelial plugs. The conidia were collected from isolates that had been incubated at 25°C for 20 days. Conidia were washed off the colonies and filtered with three layers of sterile lens wiping paper. A suspension that contained 2×105conidia mL-1was sprayed on the wounded grapevine shoots. The incubation conditions for the grapevine shoots,treatment repetitions,and disease survey were performed as described above.
3.Results
3.1.Symptoms of grapevine shoot rot in the cutting nursery
In the last two years,severe grapevine shoot rot was identified in grapevine cutting nurseries in Rizhao. Initially,water-soaked,slightly brown,nearly circle spots were observed on the new shoots. Under conditions of high humidity and high temperature in the nursery,the small spots soon enlarge and become irregular large rot lesions,which finally lead to the death of shoots or new branches (Fig.1).With the high humidity,massive growth of white hyphae was observed on the surface of the diseased grapevine cuttings and new shoots.

Fig.1 The symptoms of grapevine shoots rot in cutting seedling nurseries. The white are the hyphae on the surface of the grapevine cuttings. In the green circle,the death of the grapevine shoots caused by infection of the pathogens.
3.2.Morphological characteristics
In total,we obtained 36 fungal isolates from the 21 grapevine samples at different disease stages. Based on their morphological characteristics and ITS,the fungal isolates were first identified as 12 isolates ofSclerotium rolfsii,12Neopestalotiopsis,fourBotryosphaeriadothidea,fourFusarium,twoClonostachys,and twoBotrytiscinerea.
The isolates ofNeopestalotiopsiswere selected for further study. After incubation at 25°C for 7 days,the morphological characteristics of the colonies were surveyed.The colonies were 5.6-6.0 cm in diameter,circular with a lobate edge,white or pale yellow with aerial mycelia on the surface (Fig.2-A). After incubation for 8 days,some black conidiomata acervuli were initially observed on the surface of the colonies (Fig.2-A). The conidiomata acervuli were globose,semi-immersed,solitary,or aggregated. The morphological characters of the colonies and conidiomata acervuli of theNeopestalotiopsisisolates developed on PDA were highly similar to those ofN.eucalyptireported previously. Many conidia developed in the acervuli. The conidia were (20.43-28.57)×(5.30-8.74) μm,relatively fusiform in shape,straight to slightly curved,and divided into five cells by four septa (Fig.2-C). The apical cell was 2.85-5.10 μm long,hyaline,cylindrical,thin-and smoothwalled. The median cells were 14.91-17.76 μm long,doliiform,thick-walled,versicolored and separated by darker septa than the rest of cells. The second cell from the base was pale brown,while the third and fourth cells were honey brown. The basal cell was 3.3-6.02 μm long,conic to obconic,hyaline,thin-and smooth-walled. The conidia bore 2-4 tubular apical appendages 22.14-35.45 μm long and one basal appendage 3.15-9.07 μm long (Fig.2-E and F). The shape and size of the conidia produced by theNeopestalotiopsisisolates were similar to those ofN.eucalypti.
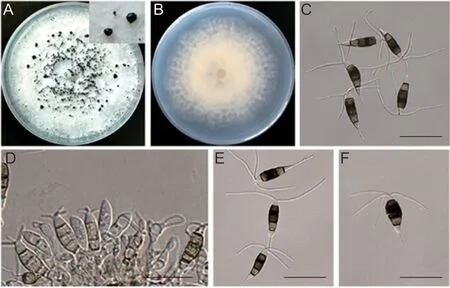
Fig.2 The morphological characteristics of the Neopestalotiopsis eucalypti. A and B,the colony of N. eucalypti on PDA medium.C,the conidia of the fungus. D,conidiogenous cells giving rise to conidia. E and F,appendages of the conidia. Bar=20 μm.
3.3.Phylogenetic analysis
Based on the similar colonies and morphology of the conidia,three of the 12Neopestalotiopsisisolates were used for phylogenetic analyses. The sequences of ITS,Tub2andTefof the three isolates QN5071 (accession numbers MZ947167,OK017475,and OK042835,respectively),QN5072 (accession numbers MZ948830,OK017474,and OK042836,respectively),and QN5073 (accession numbers MZ956121,OK017476,and OK042837,respectively) were deposited in GenBank. The sequence data are shown in the Appendix A. The phylogenetic tree was generated using the combined ITS,Tub2andTefof 16 taxa in whichP.diversiseta(MFLUCC 12-0287) served as an outgroup.The phylogenetic tree was obtained from the ML analyses.ML bootstrap values ≥50% are shown in the ML tree (Fig.3).All three isolates QN5071,QN5072 and QN5073 in this study clustered together with the strains PA3,PA6 and PA10 ofN.eucalypti(Fig.3). The result indicates that QN5071,QN5072 and QN5073 are isolates ofN.eucalypti.

Fig.3 The phylogenetic tree generated through maximum likelihood (ML) analysis with the combined sequence of ITS,Tub2 and Tef. ML bootstrap support values (BS) greater than 50% were given at the nodes. Pestalotiopsis diversiseta (MFLUCC 12-0287)was used as the outgroup. The species obtained in this study were showed in red.
3.4.Pathogenicity test of the isolates
The threeN.eucalyptiisolates QN5071,QN5072 and QN5073 were used for the pathogenicity test. In theinvitroassays,slight sunken lesions that were pale brown with a circle of dark brown edge developed on the wounded grapevine shoots after inoculation for 2 days,while no lesions were observed on the unwounded grapevine shoots and the control (Fig.4-A-C). After inoculation for 3 days,many dark conidiomata were observed on the surface of the rot lesions (Fig.4-D). Similar disease symptoms were observed in theinvivoassays (Fig.5). The results reflect that infection by the three isolates QN5071,QN5072 and QN5073 caused the grapevine shoot rot through invasion of the wound. Furthermore,based on the morphological characteristics of the colonies and conidia and the ITS sequence analyses,we successfully reisolatedN.eucalyptifrom the inoculated grapevine shoots. The results indicated thatN.eucalyptiis responsible for the grapevine shoot rot based on Koch’s postulates.

Fig.4 The symptoms caused by the Neopestalotiopsis eucalypti on the detached grapevine shoots. A,no extension on the wounds covered with PDA plugs. B,no lesion developed on the unwounded grapevine shoots covered with N.eucalypti mycelium plugs. C,lesion caused by N.eucalypti on the grapevine shoots after inoculation for 48 h. D,the acervuli generated on the surface of the necrotic lesions.
4.Discussion
In grapevine cutting nurseries in Rizhao,severe rot lesions on the new shoots were observed during the last two years. The pathogens eventually caused the death of shoots. Typically,the death rate of the new shoots >30%,which led to enormous economic losses. With the aim of developing effective strategies for the disease,we isolated and identified the causal pathogens. As a result,six genera of fungi were isolated. However,the fourFusariumisolates and twoClonostachysisolates were nonpathogenic when examined in this study with grapevine shoots (unpublished data). Thus,these two genera of fungi were not involved in the following experiments.Sclerotiumrolfsiiis the pathogen responsible for southern blight,which is associated with the death of grapevines in nurseries (Keyser and Ferreira 1988).Botryosphaeriadothideais linked to grapevine trunk disease(van Niekerket al.2006;Qiuet al.2008;Liet al.2010).Botrytiscinereais the causal agent of gray mold disease on grapevine,which commonly occurs in cutting nurseries and grape production fields. Since these three fungi had already been identified as pathogens of specific diseases,they were ruled out in subsequent experiments.Neopestalotiopsisisolates were selected for further study because they had not been previously reported as pathogens of grapevine.Moreover,it has been documented thatN.asiaticaandN.javaensisare causal pathogens of grapevine trunk disease (Maharachchikumburaet al.2016).
Although thePestalotiopsis-like members are divided into different genera based on the characteristics of their conidia,information of the morphology of conidia is not adequate for identification of the species (Liuet al.2010;Maharachchikumburaet al.2012). To fully identify species in theNeopestalotiopsisgenus,a phylogenetic analysis of the combined sequences of ITS,Tub2andTefwas performed,which has been proven to be reliable at distinguishing species in this genus (Maharachchikumburaet al.2014;Norphanphounet al.2019). Based on the phylogenetic tree,the three isolates all clustered together withN.eucalypti.This provides strong evidence that these three isolates areN.eucalypti.Neopestalotiopsiseucalyptiis a new species that was documented as a causal pathogen of stem rot of eucalyptus cuttings (Santoset al.2020). Moreover,the three isolates caused grapevine shoot rot bothinvitroandinvivo,and the same fungus was re-isolated from the inoculated shoots,providing very strong evidence thatN.eucalyptiis a causal pathogen of the disease. SinceS.rolfsii,B.dothideaandB.cinereaare all pathogens that cause similar rot symptoms on grapevine shoots,we hypothesized that the grapevine shoots could be simultaneously infected by different pathogens.
The use of cuttings is an effective method to rapidly propagate grapevines. However,the overwintering of pathogens on grapevine cuttings is an important factor that leads to the death of the new shoots. In this study,N.eucalyptiwas identified as a causal pathogen of grapevine shoot rot. To our knowledge,this is the first report thatN.eucalypticauses grapevine shoot rot in China. The finding of this study are the basis for developing management strategies to effectively control this new disease on grapevine.
5.Conclusion
In this study,N.eucalyptiwas identified based on its morphological and molecular characteristics. AndN.eucalyptiis a causal pathogen of grapevine shoots rot according to the Koch’s postulates. It is the first time to reportN.eucalyptias a pathogen on grapevine. Our finding would be the basis for developing management strategies to effectively control the new disease on grapevine.
Acknowledgements
The authors would give thanks to the financial support from the earmarked fund for China Agriculture Research System(CARS-27),the reviewers who participated in the review,as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendixassociated with this paper is available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
杂志排行
Journal of Integrative Agriculture的其它文章
- Integrated pest management and plant health
- Changes in phenolic content,composition,and antioxidant activity of blood oranges during cold and on-tree storage
- Development of a texture evaluation system for winter jujube(Ziziphus jujuba ‘Dongzao’)
- Statistical analysis of nitrogen use efficiency in Northeast China using multiple linear regression and Random Forest
- Characteristics of inorganic phosphorus fractions and their correlations with soil properties in three non-acidic soils
- Fractionation of soil organic carbon in a calcareous soil after longterm tillage and straw residue management
