Changes in phenolic content,composition,and antioxidant activity of blood oranges during cold and on-tree storage
2022-12-01ZHAOJichunAOMiaoHEXiaoqinLlWeizhouDENGLiliZENGKaifangMlNGJian
ZHAO Ji-chun ,AO Miao ,HE Xiao-qin ,Ll Wei-zhou ,DENG Li-li, ,ZENG Kai-fang,,MlNG Jian,
1 College of Food Science,Southwest University,Chongqing 400715,P.R.China
2 Research Center of Food Storage &Logistics,Southwest University,Chongqing 400715,P.R.China
Abstract Citrus fruits are rich in phenolic compounds that possess several health benefits. However,few studies have focused on the changes in phenolic compounds in citrus fruits during postharvest storage. This study dynamically monitored the phenolic content,components and antioxidant activity of ‘Tarocco’ blood oranges during a period of 12-week cold storage and on-tree storage,respectively. We investigated the alteration mechanism of phenolic compounds in blood oranges by evaluating phenylpropanoid pathway-related enzyme activities and gene expression. Results showed that flavanones were the main phenolic compounds in blood oranges. Both storage methods mainly stimulated the accumulation of phenolic acids to improve total phenolic content,which reached the maximum at week 12. Nonetheless,blood oranges had a higher phenolic content and antioxidant activity under on-tree storage than cold storage. Furthermore,the enzyme activities and gene expression of the phenylpropanoid pathway demonstrated that the accumulation of phenolics in blood oranges during storage was highly related to the activation of the phenylpropanoid pathway. These results demonstrate that on-tree storage is a potential approach for extending the supply period of blood orange from the perspective of phenolic compounds.
Keywords: ‘Tarocco’ blood orange,polyphenol components,antioxidant ability,phenylpropanoid pathway,on-tree storage
1.lntroduction
Citrus fruit is one of the most important and popular cash crops worldwide. It is rich in polyphenols such as phenolic acids,flavanones,flavones,flavonols,and isoflavones (Zouet al.2016;Yiet al.2017). Among most commercial citrus fruits,blood orange (CitrussinensisL.Osbeck) attracts increasing attention since it is the only species containing anthocyanins,which impart a deep red color and several health benefits to the fruit (M’hiriet al.2017).
As one of the late-ripening citrus fruits,the short commercial period of fresh blood orange would influence the economic benefit;therefore,its supply period needs to be extendedviacold storage (Carmonaet al.2017). The alteration in nutritional ingredients of citrus fruits during storage is worthy of attention. A growing body of studies has focused on the effects of cold storage conditions on fruit quality,anthocyanin accumulation,and certain phenolic content (Habibiet al.2020b;Stranoet al.2021a,b). Cold storage can induce anthocyanin accumulation in blood oranges,which was significantly associated with storage temperature (Carmonaet al.2017;Habibiet al.2020a). Anthocyanin biosynthesis has been identified as a cold-regulated pathway. Specifically,the higher anthocyanin content at 9°C was associated with the enhanced upregulation of cinnamate 4-hydroxylase(C4H),4-hydroxycynnamoyl CoA ligase (4CL),chalcone synthase (CHS),and chalcone isomerase (CHI) genes,compared to those at 4°C (Carmonaet al.2017).Moreover,the increase in the content of anthocyanins,flavanones,and hydroxycinnamic acids was observed in blood oranges during cold storage,and phenolic accumulation was positively correlated with antioxidant capacity (Rapisardaet al.2008). Researchers tend to focus on the anthocyanins of blood oranges during cold storage. However,there is little knowledge on the change in phenolics of blood oranges during cold storage,except anthocyanins.
In addition to traditional indoor storage,on-tree storage that keeps fruits on trees after ripening is regarded as another promising storage method (Zhanget al.2010).Although there is some argument about whether it is a strict storage approach,on-tree storage does have the same function as cold storage in preserving the edible qualities and nutritional values. Besides,compared to traditional storage,on-tree storage has several advantages,such as low cost,low carbon,and being environmentally friendly. Three pummelo cultivars by on-tree storage had better quality than those by indoor storage (Wanget al.2019). Wanget al.(2011) reported that on-tree storage improved the total sugar,soluble solids,and ascorbic acid content,increasing the quality of Newhall navel orange,compared with cold storage at 7°C.
It has also been demonstrated that on-tree storage is beneficial for regulating gene expression and controlling the dietary fiber content on navel orange (Donget al.2008). However,the changes in phenolic component and content under on-tree storage are mostly unknown.Therefore,the tracing and systematic analysis of phenolic compounds during storage have been encouraged.
This study aimed to evaluate the changes in phenolic content,composition,and antioxidant activity of blood oranges during cold storage and on-tree storage. The change mechanism of phenolic components was also investigated by monitoring the enzyme activity of the phenylpropanoid pathway and the expression of the corresponding genes. This study will provide a theoretical basis for the actual production and storage of blood oranges from the nutritional perspective.
2.Materials and methods
2.1.Plant materials
Fruits of blood orange (CitrussinensisL.Osbeck‘Tarocco’) were harvested at 0,1,2,3,4,6,8,and 12 weeks (W0 to W12) from three pest-free trees in Citrus Research Institute of Chinese Academy of Agricultural Sciences (29°76′45′′N,106°39′02′′E) from December
2018 to March 2019 for the on-tree storage group. The fruits at W0 had met the sale requirement. The daily average temperature was from 5 to 16°C,and the average relative humidity was 52-93% during 12 weeks(http://data.sheshiyuanyi.com/WeatherData/). After being harvested and delivered to our research laboratory,fruits were washed with tap water and sampled immediately.
Cold storage fruits were collected at W0 from the same batch of trees,with uniform size and color and no mechanical damage or infection,and were transported immediately to the laboratory. These fruits were washed with tap water after being dipped in 2% sodium hypochlorite solution for 2 min for surface disinfection(Habibi and Ramezanian 2017). The fruits were then soaked in 700-fold diluted citrus preservative (active ingredients: 24% prochloraz and 4% imazalil) (Zhuhai Gengreen Technology Co.,Ltd.,Guangdong,China) for 2 min and dried in air. Finally,the fruits were packed independently with PU film,randomly placed in four baskets (60-70 fruits per basket),and stored at 8-10°C and 80-85% relative humidity.
From these baskets,16 blood orange fruits were randomly selected for collecting pulp at each sampling point. The sampling slots of cold storage fruits were consistent with on-tree samples. The pulp around the equator of blood orange with a width of 2 cm,except mesocarp,was taken and randomly divided into four equal parts by quartering method. One equal part from each fruit was then pooled together,resulting in four mixed pulp groups. Of them,three mixed samples were respectively homogenized and stored at -40°C for phenolic extraction.The remaining aliquot was immediately frozen with liquid nitrogen and stored at -80°C for measuring enzyme activity and extracting RNA. All results were expressed based on the fresh weight of samples.
2.2.Chemicals and kits
Phenolic standards such as hesperidin,rhoifolin,naringin,neohesperidin,ferulic acid,gallic acid,and vanillic acid were supplied by National Institutes for Food and Drug Control (Beijing,China). Rutin,caffeic acid,chlorogenic acid,and p-coumaric acid were obtained from Beijing Solarbio Science &Technology Co.,Ltd.(Beijing,China). Naringin and didymin were purchased from Chengdu Must Bio-Technology Co.,Ltd.(Chengdu,China). p-Hydroxybenzoic acid was from Shanghai Source Leaf Biotechnology Co.,Ltd.(Shanghai,China). Sinapic acid,HPLC grade formic acid,and acetonitrile were bought from Shanghai Aladdin Biochemical Technology Co.,Ltd.(Shanghai,China). Hesperidin,1,1-diphenyl-2-picrylhydrazyl(DPPH),and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were obtained from Sigma-Aldrich (St.Louis,MO,USA). 2,2′-Azobis(2-methylpropionamidine) dihydrochloride (ABAP) was supplied by Tokyo Chemical Industry (Tokyo,Japan).The purity of all standards was above 98%. Other chemicals and reagents were at analytical grade.ELISA kits for the activity of CHS,CHI,flavone synthase(FSI),and flavonol synthase (FLS) were obtained from Shanghai Enzyme-linked Biotechnology Co.,Ltd.(Shanghai,China).
2.3.Fruit quality assessment
The change in the visual aspect of blood orange pulp was examined by comparing the image of the orange pulp equator at different time points. Weight loss was determined by weighting the fruit at the beginning stage(W0) and the sampling points (Wn).
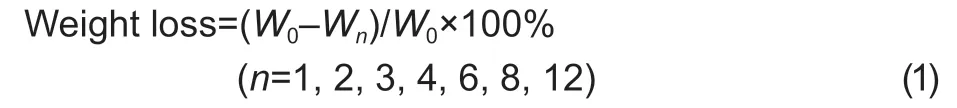
The decay rate was assayed by the visual observation of the fruits and calculated by the number of decayed fruits (Nn) and the total number of fruits (N).

Soluble solid content and titratable acidity were measured according to Stranoet al.(2021a). Blood orange juice was obtained using an electric citrus juicer and centrifuged at 4 500×g for 10 min. The soluble solid content of the supernatant was determined with a digital refractometer and expressed as a percent. Titratable acidity was measured with the titration method,adding 0.1 mol L-1NaOH to the react system until its color unchanged for 30 s. As the maturity index of the blood orange fruits,the solid-acid ratio was calculated by soluble solid content/titratable acidity.
Ascorbic acid content was determined based on the method used by Mditshwaet al.(2015).
2.4.Phytochemical extraction
The extraction method of free and bound phenolics was modified according to Sunet al.(2002). A total of 50 g of frozen pulp was homogenized with 100 mL chilled acetone solution (80%,v/v) for 6 min using a high-speed disperser (Ningbo Scientz,Ningbo,China). After the extraction,the homogenate was centrifuged at 3 500×g for 10 min instead of filtering in the original method. The above procedure was performed in duplicate. All the supernatants were pooled and evaporated at 45°C. The free phenolic extracts were re-dissolved with deionized water to 10 mL.
The residue from free phenolic extraction was digested with 40 mL NaOH (2 mol L-1) for 90 min with a magnetic stirrer at room temperature in the dark. The resulting digestive solution was adjusted to a neutral condition(pH 7.0) with hydrochloric acid and then degreased withn-hexane. The mixture was extracted five times with ethyl acetate. Then the ethyl acetate fraction was pooled and evaporated at 45°C. The bound phenolic extract was reconstituted with deionized water to 10 mL. Both free phenolics and bound phenolics were extracted from three independent mixed samples and were stored at -80°C until further analysis.
2.5.Determination of phenolic content
The free and bound phenolic content was determined using the Folin-Ciocalteu method (Singletonet al.1999)with slight modifications. We mixed 0.2 mL of the extract,0.8 mL water,and 0.2 mL Folin-Ciocalteu reagent and maintained it for 6 min. Sodium carbonate (7%,w/v)and 1.6 mL water were then added,and the mixture was kept at room temperature for 90 min in the darkness.Absorbance was determined at 760 nm using the spectrophotometer V-1000 (Shanghai AOE Instrument,Shanghai,China). The phenolic content was expressed as grams of gallic acid equivalents (GAE) per kilogram of fresh blood oranges. All measurement was conducted in triplicate.
2.6.HPLC-DAD analysis of phenolic components
Phenolic compounds were analyzed using a Shimadzu HPLC LC-20A system (Shimadzu,Tokyo,Japan),which consists of a photodiode array detector (SPD-M20A),an automatic sampler (SIL-20A),a degasser (DGU-20A3R),two pump units (LC-20AT),and a GIST C18 column(250×4.6 mm I.D.,5 μm). The mobile phase consisted of 0.1% formic acid solution (A) and 100% acetonitrile (B).The gradient was as follows: 0-3 min,7% B;3-8 min,7-8% B;8-12 min,8-8.5% B;12-17 min,9% B;17-22 min,11% B;22-32 min,13% B;32-40 min,15% B;40-45 min,16% B;45-55 min,18.5% B;55-85 min,24%B;85-95 min,26% B;95-100 min,40% B;100-110 min,7% B. The injected volume was 10 μL. The column temperature was 40°C,and the flow rate was 0.4 mL min-1. The identification and quantification of all phenolic compounds were performed at 280 nm using the external standard method.
2.7.Determination of antioxidant activity
The DPPH radical scavenging ability of phenolic extract was determined according to Gangopadhyayet al.(2016) with minor modifications. The reaction mixture was composed of extract of different concentrations(100 μL) and DPPH solution (120 μmol L-1,100 μL). All quantification was spotted onto a 96-well microtiter plate in triplicate. The mixture was then incubated at 37°C for 30 min in the dark,and the absorbance was read at 515 nm by a Synergy H1 Hybrid Multi-Mode Microplate Reader (BioTek,USA). Deionized water and ascorbic acid were used as blank and standard,respectively. The DPPH scavenging capacity of samples was indicated by the effective concentration (EC50) value.
The antioxidant activity of the phenolic extract was also measured by the oxygen radical absorbance capacity(ORAC) assay (Wolfeet al.2008;Alvarez-Jubeteet al.2010). Briefly,20 μL of phosphate buffer (pH 7.4),Trolox standard solution (6.25 μmol L-1),and the extract were mixed with sodium fluorescein solution (200 μL,0.96 μmol L-1) and incubated at 37°C for 20 min. A total of 20 μL ABAP (119 mmol L-1) was then added to the mixture.Fluorescence intensity was assayed 35 cycles every 4.5 min by Microplate Reader at 485 nm excitation and 535 nm emission. Trolox of different concentrations ranging from 6.25 to 50 μmol L-1was used as a control.Data were indicated as micromole of Trolox equivalents(TE) per gram of blood oranges. All quantification was carried out in triplicate.
2.8.Measurement of the activity of phenylpropanoid pathway enzymes
Phenylalanine ammonia lyase (PAL) activity was determined using the method described by Zhanget al.(2019). The only modification in enzyme extraction was 2 g of fresh pulp homogenized in an ice bath with 5 mL sulfhydryl alcohol sodium borate buffer (100 mmol L-1,pH 8.8) and 4% cross-linked polyvinylpyrrolidone (PVPP).Other procedures were completely consistent with the original method. One unit of PAL activity was defined as the increase of 0.01 absorbance units at 290 nm per hour per gram of fresh samples.
The measurement of C4H activity was modified based on the method of Maet al.(2018). The crude enzyme was extracted according to the original method. The reaction system consisted of Tris-HCL buffer (50 mmol L-1,pH 8.9),trans-cinnamaldehyde (2 mmol L-1),NADPNa2(0.5 mmol L-1),G-6-P-Na2(0.5 mmol L-1),and 200 μL crude enzyme solution. The mixture was incubated at 37°C for 1 h. The reaction was then stopped by the addition of 200 μL of 6 mol L-1HCl. C4H activity was evaluated by the absorbance at 340 nm. The extraction buffer was used as the control system instead of the crude enzyme solution.
4CL activity was determined by a slightly modified method (Weiet al.2017). The extracting buffer for 4CL was comprised of β-mercaptoethanol (5 mmol L-1),ethylenediaminetetraacetic acid (5 mmol L-1),ascorbic acid (5 mmol L-1),leupeptin (10 μmol L-1),PMSF(1 mmol L-1),PVP (0.15%,w/v),and glycerol (10%).Fresh pulp (1 g) was mixed with the extraction buffer(4 mL) by vortex stirring for 1 min,followed by ultrasonic extraction for 2 min in an ice bath. The mixture was centrifuged at 12 000×g for 30 min at 4°C,and the supernatant was collected to assay 4CL activity. The enzymatic reaction system was composed of coumaric acid (5 mmol L-1),COA-SH (1 μmol L-1),MgSO4·7H2O(15 μmol L-1),ATP (50 μmol L-1),and 0.5 mL crude enzyme solution. The mixture was incubated at 40°C for 10 min,and the absorbance was measured at 333 nm.The extraction buffer was used as the control instead of the crude enzyme solution.
CHS,CHI,FSI,and FLS activities were measured using an ELISA kit according to the manufacturer’s instructions.
2.9.Measurement of gene expression by RT-PCR
Extraction of total RNA and cDNA synthesisTotal RNA from blood orange pulp was extracted using the UNlQ-10 Column Trizol Total RNA Isolation Kit (Sangon Biotech Co.,Ltd.,Shanghai,China). First,800 μg of total RNA,1 μL Random Primer p(DN)6,and 1 μL dNTP Mix were mixed and diluted to 14.5 μL with Rnase-free ddH2O,and then kept at 65°C for 5 min. Subsequently,4 μL 5×reaction buffer,0.5 μL Ribolock RNase inhibitor (20 U),and 1 μL Maxima Reverse Transcriptase (200 U) were added under ice bath conditions. After mixing,the cDNA was synthesized at 25°C for 10 min,50°C for 30 min,and 85°C for 5 min.
Quantitative RT-PCR analysisQuantitative real-time was carried out by a StepOne Plus Real-Time PCR System (Applied Biosystem,Foster,CA,USA). The RTPCR system (20 μL) comprised 2 μL 10-fold diluted cDNA sample,10 μL SYBR Green qPCR Master Mix,0.4 μL primer F,0.4 μL primer R,and 7.2 μL ddH2O. The RTPCR procedure consisted of 95°C for 3 min,followed by 45 cycles at 95°C for 5 s and 60°C for 30 s. The relative quantitation of gene expression between on-tree storage and control orange samples was calculated using the comparative threshold (CT) method (Carmonaet al.2017). Three independent quantitative PCR experiments were performed for each gene. The housekeeping gene elongation factor (EF-1α) was used as an endogenous reference. ΔCTwas computed by subtractingEF-1αCTfrom CTof the gene of interest to obtain ΔCTof all samples. Subsequently,the comparative expression level of genes is given by formula 2-ΔΔCT,where ΔΔCTis calculated by subtracting the baseline ΔCTfrom the sample ΔCT,while the baseline represents the expression level of W0. The primer sequences were designed using Primer Premier 5.0 and are listed in Appendix A.
2.10.Statistical analyses
Data were shown as mean±standard deviation (SD) of three independent experiments. Differences among different storage times under the same condition were evaluatedviaDuncan’s multiple comparison test by SPSS 20 (SPSS Inc.,Chicago,IL,USA). Statistical significance between the two storage methods was assessed based on Student’st-test. Differences atP<0.05 were considered statistically significant. Correlation coefficient between the antioxidant activity,evaluated by DPPH radical scavenging ability and ORAC assay,and the content of phenolic compounds,determined by HPLC,were analyzed by Pearson’s correlation in SPSS 20. The charts were drawn by Origin 8.1 Software (OriginLab,Northampton,Massachusetts,USA).
3.Results
3.1.Fruit storage quality
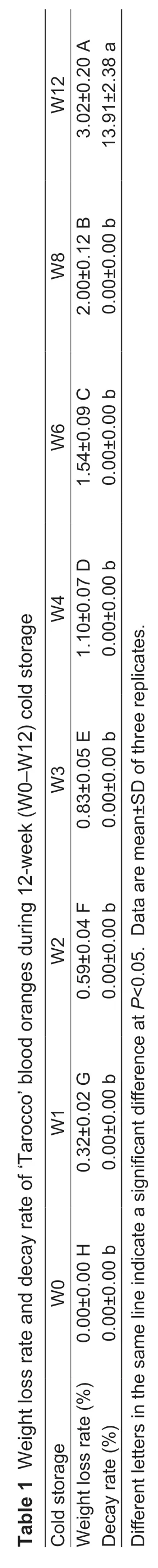
The fruit quality of stored blood orange was characterized by changes in the visual aspect of pulp (Fig.1-A),soluble solids (Fig.1-B),titratable acid (Fig.1-C),solid-acid ratio (Fig.1-D),ascorbic acid content (Fig.1-E),weight loss,and decay rate (Table 1). Both storages induced an increase in pigments,soluble solids concentration,and solid-acid ratio. The color of on-tree oranges turned deep red gradually,and the pigments distributed equally,whereas the color of cold storage orange increased remarkably after four weeks. The ascorbic acid content showed an increase-decrease trend during 12-week cold and on-tree storage. Besides,the cold storage group had lower titratable acid content and higher solid-acid ratio than the on-tree group. For cold storage samples,weight loss increased gradually from 0.32 to 3.02% after 12-week storage. No decayed fruit was found in the cold storage group until the 12th week (13.91%).
3.2.Changes in phenolic content during storage
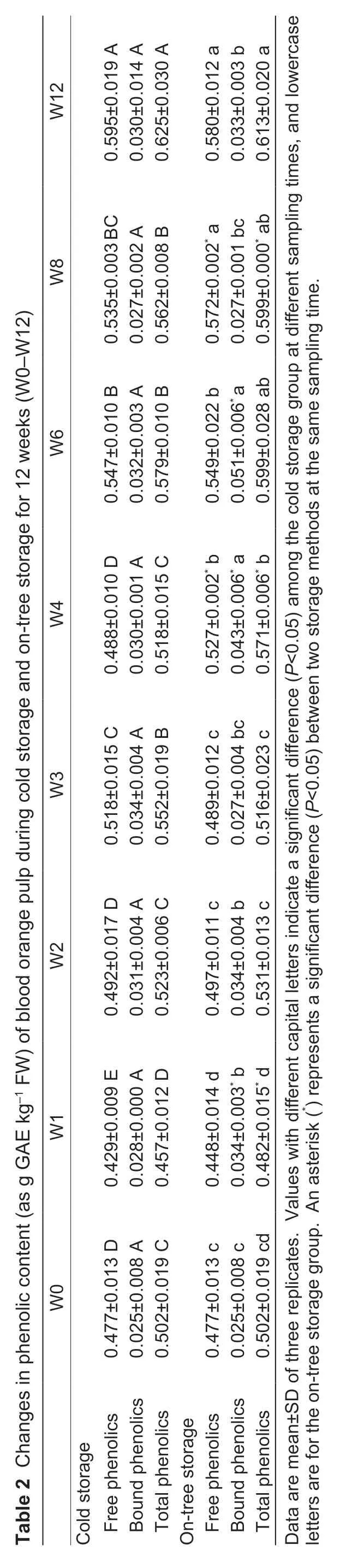
Total phenolic content (TPC) tended to increase during orange storage,although there was a relatively large fluctuation in the first four weeks (Table 2). TPC of ontree storage oranges increased more sharply and was higher than that of cold storage ones at most sampling points,especially at W1,W4,and W8 (P<0.05). They both reached the maximum value at W12,which were 22.03 and 24.46% higher than those at W0 (P<0.05),respectively. The alteration tendency of free phenolic content in storage samples was consistent with TPC,whereas there was a slight change in bound phenolics.Obviously,free phenolic alteration mainly contributed to the increase in TPC of both groups.
3.3.Variation of phenolic composition during blood orange storage
Sixteen phenolic compounds,including eight phenolic acids,six flavanones,one flavone,and one flavonol,were identified by the HPLC-DAD method (Appendix B).An increase in the total content of quantified phenolics(TQPC) was observed in both storage fruits during 12 weeks,and on-tree fruits had overall higher TQPC than cold storage ones. There was a significant difference between on-tree storage (445.08±1.92 mg kg-1) and cold storage (423.52±4.93 mg kg-1) (P<0.05) at W12 (Fig.1-F).In addition,the content of flavanones,phenolic acids,flavonol,and flavone in the pulp constituted 63.05,33.8,2.55,and 0.60% of TQPC during the whole storage (data not shown),respectively.
Changes in the content of flavone and flavonolOnly free state rutin (flavonol) and rhoifolin (flavone) were detected,the highest content of which corresponded to the samples at week 12 and during the first three weeks,respectively (Fig.1-G). For cold storage fruits,the rhoifolin content decreased from W1 to W4,then increased and reached the maximum until week 12(2.96 mg kg-1). Conversely,the rutin content had a rising trend in the first three weeks and reached the highest value (12.27 mg kg-1),and then reduced slightly in the remaining weeks. A similar change trend was also found in the on-tree storage group.
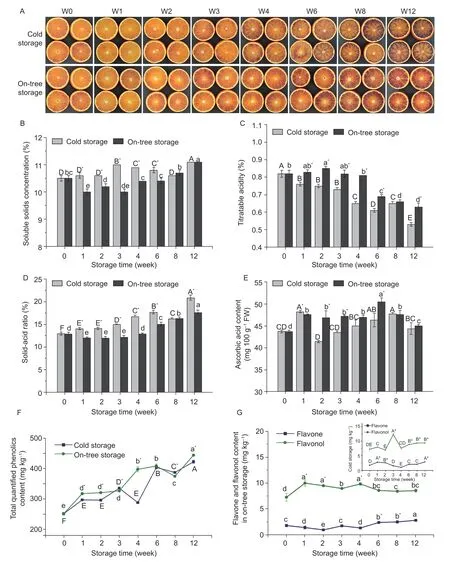
Fig.1 Internal appearance of pulp (A),soluble solids(B),titratable acid (C),solid-acid ratio (D),ascorbic acid content (E),TQPC(F),and average content of flavone and flavonol (G) in blood orange pulp during 12-week (W0-W12) cold storage and on-tree storage. Values are mean±SD of three independent experiments. Different capital letters indicate significantly different (P<0.05)among cold storage samples;lowercase letters for on-tree storage samples. An asterisk (*) indicates a significant difference(P<0.05) between two storage methods at the same sampling time.
Changes in the flavanone contentTotal flavanone subclass accounted for 59.98-67.69% and 58.72-67.69% of TQPC in the cold storage and on-tree storage periods,respectively.The content of flavanone increased gradually among the storage periods and achieved a maximum at week 6 and week 12 for cold storage and on-tree storage (269.96 and 269.49 mg kg-1),respectively. Flavanone subclass includes free and bound states,of which the free state is predominant.The content of free-state hesperidin was the highest,followed by narirutin and didymin (Fig.2-A;Appendix C).
All flavanone components firstly increased and then decreased in the cold storage group except for didymin,which increased continuously. Although the bound flavanone content was not as high as the free state,it increased remarkably.For example,the content of bound naringin,hesperidin,and narirutin had the greatest fold changes (2.4,3.73,and 9.91,respectively) between week 0 to week 8. The variation trend of flavanones in the on-tree storage group was different from the cold storage group. Most free flavanone components increased with storage time,while bound flavanone content decreased after peaking around week 8. It is worth noting that the content of free narirutin,hesperidin,and didymin continued to increase in the late storage stage,whereas the bound state decreased continuously,which might indicate that the fragmentation and dissociation of the bound flavanones in the later storage period promoted the enrichment of the free state. Conclusively,both storage methods promoted the accumulation and synthesis of flavanones,and there was no significant difference between them.
Changes in the phenolic acid contentPhenolic acids were the second most abundant subclass of the studied phenolics,accounting for 28.72-37.00% and 38.38% of TQPC under cold storage and on-tree storage,respectively. The content of phenolic acids increased throughout the storage period and reached 147.93 and 164.25 mg kg-1under cold storage and on-tree storage,respectively,2.04 and 2.26 times higher than those at week 0 (P<0.05) (Appendix C). Gallic acid was the main phenolic acid in pulps. Chlorogenic acid was the next major one,followed by ferulic acid and erucic acid,while p-hydroxybenzoic acid was the least. All the relative changes in phenolic acids are illustrated in Fig.2-B.
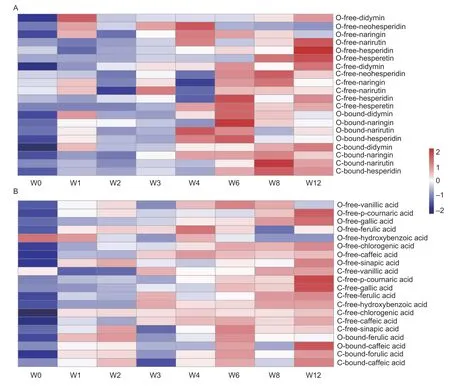
Fig.2 Changes in the content of flavanone (A) and phenolic acid (B) components of blood orange pulp extracts during 12-week(W0-W12) cold storage and on-tree storage. The capital letters “C” and “O” represent cold storage and on-tree storage,respectively.“free” and “bound” represent phenolics of free and bound states,respectively.
The content of other phenolic acids increased continuously during cold storage except for erucic acid. Caffeic acid and gallic acid showed a more efficient enrichment advantage than the on-tree storage group during the late storage period(weeks 6 to 12). Phenolic acids with higher content displayed a rising trend during on-tree storage,such as gallic acid,chlorogenic acid,and erucic acid. Ferulic acid,caffeic acid,and vanillic acid content reached the maximum levels at week 4 and then decreased slightly. Notably,the content of p-hydroxybenzoic acid displayed a declining trend and could not be detected after week 6,resulting in a higher p-hydroxybenzoic acid level in the cold storage group than in the on-tree storage group (P<0.05). The content of free p-coumaric acid,erucic acid,chlorogenic acid,vanillic acid,and bound ferulic acid was higher than those of the cold storage group during the whole on-tree storage period (P<0.05). In short,both storage methods promoted the synthesis of phenolic acids,and on-tree storage was more conducive to the accumulation of phenolic acids in blood orange fruit.
3.4.Total antioxidant activity
DPPH free radical scavenging capacityIt was shown that the DPPH radical scavenging capacity of free (Fig.3-A) and bound phenolic extracts (Fig.3-B) of blood oranges during storage. The DPPH scavenging capacity of free phenolics remained at a high level from W4 to W8,and cold storage and on-tree storage groups reached the maximum value at W4 (EC50=3.74 mg L-1)and W8 (EC50=3.48 mg L-1),respectively. Moreover,the DPPH radical scavenging capacity of bound phenolics of cold storage and on-tree storage group kept a high level throughout the whole storage stage except for W8,which reached their maximum value at W6(EC50=5.13 mg L-1)and W1 (EC50=5.05 mg L-1),respectively (Fig.3-B). In addition,the orange polyphenols of the on-tree storage group showed a higher DPPH scavenging ability during W4 to W8 than the cold storage group (P<0.05).
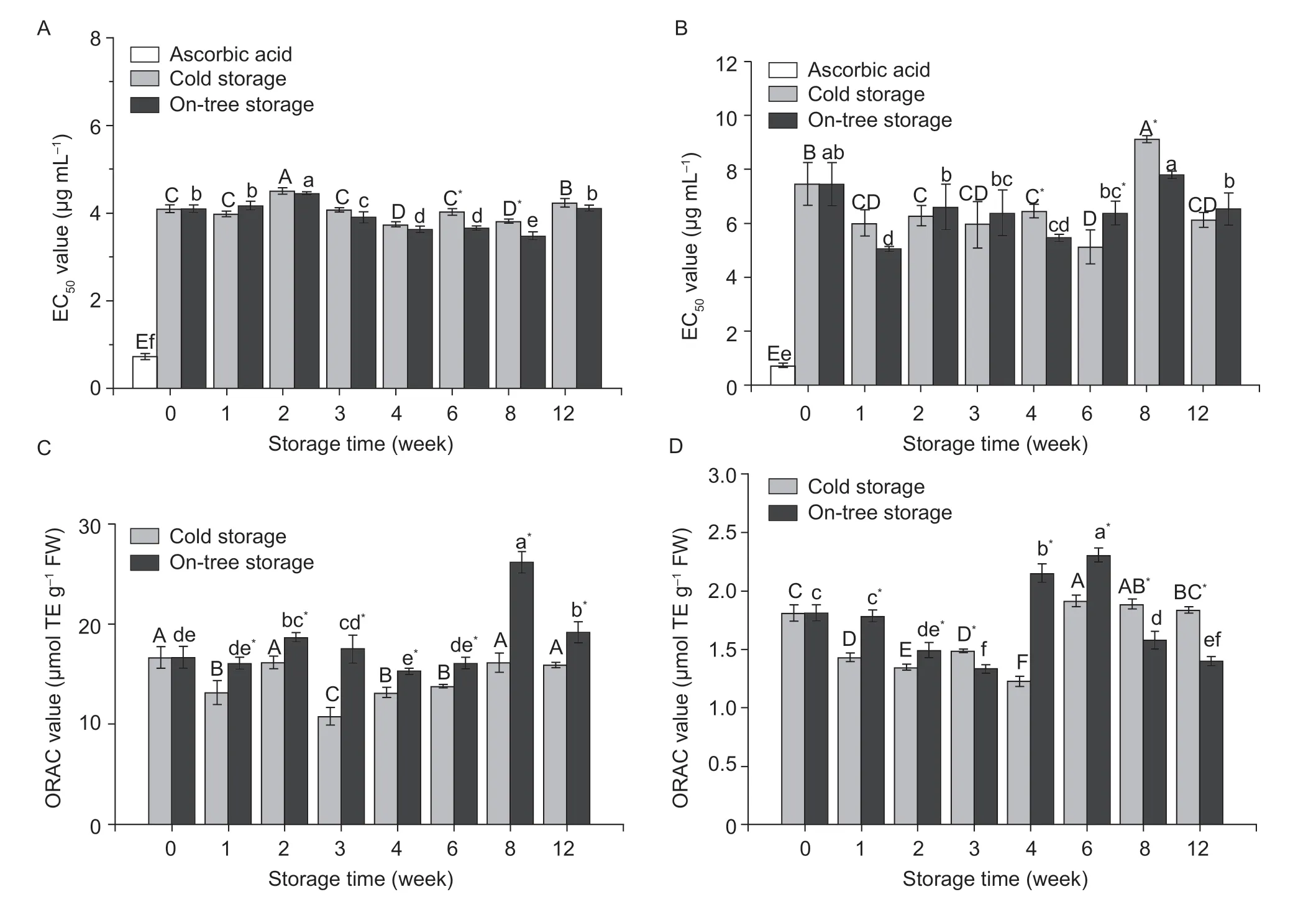
Fig.3 EC50 values and oxygen radical absorbance capacity (ORAC) values of free (A and C) and bound (B and D) phenolics in blood orange pulp during cold storage and on-tree storage. TE,trolox equivalents. Values are mean±SD of three replicates. Different capital letters indicate the difference (P<0.05) among cold storage samples;lowercase letters for the on-tree storage group. An asterisk (*) indicates a significant difference (P<0.05) between two storage methods at the same sampling time.
ORACIt has been found that the ORAC values of polyphenol extracts had a fluctuated change during 12-week storage. ORAC values of free phenolics remained low during cold storage (Fig.3-C),and of bound phenolics gradually decreased in the first four weeks and returned to the initial level after peaking at W6 (1.91 μmol g-1)(Fig.3-D). The change tendency in ORAC of the on-tree storage group was slightly different from that of the cold storage group. ORAC values of free and bound phenolics under on-tree storage reached their maximum value at W8 (26.23 μmol g-1) and W6 (2.31 μmol g-1),respectively,and then decreased gradually. Both methods were conducive to the increase of ORAC value,and on-tree storage had a greater effect on improving the antioxidant capacity.
3.5.Activity of phenylpropanoid pathway associated enzymes
The activity of enzymes involved in the phenylpropanoid pathway was measured and is shown in Fig.4-A-G. The activity of enzymes increased firstly and then decreased during storage,except for CHS under cold storage and C4H under on-tree storage. It is noteworthy that the enzyme activity of on-tree storage fruits was significantly higher than those of the cold storage group (P<0.05).Moreover,the enzyme activity of the on-tree storage group peaked at W4,while that of the cold storage group mainly peaked at W6 and W8.
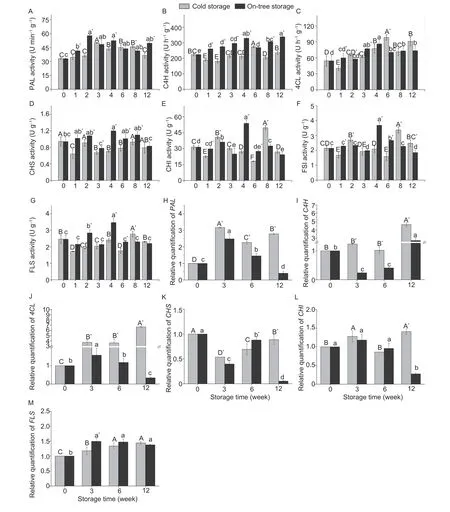
Fig.4 Activity of phenylalanine ammonia lyase (PAL) (A),4-hydroxycynnamoyl CoA ligase (4CL) (B),cinnamate 4-hydroxylase (C4H) (C),chalcone synthase(CHS) (D),and chalcone isomerase (CHI) (E),flavone synthase (FSI) (F),and flavonol synthase (FLS) (G) and relative quantification of the expression of PAL(H),4CL (I),C4H (J),CHS (K),CHI (L),and FLS (M) in blood orange pulp during cold storage and on-tree storage. Values are mean±SD of three replicates.Different capital letters indicate the difference (P<0.05) among the cold storage group;lowercase letters for the on-tree storage group. An asterisk (*) indicates a significant difference (P<0.05) between two storage methods at the same sampling time.
3.6.Changes in the expression of PAL,4CL,C4H,CHS,CHI,and FLS in blood orange pulp
The expression of genes involved in the phenylpropanoid pathway was up-regulated differently under cold storage and on-tree storage,except forCHS(Fig.4-K). The expression ofPALand4CLmarkedly increased after three weeks of low-temperature exposure,reaching the maximum value in week 3 and week 12,respectively(Fig.4-H and J).4CLwas the highest one (increased by 6.68 folds) during cold storage. The expression ofPAL,4CL,andFLSincreased at week 3 during on-tree storage,and thenPALand4CLdecreased gradually after week 3,whileFLSremained unchanged. In addition,the expression of most genes under cold storage oranges was higher than those under on-tree storage (P<0.05).Overall,both methods induced the up-regulated expression of genes related to the phenylpropanoid pathway and promoted the synthesis of polyphenols at the molecular level. Moreover,cold storage had a stronger induction effect compared with on-tree storage.
4.Discussion
After 12-week storage,the color of blood oranges turned deep red from yellow due to the accumulation of anthocyanin in oranges (Stranoet al.2021b). Both treatment fruits kept high content of ascorbic acid and soluble solid for 12 weeks,indicating that the orange had good edible quality (Zhuet al.2021). The gradual increase in the solid-acid ratio suggested that the current storage treatment resulted in a higher maturity index and improved the flavor of blood oranges. Moreover,cold storage exerted a more potent effect on the maturity than on-tree storage.
Our study aimed to assess the changes in polyphenol content,composition,and antioxidant activity of blood oranges during two storages. Phenolic substances not only impart much color and flavor to fruits but also play a critical role in resisting environmental stress. The current study revealed that the TPC of blood orange pulp increased during storage,and on-tree storage was significantly higher than cold storage at certain sampling points,although the fluctuation of TPC was observed in the beginning storage. These findings may result from the balance of two antithetic processes: the degradation process and the biosynthesis of defense molecules induced by external stress (Pannitteriet al.2017). The results are consistent with the findings reported by Habibi and Ramezanian (2017) and the study of kiwi stored on trees (Gulloet al.2016) and the results of Gannan navel orange (Zhanget al.2022). The changing trend of phenolics indicated that cold storage promoted the significant increase of TPC by stimulating the synthesis of free phenolics,while the increase in TPC of the on-tree storage group resulted from the change of both free and bound phenolics.
The difference in TQPC between the cold storage and on-tree storage groups at W4 and W8 revealed that the phenolic enrichment process of blood oranges under ontree storage was more stable than that under cold storage.The proportion of flavone,flavonol,and flavanones in TQPC declined during storage,while the percentage of phenolic acids,such as gallic acid,caffeic acid,p-cournaric acid,and chlorogenic acid,increased under both storage conditions. These results indicated that phenolic acids were the phenolic substance mainly synthesized by blood oranges during storage. In addition,on-tree storage has a better effect on the synthesis of phenolic acid than cold storage.
There are several reasons for the continuous increase in phenolics content of blood oranges under both storage treatments. In contrast to the changing trend of phenolics,the gradual decrease in titratable acids was observed during 12-week storage,which can be attributed to those organic acids,such as citric acid and ascorbic acid,providing carbon skeleton for the synthesis of phenolics(Mathabaet al.2014). The biosynthesis of flavonoids responds to physical damage,infection,stress,or ultraviolet light (Ledesma-Escobaret al.2018). Pathogen attack or animal ingestion can alter the phytochemical profile of plants (Brovelli 2006). Therefore,the feeding behavior of wild birds and the invasion of microorganisms may be one of the reasons for the high TPC at W4 and W8 of the on-tree storage. Moreover,the nutrients that fruits constantly absorb from the tree body provide the substrate for the synthesis of phenolics (Guoet al.2007).Some phenolics respond actively to higher altitudes and ultraviolet radiation (Senicaet al.2019). Ultraviolet B induces flavonol biosynthesis through activation of the transcription factor BBX-ELONGATED HYPOCOTYL 5 module and inhibition of BRI1-EMS-SUPPRESSOR1(Dong and Lin 2021). Furthermore,UV-B specifically induces the expression ofOsUGT706C2which encodes a UDP-dependent glycosyltransferase and regulates phenylpropanoid metabolism,including flavonoid content in rice at both the transcript and metabolite levels (Zhang Fet al.2020). The temperature difference between day and night during on-tree storage can be up to 13°C.The large difference is conducive to the accumulation of nutrients such as carbohydrate (Tanget al.2019),which can regulate the developmental and metabolic processes such as polyphenol biosynthesis (Dong and Lin 2021). Consequently,the TQPC under on-tree storage was higher than that under cold storage. As for phenolic acid,the content of free hydroxycinnamic acids such as ferulic acid,p-coumaric acid,sinapic acid,and caffeic acid increased under both storage methods,which indicated that most derivatives of hydroxycinnamic acid were hydrolyzed to free state. The hydrolysis condition significantly affected the degradable phenolic acids (Kimet al.2006). Alkaline hydrolysis was commonly used to release bound phenolic acids,and this could further explain that only two kinds of bound phenolic acids were identified in our research.
The correlation analysis showed that the antioxidant activity of blood orange fruits during storage was closely related to the content of total phenolics and phenolic components (Table 3). More phenolic components were involved in the scavenging behavior of DPPH radical and oxygen free radicals under on-tree storage,and most of these phenolics equipped the antioxidative dominantstructure. Their contribution to the antioxidant activity resulted in the superiority of the on-tree storage group.The ORAC values of bound phenolics were positively correlated with their total content (P<0.01). Specifically,the content of bound narirutin and hesperidin contributed to the ORAC values under two storage methods,whereas free hesperetin,rhoifolin,and gallic acid contributed to the ORAC values only under on-tree storage.
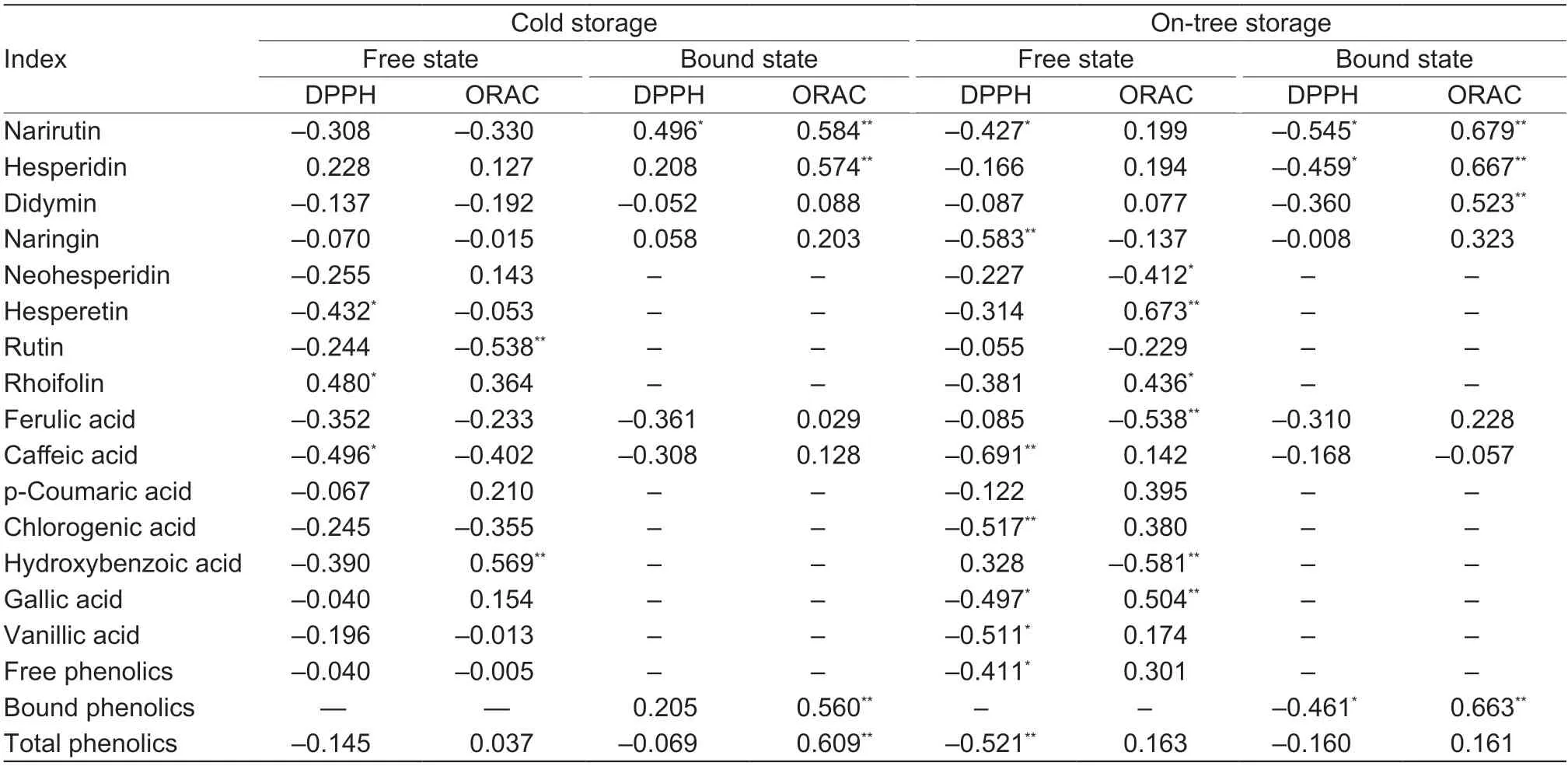
Table 3 Correlation coefficients between individual phenolic content,free phenolic content,bound phenolic content,total phenolics content,1,1-diphenyl-2-picrylhydrazyl (DPPH),and oxygen radical absorbance capacity (ORAC) in ‘Tarocco’ blood orange pulp during cold storage at 6-10°C and 80-85% RH and on-tree storage for 12 weeks
The antioxidant activity of phenolic extracts depends on the composition and content of phenolic compounds. The position and amount of hydroxyl groups in the structure of phenolics generally determine the strength of their antioxidant activities (Chen and Ho 1997). The presence of 5-hydroxyl structure in the A-ring and a catechol structure,a 5′-hydroxyl group,or multiple hydroxyl groups in the B-ring can enhance the antioxidant activity of flavonoid (Gülcin 2012). In addition,a large number of hydroxy substitutions,the presence of a carbonyl group,ester,or lactone (Gülcin 2012),and the substitution of methoxy groups (Dziedzic and Hudson 1984;Göçer and Gülçin 2011) improved the antioxidant activity of phenolic acids. Therefore,in theory,rutin and sinapic acid have the strongest antioxidant activity,followed by hesperetin,ferulic acid,caffeic acid,and chlorogenic acid. In the current study,the antioxidant activity of polyphenol extracts was higher in the on-tree storage group than in the cold storage group (P<0.05). It can be explained that the content of rutin,hesperidin,naringin,sinapic acid,and caffeic acid was higher under on-tree storage than cold storage (P<0.05). The changes in the content of these individual phenolic compounds led to the alteration of antioxidant activity (Liuet al.2015). The structure of all identified phenolic compounds can be seen in Appendices D and E. Moreover,the alteration in the percentage of phenolic acid was similar to the antioxidant activity to some extent,so the antioxidant activity seemed to be primarily related to the phenolic acids.
Furthermore,the difference in phenolic content between cold storage and on-tree storage may result from the differences in the activity and expression of enzymes involved in the phenylpropane pathway. The phenylpropanoid pathway is mainly responsible for the biosynthesis of secondary metabolites such as flavonoids,which are catalyzed by PAL,C4H,4CL,CHS,CHI,FSI,FLS,and other enzymes (Appendix F) (Chaudharyet al.2016). The biosynthesis of phenolics plays an important role in plant resistance to oxidative stress and defense against pathogens (Yiet al.1997;Saberiet al.2018;Liet al.2019). The increase in phenylpropanoid pathway enzyme activities effectively facilitated the accumulation of phenolics (Geet al.2019). The activity of phenylpropanoid pathway enzymes of the on-tree storage group was higher than that of the cold storage group(P<0.05),indicating that the on-tree storage condition was more beneficial to promoting the progress of the phenylpropane pathway. External stimulating factors,low temperature,mechanical damage,light,pathogen infection,and insect feeding can induce PAL expression(Huang and Zhao 2017). Multiple factors of the external environment may be the reasons for the early peak of the activity of enzymes in the on-tree storage group. A previous study revealed that the activity of CHI increased during fruit coloring and decreased during fruit ripening(Wanget al.2017). The change in CHI activity might suggest that blood oranges entered the turning stage at weeks 8 and 4 under cold storage and on-tree storage,respectively.
The gene expression of phenylpropanoid biosynthetic pathway-related enzymes played an important role in the synthesis of polyphenols. In our study,the expression of most measured genes was up-regulated,which was consistent with the increasing trend of phenolics content,indicating the positive regulatory effect on phenolics biosynthesis. The level of gene expression of the cold storage group was higher than that of the on-tree storage group,which was different from the results of TQPC and enzyme activities. Tsaniklidiset al.(2017) reported the non-consistent patterns of the transcription and enzyme activity of PAL and the concentrations of total phenolics of sweet cherry in postharvest storage. Most enzymes are essential proteins,and the transcription and translation of proteins have time-space specificity,and proteins are usually modified,resulting in changes in localization function,stability,and activity (Chenet al.2017). Specifically,phenylpropanoid metabolism is extremely complex and regulated by diverse signaling pathways and regulation mechanisms such as transcriptional regulation,posttranscriptional regulation,post-translational regulation,and biotic and abiotic stresses (Tsaniklidiset al.2017;Dong and Lin 2021). MYB transcription factors (TFs),MBW ternary complex and additional TFs play a central role in the transcriptional regulation of phenylpropanoid metabolism,especially the structural genes of flavonoid biosynthesis (Yuan and Grotewold 2020). Multiple micro RNAs (miRNAs) have been identified as the post-transcriptional regulators of the phenylpropanoid metabolism pathway that target the structural genes(Zhang Jet al.2020),which leads to the inconsistency between the levels of mRNA transcription and enzyme activities of these phenolic genes. Furthermore,all the studied enzymes have been demonstrated to be involved in polyphenol accumulation in blood oranges during cold storage. However,there is little knowledge on polyphenol synthesis in blood oranges under on-tree storage. Therefore,we speculated that the regulation patterns and regulators of the synthesis of phenolic compounds under on-tree storage might be different from the cold storage group,and there are other phenolic biosynthesis pathways or more important transcription factors under on-tree storage,which need to be further explored in future.
5.Conclusion
Our study suggested that the storage period of fresh blood oranges can be effectively extended by 12 weeks through on-tree storage. Postharvest cold storage and on-tree storage mainly facilitated the increase of total phenolics by stimulating the synthesis of phenolic acid,resulting in antioxidant activity changes in ‘Tarocco’blood oranges. On-tree storage was more conducive to accumulating fruit pulp polyphenols and enhancing antioxidant activity. In addition,postharvest cold storage accelerated the accumulation of phenolic substances in blood oranges by activating the phenylpropane pathway-related enzyme activity and gene transcription,while the pathway has no obvious effect under on-tree storage. This finding indicates a complex regulation of the biosynthesis of phenolic substances in blood oranges under on-tree storage. Future research should study the phenolic biosynthesis key pathway and other important transcription factors in blood oranges under on-tree storage.
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFD0401301) and the Chongqing Postgraduate Scientific Research Innovation Project,China (CYS18120).
Declaration of Competing lnterest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
杂志排行
Journal of Integrative Agriculture的其它文章
- Integrated pest management and plant health
- Neopestalotiopsis eucalypti,a causal agent of grapevine shoot rot in cutting nurseries in China
- Development of a texture evaluation system for winter jujube(Ziziphus jujuba ‘Dongzao’)
- Statistical analysis of nitrogen use efficiency in Northeast China using multiple linear regression and Random Forest
- Characteristics of inorganic phosphorus fractions and their correlations with soil properties in three non-acidic soils
- Fractionation of soil organic carbon in a calcareous soil after longterm tillage and straw residue management
