Preparation and characterization of HMX/NH2-GO composite with enhanced thermal safety and desensitization
2022-11-28YulanSongQiHuangBoJinRufangPeng
Yu-lan Song,Qi Huang,Bo Jin,Ru-fang Peng
State Key Laboratory of Environment-friendly Energy Materials,School of Materials Science and Engineering,Southwest University of Science and
Technology,Mianyang,621010,China
Keywords:HMX GO Ammonia Surface coating technology
ABSTRACT To improve the safety of HMX,HMX/NH2-GO composite was prepared with aqueous ammonia functionalized graphene oxide(NH2-GO).The composite was characterized by SEM,Zeta potential,XPS,Raman spectrum,XRD,HPLC,DSC and BAM sensitivity test.The results indicated that the functionalization with aqueous ammonia can enhance the interaction between GO and HMX,and more efficiently desensitize the explosive.The optimal impact sensitivity of the HMX/NH2-GO composite can be not less than 40 J,which is also the most insensitivity compared to the previous reports prepared by coating desensitization with non-energetic desensitized material.Moreover,the potential reason for the different impact and friction sensitivity was also discussed,which may bring a novel perspective to achieve the desensitization of energetic material.
1.Introduction
Energetic materials are a kind of functional materials containing a lot of chemical energy,such as:hexahydro-1,3,5-trinitro-1,3,5-triazine(RDX),1,3,5,7-tetranitro-1,3,5,7-tetrazocane(HMX),2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane(CL-20).It is widely used in civil and military fields because it can quickly react and release a lot of gas and heat[1-6].Among them,HMX is a kind of high explosive,which is widely used at present[7].It has some significant advantages,such as high density,burning rate,detonation velocity and detonation pressure[8,9].However,it is sensitive to external stimuli(such as impact,friction and heat),which may lead to unexpected explosion in manufacture,transportation,storage and usage[10,11].Therefore,the sensitivity of HMX is still the limiting factor for its application.
In the previous research,several strategies have been utilized to solve the contradiction between safety and energetic performance,such as the design of new insensitive energetic compounds.However,the cost of the synthesis will be expensive and a lot of time also will be needed[12-14].Optimization of energetic crystal is another method to desensitize the energetic material.Unfortunately,this strategy needs to customize optimization for every energetic compound respectively,and some energetic crystal is hard to obtain the optimization[15].Co-crystal is a novel strategy to balance the safety and energetic performance.However,this method is difficult to prepare on a large scale[16,17].Therefore,surface coating technology might be the most feasible and practical way to improve the safety of energetic materials[18,19].
For surface coating technology,the selection of the coating materials has a great influence on the effect of desensitization.Therefore,it is very important to choose the appropriate materials.Many research results have proved that the mechanical sensitivity of energetic materials could be decreased when they are coated with wax[20],polymer[12],stearic acid[21],energetic desensitizer[22],graphite[23],and carbon nanomaterials[24].Among these materials,carbon nanomaterials(graphene,GO,reduced graphene oxide(r-GO),etc.)have been widely studied because of their unique structure and excellent properties.GO,as a new type of two-dimensional nanomaterial,has excellent electrical,mechanical and thermal properties[25].Because its surface has a wide range of oxygen-containing functional groups(phenol,hydroxyl,epoxide and carboxyl),which can be easily functionalized[11].Therefore,GO is more suitable for coating applications in energetic materials than others’carbon nanomaterials.According to previous reports,GO could stabilize energetic materials such as HMX,RDX,CL-20,etc.[26-28].But it still has some shortcomings,such as low coating rate,weak interface force between coating material and energetic material,etc.[2,29,30].
In our previous work,CL-20 was coated by in-situ reduced-GO reacted with hydrazine hydrate[24].The possible mechanism was also proposed,which has indicated the reduction of carboxyl group is benefit for the desensitization.Moreover,the introduction of amino group can improve the interaction between GO and CL-20,which is more important to enhance the safety.Inspired by this mechanism,we utilized aqueous ammonia to improve the amino functionalization of GO,and enhance the interaction between GO and energetic material in this work.The impact sensitivity of the coating composite was more than 40 J by BAM method,which is the best impact desensitization of HMX by coating technology with non-energetic desensitized material so far.Moreover,the potential reason of different mechanical sensitivity was also discussed,which may bring a novel perspective to achieve the desensitization of energetic material.
The morphology and content of the energetic composite were determined by SEM and HPLC.The thermal stability and nonisothermal kinetics were evaluated by DSC,Kissinger and Ozawa methods.Moreover,the mechanical sensitivity of raw materials HMX and HMX/NH2-GO was determined by BAM sensitivity testers.The results have proved that the functionalization of GO with aqueous ammonia can effectively enhance the interface effect and reduce the sensitivity of energetic materials.
2.Experimental
2.1.Materials
The raw HMX was provided by the Institute of Chemical Materials,Chinese Academy of Engineering Physics.GO was obtained from Shanghai Aladdin Reagent Factory(Shanghai,China).Aqueous ammonia(28 wt%)was supplied by Chengdu Kelong Chemical Reagent Factory(Chengdu,China).Ultrapure water(18.25 MΩ cm)was prepared by a Millipore Milli-Q system and used throughout the experiment.
2.2.Preparation of NH2-GO
Ammonia water(35 μL,28 wt% in water)was added to the GO aqueous suspension(50 mL,0.2 mg/mL).The mixture was placed in a 100 mL round bottom flask and heated to 100°C under magnetic stirring for 2,4,6 and 8 h,respectively.Then,freeze-drying is carried out.Finally,NH2-GO with different reaction time(labeled as NH2-GO-xh,xas the reaction time)was obtained.
2.3.Preparation of HMX/NH2-GO composite
The preparation of HMX/NH2-GO composites was shown in Fig.1.10 mg GO was put into 30 mL ultrapure water,and ultrasonic dispersion was carried out for 2 h.Then,490 mg HMX and 20 mL ultra-pure water were added into the mixed solution,and ultrasonic dispersion was continued for 1 h 35 μL of aqueous ammonia water was added.The mixed solution was stirred and reacted at 100°C for 2,4,6 and 8 h,respectively.After filtration,washing and vacuum drying at 50°C,HMX/NH2-GO-xh(xas the reaction time)composite was obtained.
2.4.Characterization
Scanning electron microscope(SEM)for measuring the morphology of the samples was made on an Ultra 55 microscope system(Zeiss,Germany).X-ray photoelectron spectroscopy(XPS)was acquired from Thermo VG 250(USA).X-ray diffraction(XRD)patterns were obtained by using an X"Pert Pro X-ray diffractometer(PAN analytical,Netherlands).Raman spectra were recorded using an Invia Raman Spectrometer(Renishaw,England)with an excitation wavelength of 514.5 nm and the wavelength range of 100 cm-1to 3000 cm-1.Differential scanning calorimeter(DSC)curves were conducted with a TA Instruments DSC Q200.Highperformance liquid chromatography(HPLC)was employed to detect HMX content in coated samples at a wavelength of 254 nm.Acetonitrile was used as the mobile phase in the experiment at a flow rate of 0.5 mL/min.The sensitivities to impact(IS)and friction(FS)were determined using a standard BAM Fall hammer tester and a BAM friction tester.
3.Results and discussion
3.1.Preparation and characterization
3.1.1.N1s analysis
The introduction of amino group can improve the interaction between GO and CL-20,and enhance the desensitization and thermal stability[24].Therefore,X-ray photoelectron spectroscopy(XPS),first of all,was utilized to determine the optimal preparation condition of NH2-GO.As shown in Fig.2a,a new peak of N1s arises in NH2-GO-2h,which indicates that GO was successfully functionalized by aqueous ammonia.To know the specific existence form of N element,the N1s of NH2-GO-2h was also analyzed in Fig.2b.The N1s spectrum has characteristic peaks at 398.5 eV,400.0 eV and 401.7 eV,which is corresponding to C-N=C,C-NH2and C-NH-C=O bonds respectively[31].
With the treat of aqueous ammonia,amino group can be successfully introduced into GO.To verify the relationship between the content of amino group and reaction condition,the N1s of all prepared NH2-GO-xh was also analyzed and the data was presented in Table 1.When the reaction temperature is 100°C and the reaction time is 2 h,the NH2-GO-2h has the most content of amino group.Therefore,100°C and 2 h are determined as the preparation condition of HMX/NH2-GO composite in the following study.
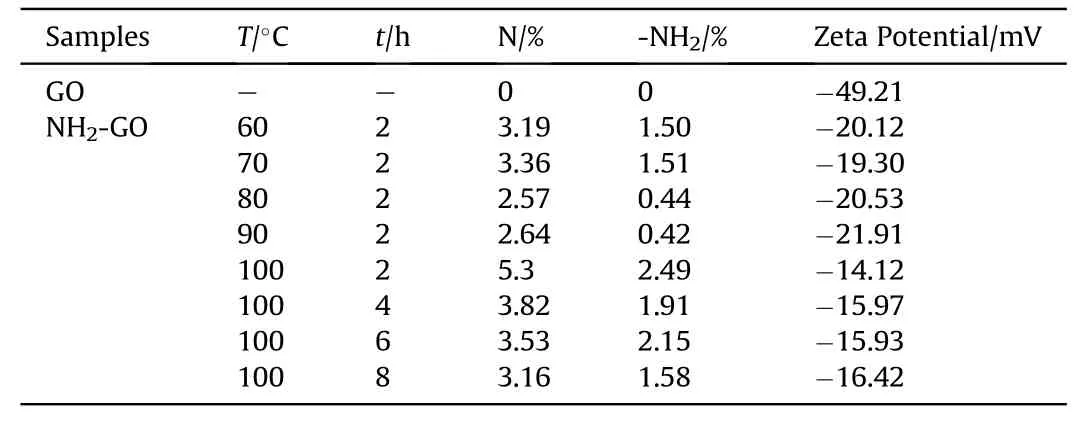
Table 1Zeta potential,N content and amino content of GO and NH2-GO.
3.1.2.Morphological analysis
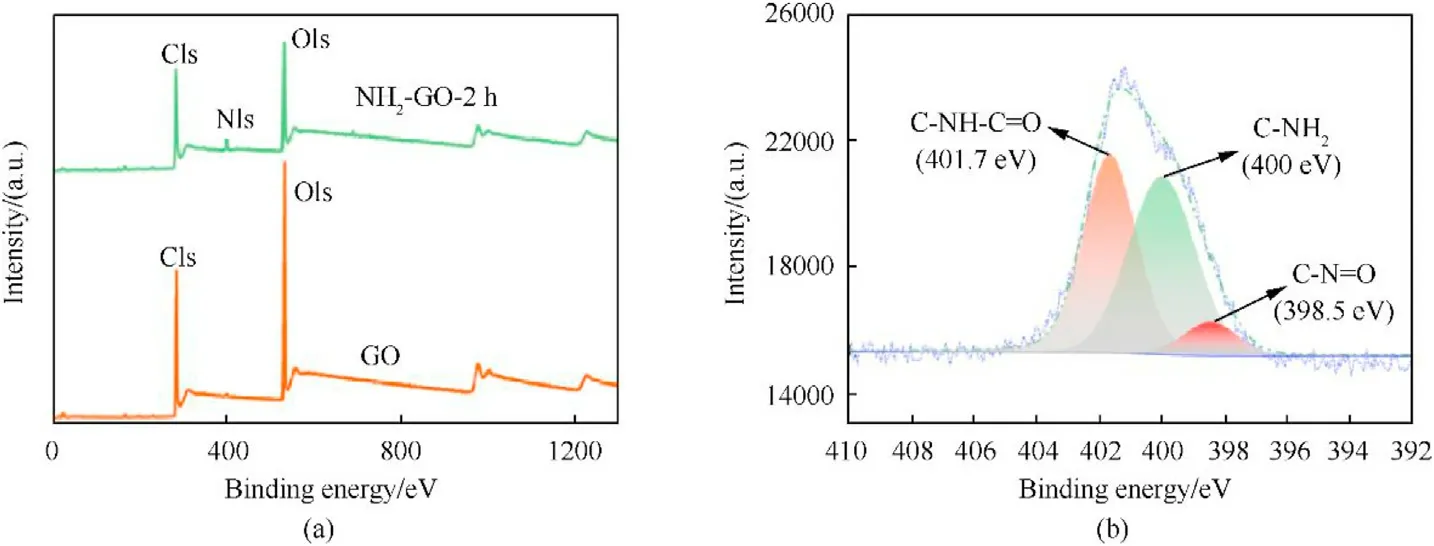
Fig.2.XPS spectra of GO and NH2-GO-2h(a)and the N1s spectra of NH2-GO-2h(b).
To verify the preparation of HMX/NH2-GO composite,the morphology of the raw HMX,GO,NH2-GO-2h and HMX/NH2-GO-2h composite was investigated in detail by SEM images.From the SEM images of GO and NH2-GO-2h,it can be seen that there is no special change in both of them,and both of them have fold morphology.It can be seen that the raw HMX has not processed in Fig.3c,which presents uneven particle size and irregular morphology.After the treatment with NH2-GO-2h,there is obviously wrinkled shape of GO sheet coating on HMX particles,which indicates the successfully preparation of HMX/NH2-GO-2h composite.Unfortunately,due to the strictly addition amount of GO by 2 wt%,not all the particles can be covered by the GO sheet.
3.1.3.Raman analysis
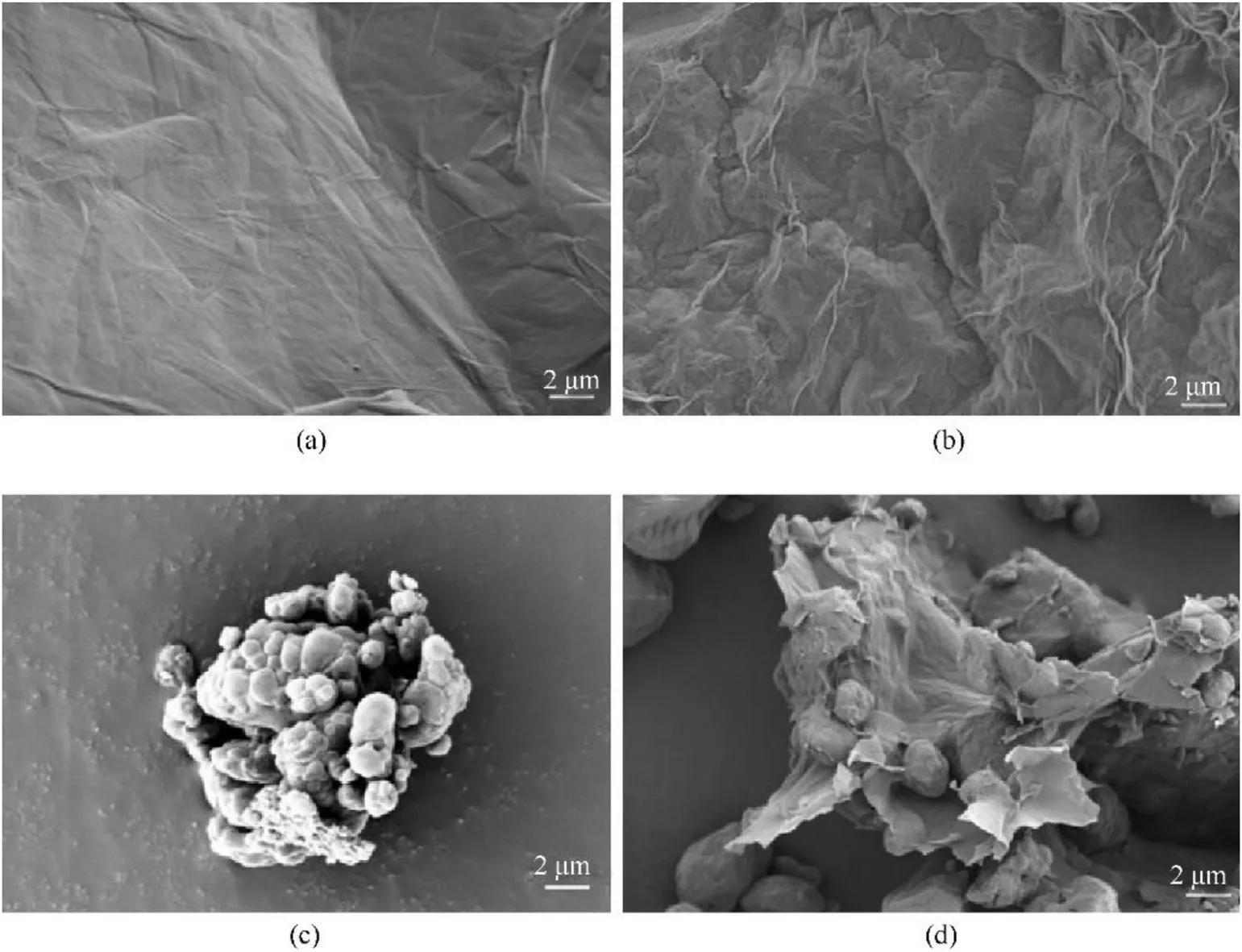
Fig.3.The SEM images of GO(a)and NH2-GO-2h(b),raw HMX(c),HMX/NH2-GO-2h(d).
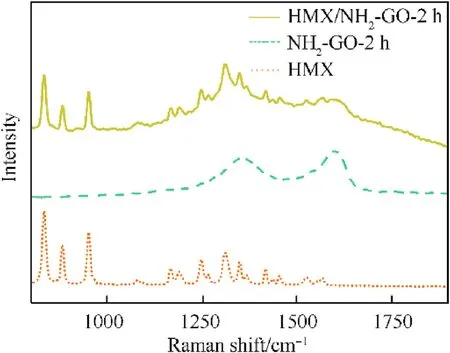
Fig.4.Raman spectra patterns of NH2-GO,HMX,HMX/NH2-GO-2h.
Raman spectrum can provide detailed chemical information without destroying material structure.Therefore,the Raman spectrum of raw materials and HMX/NH2-GO-2h composites were presented in Fig.4.For the Raman spectrum of HMX,the peaks between 830 and 970 cm-1are contributed by ring stretching of the structure.The peaks between 1248 and 1420 cm-1are attributed to the symmetric vibration stretching of the NO2and N-N group.The peaks between 1525 and 1570 cm-1are corresponded to the anti-symmetric vibration stretching of the NO2group.The results are also consistent with the previous report[18].The Raman spectrum of NH2-GO-2h was mainly composed of G and D peaks,which is around 1580 cm-1and 1370 cm-1respectively.The G peak is the main characteristic peak of graphene,which is caused by the in-plane vibration of sp[2]carbon atom[32].The D peak is caused by the structural defects of functional groups,edge effects,ripples and charge pits on the carbon substrate[33,34].Therefore,the D peak can be considered as the disordered vibration peak of graphene.These peaks are specific signals of graphene in Raman spectrum,which are not present in pure HMX.However,the HMX/NH2-GO-2h composite not only presents the characteristic peaks of pure HMX,but also has similar D and G signal with NH2-GO-2h,which indicates the successfully preparation of HMX/NH2-GO-2h composite.
3.1.4.C1s analysis
To further verify the preparation of HMX/NH2-GO-2h composite,the C1s spectrum of original HMX and HMX/NH2-GO-2h was also analyzed to understand the change of surface element content and chemical state.As shown in Fig.5a,the C1s spectrum of raw HMX presents characteristic peaks at 284.6 eV and 287.7 eV,which is corresponding to C-H and N-C-N bonds respectively[18].After the treatment with NH2-GO-2h,the C1s spectrum of HMX/NH2-GO-2h not only has the characteristic signal of HMX,but also presents new peaks at 284.3 eV,285.1 eV,287.3 eV,288.0 eV and 288.5 eV.These peaks are corresponding to C-C/C=C[35],C-OH[36],C=O[37],C-NHR and C-C=O bonds[37]respectively,which is also the characteristic peaks of NH2-GO-2h.All these results indicate the successfully preparation of HMX/NH2-GO-2h composite.
3.1.5.XRD analysis
The crystal form will affect the energy content and safety of the explosives.Therefore,X-ray diffraction(XRD)was utilized to characterize the crystal form of raw HMX and HMX/NH2-GO-2h composite.As shown in Fig.6,the XRD spectrum of HMX possessed the characteristic diffraction peaks of the β-HMX crystal form,which has been proved by previous literature(JCPDS Card No.42-1768)[8].After the treatment with NH2-GO-2h,the diffraction peaks of HMX/NH2-GO-2h still present similar signal with raw β-HMX.This result indicates that the preparation of HMX/NH2-GO-2h composite will not cause the phase transition of crystal form.
3.1.6.HPLC analysis
To ensure no damage of the structure during the preparation,high-performance liquid chromatography(HPLC)was utilized to determine the retention time and content of HMX in the HMX/NH2-GO-2h composite.As shown in Fig.7,the retention time of HMX in the composite is consistent with that of the raw HMX,which indicates that the molecular structure of the explosive will not be destroyed during the preparation.The standard curve of the concentration integral area of raw material HMX was also obtained and presented in Fig.7.By this standard curve,the concentration of HMX in HMX/NH2-GO-2h composite material was determined to be 98%,which was consistent with the addition amount in the preparation.

Fig.5.The C1s spectra of raw HMX(a),the C1s spectra of HMX/NH2-GO-2h(b).
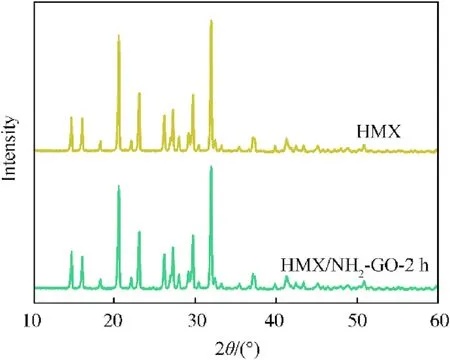
Fig.6.XRD spectra patterns of HMX,HMX/NH2-GO-2h.
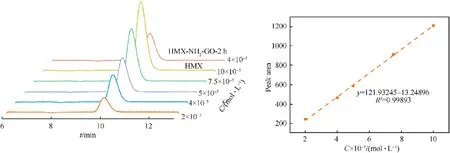
Fig.7.HPLC patterns of the raw HMX and HMX/NH2-GO-2h(left),concentration-integral area standard curve of raw HMX(right).
3.1.7.Mechanical sensitivity
Mechanical sensitivity is an important index to evaluate the safety of explosives.It directly affects the storage,transportation,manufacturing and application of energetic materials.To determine the impact and friction sensitivity of HMX and HMX/NH2-GO-2h composite,the mechanical sensitivity of the samples was characterized by standard BAM method.The results were listed in Table 3.The impact sensitivity and friction sensitivity of raw HMX are 10 J and 96 N,respectively.After the treatment with NH2-GO-2h,the impact sensitivity of HMX/NH2-GO-2h composite is not less than 40 J and the friction sensitivity is 144 N,which is obviously more desensitization than that of raw HMX.And to our best knowledge,this impact sensitivity is the most insensitivity compared to the previous reports prepared by coating desensitization with nonenergetic desensitized material in Table 2[2,5,19,38-40].Especially,the addition amount of desensitized material in this preparation is strictly controlled to 2 wt%,which is also less than most previous reports(see Table 2).
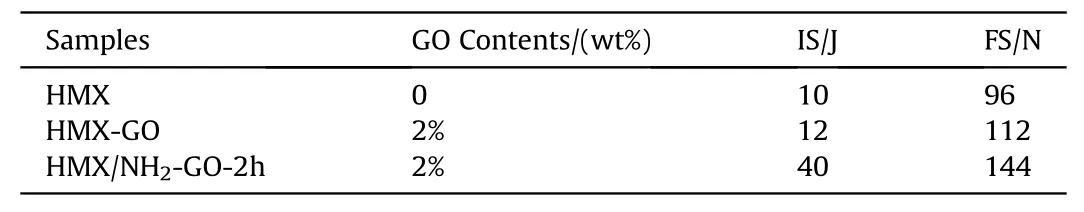
Table 3The impact sensitivity and friction sensitivity of raw HMX and the composites.
To compare the desensitization introduced by aqueous ammonia,the HMX-GO composite was also prepared only without the addition of aqueous ammonia.The impact sensitivity and friction sensitivity of HMX-GO composite is 12 J and 112 N respectively,which is less desensitization than that of HMX/NH2-GO-2h composite.This result indicates the functionalization introduced by aqueous ammonia can enhance the desensitization of GO and contribute to the insensitivity of the explosive.
3.1.8.Thermal analysis
The thermal stability of the samples was also studied by differential scanning calorimetry(DSC).As shown in Fig.8,the peaktemperature of decomposition of raw HMX is 280.71°C at the heating rate of 5°C/min.The peak temperature of decomposition of HMX-GO is 281.46°C,which is slightly higher than that of HMX[34].After the treatment of NH2-GO-2h,the peak temperature of decomposition of HMX/NH2-GO-2h is 281.82°C,which indicatesthe NH2-GO-2h can further enhance the thermal stability of raw HMX.
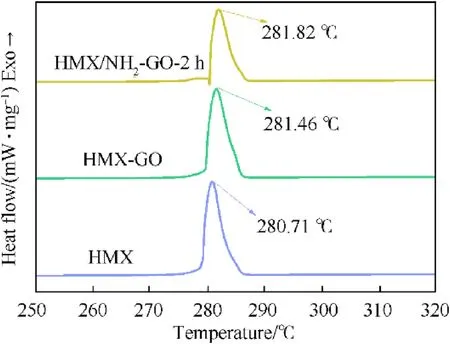
Fig.8.DSC curves of different samples at the heating rate of 5 °C/min.
To further understand the effect of NH2-GO-2h on the decomposition of HMX,non-isothermal kinetics studies were carried out on raw HMX,HMX-GO and HMX/NH2-GO-2h composite at different heating rates(5,10,15 and 20°C/min)by DSC.The results are shown in Fig.9 and Table 4.And Kissinger-Akahira-Sunose(KAS)method and Flynn-Wall-Ozawa(FWO)method[41-43]were used to obtain the kinetic parameters of the three samples.
Kissinger-Akahira-Sunose(KAS)method:

Flynn-Wall-Ozawa(FWO)method:

In these equations,according to KAS method and FWO method,EKASandEFWOpresent activation energy.G(α)is the mechanism function.Ais the preexponential factor.α is the conversion degree.β is the different heating rate.Ris the gas constant with the value of 8.314 J·mol-1K-1.TPis the peak temperature of the DSC curve.
Fig.9(d)and 9(e)showed the fitting results of kinetic parameters of pure HMX,HMX-GO and HMX/NH2-GO-2h composites by KAS method and FWO method,respectively.The value ofEKAS(385.5701 kJ/mol)by KAS method was in good accord with that obtained by FWO method(EFWO=375.4862 kJ/mol).Meanwhile,the liner correlation coefficients were all more than 0.99,which ensure the reliability of the result.The activation energy of HMX/NH2-GO-2h is higher than that of HMX and HMX-GO composite in both of two methods,which indicates the functionalization of aqueous ammonia not only can improve the desensitization,but also can enhance the thermal stability of the explosive.
3.2.Possible mechanism of impact sensitivity and friction sensitivity
3.2.1.Impact sensitivity
Zeta potential,also known as surface potential,is the characterization of the type and quantity of charge on the surface of particles.It can directly reflect the change of surface charge.To study the effect of aqueous ammonia on the surface charge of GO,Zeta potential of pure GO and NH2-GO-xh were measured.As shown in Table 5,the Zeta potential of HMX and GO is-16.28 mV and-49.21 mV,respectively.Due to the repulsive force of negative charge,the coating of GO and corresponding desensitization is limited.After the treatment of aqueous ammonia,the Zeta potential of NH2-GO-2h is-14.12 mV,which is much less negative than that of GO and benefit for the coating on HMX.
To further understand the reason of desensitization,several NH2-GO-xh and corresponding HMX/NH2-GO-xh composites were also prepared.As shown in Table 5,the change of Zeta potential is consistent with the content of amino group in NH2-GO-xh.And when the reaction time is 8 h,the NH2-GO-8h has the least content of nitrogen and amino group,which endows this sample with most negative charge.Meanwhile,the impact sensitivity of corresponding HMX/NH2-GO-8h composite is the most sensitive as shown in Table 5.
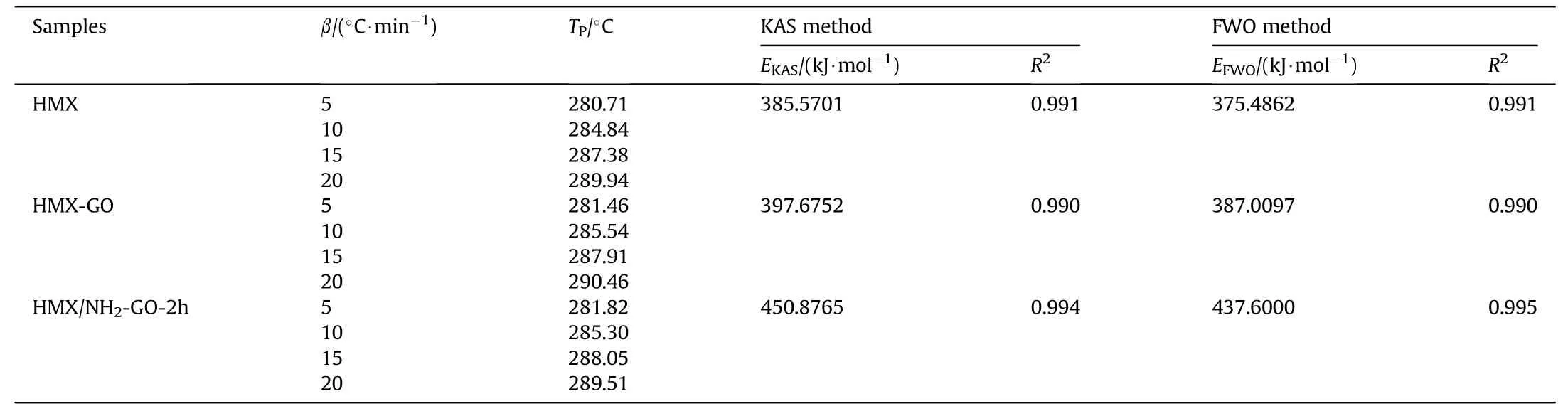
Table 4Kinetic parameters of raw HMX、HMX/NH2-GO-2h and HMX-GO composites obtained by DSC curves at different heating rates.
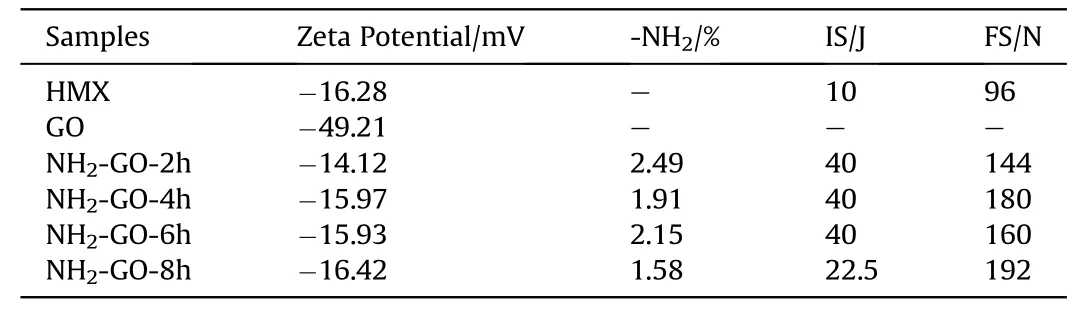
Table 5Zeta potential,amino content,impact sensitivity and friction sensitivity of HMX,GO and NH2-GO-xh.
Therefore,the possible mechanism of formation of HMX/NH2-GO-xh composite was proposed in Fig.10.With the functionalization of aqueous ammonia,amino group is introduced in GO.The new amino group not only can neutralize the negative charge of GO and decease the repulsive force with negative HMX,but also can offer electron donor to form H-bond with the nitro group of HMX.All these effects more efficiently promote NH2-GO-xh to coat HMX and transfer the generated“hot spot”,and then enhance the desensitization and thermal stability.

Fig.9.DSC curves of HMX(a)、HMX/NH2-GO-2h(b)and HMX-GO(c)composites at different heating rates,and fitting results of HMX、HMX/NH2-GO-2h and HMX-GO composites with Kissinger(d)and Ozawa(e)methods.
3.2.2.Friction sensitivity
The impact sensitivity is consistent well with the change of Zeta potential and amino group.However,the friction sensitivity does not satisfy the regularity.To explore the reason for this difference,the Raman spectra of NH2-GO-xh obtained by different reaction condition were presented in Fig.11 and Table 6.The G peak is caused by the in-plane vibration of sp[2]carbon atom,which represents the order degree of graphene.The D peak is caused by the structural defects,which represents the disorder of graphene.Therefore,theId/Igcan represent the defect density of the material.Meanwhile,the defect of GO will decrease its lubrication performance and increase friction coefficient[44].That"s means moreId/Igwill endows GO with less lubrication performance,and more“hot spot”will generate during the friction stimuli,which then will contribute to higher friction sensitivity.Therefore,when the reaction time is 8 h,the NH2-GO-8h has the lowestId/Igand the corresponding HMX/NH2-GO-8h still presents the best desensitization of friction even with the most sensitivity of impact.

Table 6The Id,Ig and Id/Ig of NH2-GO at different reaction time.
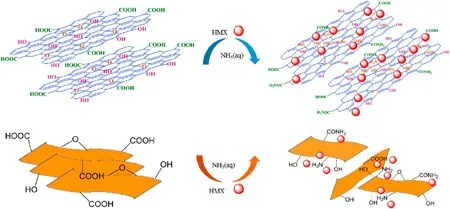
Fig.10.Possible mechanism of formation of HMX/NH2-GO-xh composite.
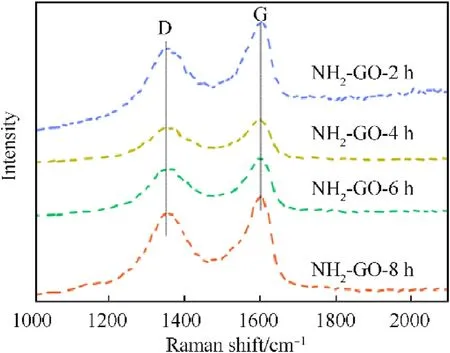
Fig.11.Raman spectra patterns of NH2-GO at different reaction time.
4.Conclusions
To improve the mechanical sensitivity and thermal stability of HMX,the HMX/NH2-GO composite was prepared by coating HMX with aqueous ammonia functionalized GO.XPS,SEM and Raman analysis proved the successfully preparation of the HMX/NH2-GO composite.XRD and HPLC analysis ensured the crystal form and content would not change during the coating process,which is benefit to maintain of the energetic performance of the composite.By the analysis of mechanical sensitivity and thermal stability,the HMX/NH2-GO composite has presented enhanced safety than that of raw HMX and HMX/GO composite,which proved the availability of the functionalization with aqueous ammonia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China(No.51972278),the Natural Science Foundation of Southwest University of Science and Technology(No.18zx7138)and the Project of State Key Laboratory of Environment-friendly Energy Materials,Southwest University of Science and Technology(project no.20fksy16).
杂志排行
Defence Technology的其它文章
- Defence Technology
- The SSA-BP-based potential threat prediction for aerial target considering commander emotion
- Visual-simulation region proposal and generative adversarial network based ground military target recognition
- Interception probability simulation and analysis of salvo of two electromagnetic coil launched anti-torpedo torpedoes
- Damage behavior of the KKV direct hit against fluid-filled submunition payload
- Formation of adiabatic shearing band for high-strength Ti-5553 alloy:A dramatic thermoplastic microstructural evolution
