miR-889-3p调控vimentin乙酰化对妊娠糖尿病胎盘滋养层细胞侵袭功能的影响*
2022-10-13杨伟辉贺鑫丁丹妮
杨伟辉, 贺鑫, 丁丹妮
miR-889-3p调控vimentin乙酰化对妊娠糖尿病胎盘滋养层细胞侵袭功能的影响*
杨伟辉△, 贺鑫, 丁丹妮
[湖南省人民医院(湖南师范大学附属第一医院)产科,湖南 长沙 410000]
探讨微小RNA-889-3p(miR-889-3p)调节妊娠糖尿病(GDM)胎盘滋养层细胞侵袭功能的作用机制。将2020年1~12月的30名GDM产妇和30名正常葡萄糖耐量产妇选入本研究。通过qRT-PCR检测miR-889-3p在产妇胎盘、人肿瘤细胞系、人滋养层HTR-8/SVneo细胞和人脐静脉内皮细胞(HUVEC)中的表达水平。进行细胞培养、细胞转染、MTT测定、EdU检测、伤口划痕实验和Transwell实验,以确定miR-889-3p的沉默和过表达对HTR-8/SVneo细胞生物功能的影响。通过生物信息学分析确定miR-889-3p与波形蛋白(vimentin)的结合关系。分别使用双萤光素酶报告基因检测系统和蛋白质印迹分析miR-889-3p过表达对双萤光素酶活性和vimentin乙酰化的影响。HTR-8/SVneo细胞的相对miR-889-3p表达水平高于其他肿瘤细胞和HUVEC,GDM产妇胎盘的相对miR-889-3p表达水平低于正常葡萄糖耐量产妇胎盘(<0.05)。miR-889-3p过表达抑制HTR-8/SVneo细胞生长、迁移和侵袭(<0.05),而miR-889-3p敲减促进细胞生长、迁移和侵袭(<0.05)。通过生物信息学分析确定vimentin是miR-889-3p的直接靶点。在转染vimentin-WT的HTR-8/SVneo细胞中,miR-889-3p模拟物的施用抑制了萤光素酶活性(<0.05),并显著提高vimentin乙酰化水平(<0.05)。上调vimentin部分拮抗miR-889-3p过表达对细胞生长、迁移和侵袭的抑制作用(<0.05),而下调vimentin减弱了由miR-889-3p敲减诱导的细胞生长、迁移和侵袭增强(<0.05)。胎盘组织中miR-889-3p的下调参与GDM病理过程。miR-889-3p通过促进vimentin乙酰化来减弱滋养层细胞的侵袭功能。
微小RNA-889-3p;波形蛋白;妊娠糖尿病;胎盘;细胞侵袭
妊娠糖尿病(gestational diabetes mellitus, GDM)是妊娠期最常见的并发症之一,定义为妊娠期出现或首次出现的任何程度的葡萄糖不耐受[1]。GDM有多种致病因素,可能是遗传因素和环境因素共同作用的结果。然而,GDM的确切发病机制仍需进一步研究。研究表明,胎盘功能障碍与GDM的发病机制有关,因为GDM相关的高血糖可以在胎盘分娩后得到解决[2]。绒毛外滋养层细胞(extravillous trophoblast, EVT)是胚胎的重要组成部分,它侵入母体蜕膜和子宫内膜以重塑螺旋动脉[3]。具有正常生物学功能的EVT对胎盘发育至关重要;如果EVT的功能过度或不足,可能会导致妊娠并发症,包括GDM[4]。因此,针对EVT功能障碍的研究可能是探索GDM发病机制的有效策略。微小RNA(microRNA, miRNA, miR)是一类单链小分子非编码RNA,在进化过程中高度保守,并在表观遗传学水平上发挥负调控作用[2]。近年来,越来越多研究证实miRNA在GDM的发病机制中发挥重要作用[5-6]。Shah等[7]使用miRNA芯片筛选GDM和正常对照组胎盘中差异表达的miRNA,发现miR-889-3p在胎盘中差异表达,但miR-889-3p在GDM中的功能和分子机制尚不清楚。已有研究证实,miR-889-3p通过靶向波形蛋白(vimentin)参与抑制肺癌细胞的侵袭[8]。vimentin是参与细胞滋养层侵袭过程的最保守和最丰富的蛋白质之一,并经历各种重要的翻译后修饰,如磷酸化、糖基化、磺酰化和乙酰化[9]。然而,尚未明确miR-889-3p介导的vimentin在滋养层中的作用。因此,本研究分析了GDM患者胎盘组织中miR-889-3p水平的变化,并进一步观察其对滋养层细胞生物学行为的影响,以期为GDM的治疗提供参考资料。
材料和方法
1 胎盘样本采集
本研究于2020年1~12月,参照美国糖尿病协会推荐的诊断标准[10],共纳入30例GDM产妇。诊断后,所有GDM孕妇都接受饮食和锻炼的指导。孕妇自行调整饮食活动1周后,抽静脉血查血糖,根据血糖值进一步指导调整膳食及体力活动方案,并进行家庭血糖监测。若空腹血糖和餐后2 h的血糖水平控制不满意(空腹血糖>5.3 mmol/L,餐后2 h血糖>6.7 mmol/L),则进行胰岛素治疗。本研究无GDM孕妇接受胰岛素治疗。此外,选取大约在同一时间怀孕,口服葡萄糖耐量试验(oral glucose tolerance test, OGTT)结果正常,并且没有产科并发症的30例产妇作为对照。所有研究对象均对本研究知情同意,本研究获湖南省人民医院(湖南师范大学附属第一医院)伦理委员会批准同意。
收集位于脐带插入部位近端2 cm处的胎盘小叶(从基底层到滋养层)并用等渗溶液冲洗,然后将这些组织块在液氮中冷冻并储存在-80 ℃用于提取总RNA。为确保样本间的可比性和均一性,所有胎盘样本均采集自分娩足月婴儿后的产妇,并因母体要求、臀位或子宫瘢痕而接受剖宫产且无其他相关疾病(如高血压、孕前糖尿病等)。
2 细胞培养
人滋养层细胞系HTR-8/SVneo购自中国科学院生物化学与细胞生物学研究所,维持在含有10%胎牛血清和1%青霉素-链霉素的RPMI-1640培养液中。该细胞系最初由孕早期的绒毛外植体产生,被认为是人类绒毛外滋养层的代表性模型[11],已被用作替代模型来研究妊娠和妊娠相关疾病中的胎盘功能。人脐静脉内皮细胞(human umlibical vein endothelial cells, HUVEC)购自ATCC,接种于含10%胎牛血清和1%青霉素-链霉素的DMEM培养液。人乳腺癌细胞系MDA-MB-231和HCC1937,人卵巢癌细胞系OVCAR3和SKOV3,以及人胰腺癌细胞系MIAPACA2和BXPC3均购自中国科学院生物化学与细胞生物学研究所,接种于含10%胎牛血清和1%青霉素-链霉素的RPMI-1640培养液。所有细胞进行传代培养,取第3代用于实验。
3 主要试剂和仪器
胎牛血清、青霉素-链霉素和RPMI-1640培养液均购自Gibco;pGL3对照载体、Trizol试剂和Lipofectamine 2000均购自Invitrogen;TaqMan MicroRNA Reverse Transcription Kit和TaqMan Univel PCR Master Mix均购自Applied Biosystems;MTT购自Promega;二甲基亚砜、苏木精和伊红均购自Sigma-Aldrich;Cell-LightTMEdU Apollo®567体外成像试剂盒购自广州锐博生物技术有限公司;Transwell小室购自Corning;Matrigel购自BD;PVDF膜购自Amersham;兔抗vimentin、沉默信息调节因子1(silent information regulator 1, Sirt1)和乙酰化vimentin(acetylated vimentin, Ac-vimentin)多克隆抗体均购自Cell Signaling Technology;小鼠抗GAPDH单克隆抗体购自Abcam;辣根过氧化物酶(horseradish peroxidase, HRP)偶联的山羊抗兔IgG或山羊抗小鼠IgG购自Jackson ImmunoResearch Laboratories;ECL试剂购自Millipore;pmirGLO载体和双萤光素酶报告基因检测系统购自Promega。
LSM 710 META激光扫描共聚焦显微镜购自Carl Zeiss;BX61显微镜购自Olympus;X射线胶片购自Kodak;Model 3550 96孔板读数器和Quantity One分析系统购自Bio-Rad。
4 主要方法
4.1质粒构建和转染将野生型(wild-type, WT)vimentin 3'非翻译区(3'-untranslated region, 3'-UTR)和vimentin 3'-UTR突变(mutant, MUT)序列通过PCR扩增(vimentin-WT的上游引物序列为5'-CCGCTCGAGCCCTCCAGACATGCACTTAC-3',下游引物序列为5'-GCTCTAGACCTACCAAGGTGAGGTCTTTATG-3';vimentin-MUT的上游引物序列为5'-GCTTTCTTTCTACAGATTTTACTACTCTTGGTCT-3',下游引物序列为5'-TAGTAAAATCTGTAGAAAGAAA‑ GCTGGGGGGTAA-3')。用I和I双酶切后,将PCR产物克隆到pGL3对照载体。将vimentin序列的编码区扩增并克隆到pcDNA3.1载体中,命名为pcDNA3.1-vimentin。所有构建体均通过DNA测序验证。用于RNA干扰实验的vimentin siRNA和scrambled siRNA购自广州锐博生物技术有限公司。miR-889-3p mimic、mimic control、miR-889-3p inhibitor和inhibitor control由上海吉玛制药技术有限公司合成,并通过Lipofectamine 2000转染到细胞中。
4.2qRT-PCR检测miR-889-3p表达用Trizol试剂分离来自人胎盘的总RNA,然后用TaqMan MicroRNA Reverse Transcription Kit和TaqMan Univel PCR Master Mix通过qRT-PCR检测miR-889-3p的表达。通过2-ΔΔCt法将目标基因的表达水平归一化为相对于核小RNA U6的表达水平。miR-889-3p的上游引物序列为5'-GTTGCTCCTGTCAGTTTGTCAAA-3',下游引物序列为5'-TATGGTTGTTCACGACTCCTTCAC-3';U6的上游引物序列为5'-ATTGGAACGATACAGAGAAGATT-3',下游引物序列为5'-GGAACGCTTCACGAATTTG-3'。
4.3MTT法检测HTR-8/SVneo细胞活力将HTR-8/SVneo细胞接种在96孔板中(每孔5 000个细胞)并使其附着过夜。用50 nmol/L miR-889-3p模拟物、miRNA模拟物阴性对照、miR-889-3p抑制物或miRNA抑制物阴性对照转染细胞。48 h后,向每个孔中加入20 μL MTT(5 g/L)并在37 ℃下孵育4 h。然后去除上清液,并将150 μL二甲基亚砜添加到每个孔中。用96孔板读数器在570 nm处记录吸光度值。
4.4EdU法检测HTR-8/SVneo细胞增殖能力使用Cell-LightTMEdU Apollo®567体外成像试剂盒。转染50 nmol/L miR-889-3p模拟物、miRNA模拟阴性对照、miR-889-3p抑制物或miRNA抑制物阴性对照48 h后,将HTR-8/SVneo细胞在37 ℃下暴露于50 μmol/L EdU 2 h。然后用Hoechst 33342对细胞核进行染色。在LSM 710 META激光扫描共聚焦显微镜下观察样品并成像。在随机选择的3个视野(×400)中分别计算增殖细胞和总细胞数量,结果表示为增殖细胞数量与总细胞数量的比率。
4.5伤口划痕实验评估HTR-8/SVneo细胞在体外的迁移能力将用50 nmol/L miR-889-3p模拟物、miRNA模拟物阴性对照、miR-889-3p抑制物或miRNA抑制物阴性对照转染的HTR-8/SVneo细胞接种在6孔培养皿中,每孔约2×106个细胞。孵育24 h后,用无菌的200 μL移液器尖端垂直制作水平伤口。每个孔用1 mL新鲜生长培养液洗涤2次以去除脱落的细胞,然后在37 ℃的培养箱中培养。用数码相机系统获取伤口在同一点制作后0 h和48 h的图像,使用标准卡尺定量评估伤口宽度。
4.6Transwell小室测定HTR-8/SVneo细胞侵袭能力将用50 nmol/L miR-889-3p模拟物、miRNA模拟物阴性对照、miR-889-3p抑制物或miRNA抑制物阴性对照转染的HTR-8/SVneo细胞接种在24孔板的顶室中,加入40 μL 1 g/L Matrigel。孵育24 h后,用棉签小心去除小室顶部未浸润的细胞,并用4%甲醛固定小室底部的侵袭细胞。然后用苏木精和伊红对细胞进行染色。在BX61显微镜下观察,以随机选择3个不同的视野(×400)来计算侵袭细胞的数量,结果表示为每个视野的平均细胞数。
4.7蛋白质印迹分析提取的蛋白质在SDS/β-巯基乙醇样品缓冲液中煮沸。60 μg蛋白质在6%~10%梯度聚丙烯酰胺凝胶上电泳并转移到PVDF膜。将膜与兔抗vimentin多克隆抗体(1∶1 000)、兔抗Ac-vimentin多克隆抗体(1∶500)、兔抗Sirt1多克隆抗体(1∶1 000)或小鼠抗GAPDH单克隆抗体(1∶1 000)在27 ℃下孵育2 h。然后将膜与HRP偶联的山羊抗兔IgG或山羊抗小鼠IgG (1∶10 000)在37 ℃下孵育1 h。将膜与ECL试剂一起温育1 min,并暴露于X射线胶片。GAPDH蛋白用作上样对照。使用Quantity One分析系统分析条带。蛋白质水平表示为目标信号与GAPDH信号的相对比率。
4.8双萤光素酶报告基因实验将vimentin 3'-UTR的部分序列和vimentin 3'-UTR中突变的miR-889-3p靶位点分别克隆到pmirGLO载体中萤火虫萤光素酶基因的下游。将HTR-8/SVneo细胞接种在48 孔板中孵育过夜,然后使用Lipofectamine 2000将50 nmol/L miR-889-3p模拟物或miRNA模拟物阴性对照转染细胞48 h。使用双萤光素酶报告基因检测系统测量双萤光素酶活性。
5 统计学分析
所有统计分析均采用SPSS 22.0软件进行。数据表示为均数±标准差(mean±SD)。使用双尾检验进行两组(配对和非配对)间的比较;单因素方差分析和Turkey事后检验用于多组间的比较。以<0.05为差异有统计学意义。
结果
1 miR-889-3p在HTR-8/SVneo细胞和GDM产妇胎盘样本中的表达
通过qRT-PCR定量miR-889-3p在不同细胞中的表达,包括HTR-8/SVneo细胞、HUVEC、乳腺癌细胞、卵巢癌细胞和胰腺癌细胞。结果显示,miR-889-3p在HTR-8/SVneo细胞中的表达最高(图1A)。为了进一步证实miR-889-3p的异常表达是否与妊娠滋养层细胞功能异常有关,我们检测了产妇胎盘中miR-889-3p的表达水平。表1列出了研究对象的临床特征,与正常葡萄糖耐量产妇相比,GDM产妇OGTT-fast、OGTT-1h、OGTT-2h和HbA1c的差异均有统计学意义(<0.05)。与正常葡萄糖耐量组相比,GDM组胎盘组织中的miR-889-3p水平显著降低(<0.01),见图1B。
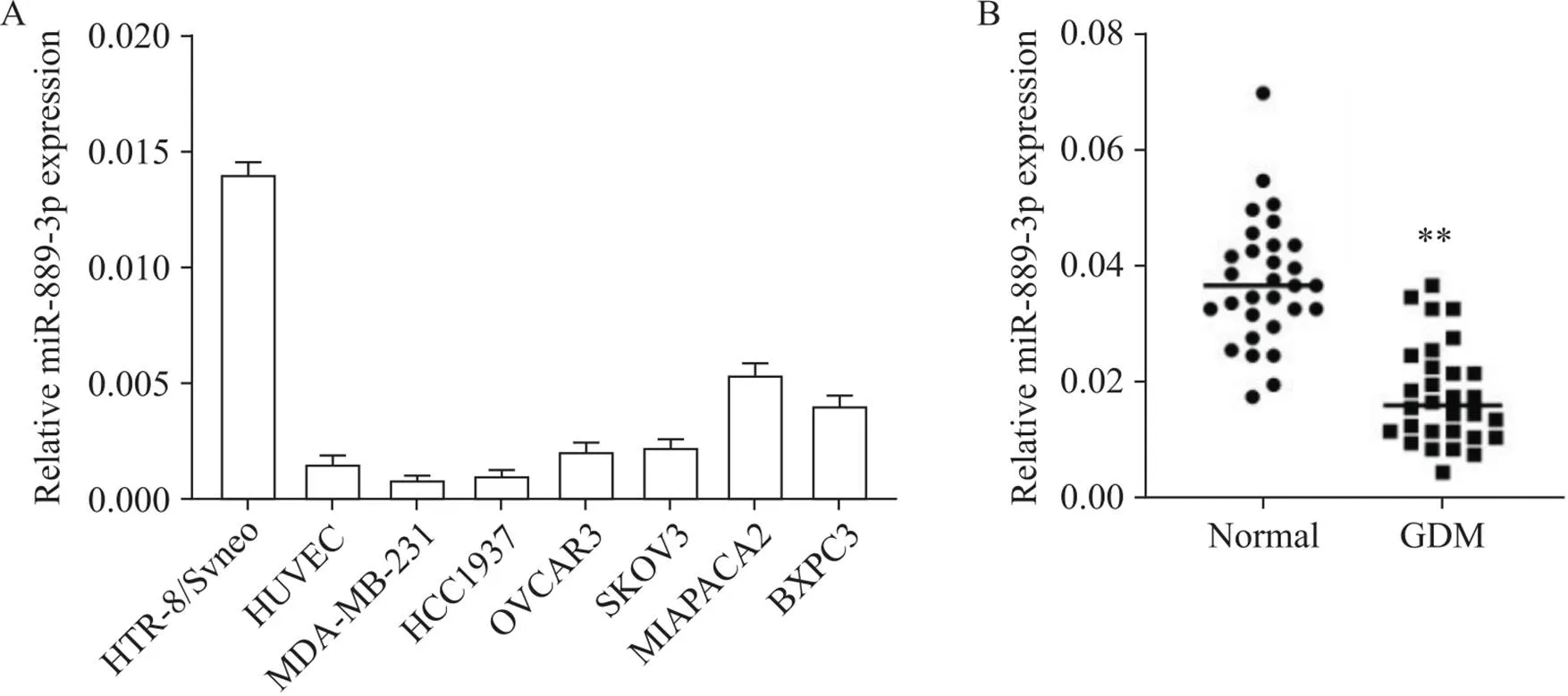
Figure 1. Expression of miR-889-3p in HTR-8/SVneo cells and GDM placental samples. A: qRT-PCR analysis of miR-889-3p expression in HTR-8/SVneo cells, human umlibical vein endothelial cells (HUVEC), breast cancer MDA-MB-231 and HCC1937 cells, ovarian cancer OVCAR3 and SKOV3 cells, and pancreatic cancer MIAPAPA2 and BXPC3 cells (n=3); B: qRT-PCR analysis of miR-889-3p expression in placental tissues from GDM group and normal glucose tolerance group (n=30). Mean±SD. **P<0.01 vs normal (glucose tolerance) group.

表1 GDM和正常葡萄糖耐量产妇和胎儿的临床特征
2 miR-889-3p对HTR-8/SVneo细胞生长的影响
为了检测miR-889-3p模拟物和抑制物的转染效率,通过qRT-PCR检测转染后HTR-8/SVneo细胞中的miR-889-3p表达水平。miR-889-3p模拟物显著增强miR-889-3p表达水平(<0.01),而miR-889-3p抑制物显著降低miR-889-3p表达水平(<0.01),见图2A、B。通过MTT法和EdU法分析miR-889-3p对HTR-8/SVneo细胞生长的影响。结果显示,与对照组相比,miR-889-3p模拟物降低细胞活力,抑制细胞增殖(<0.05),而miR-889-3p抑制物增强细胞活力,促进细胞增殖(<0.05),见图2C~F。
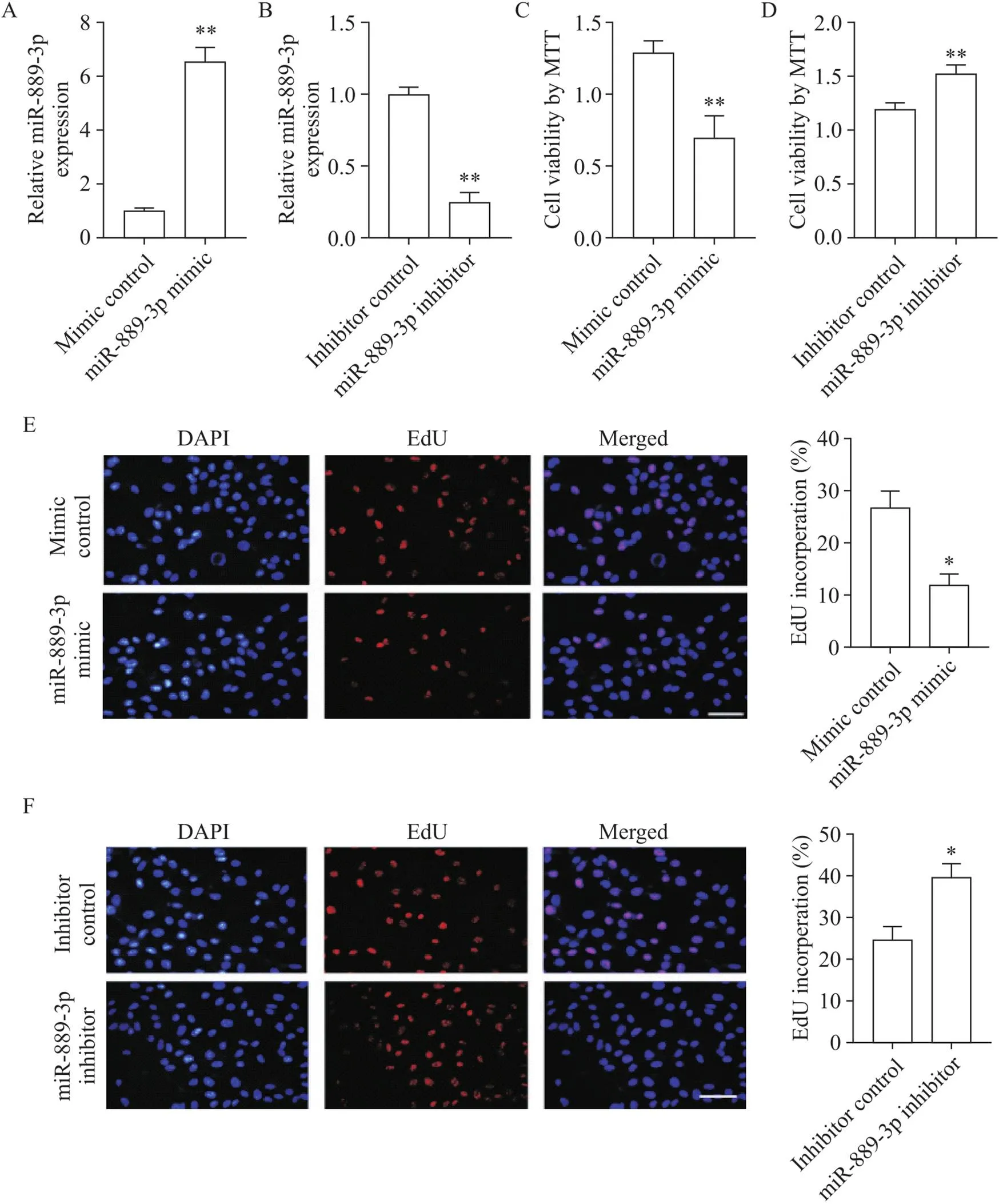
Figure 2. The effect of miR-889-3p on the growth of HTR-8/SVneo cells. A and B: miR-889-3p expression was detected after HTR-8/SVneo cells were transfected with miR-889-3p mimic or inhibitor; C and D: MTT assay was used to detect the viability of HTR-8/SVneo cells 48 h after transfection with miR-889-3p mimic or inhibitor; E and F: EdU method was used to detect the proliferation of HTR-8/SVneo cells 48 h after transfection with miR-889-3p mimic or inhibitor (scale bar=50 μm). Mean±SD. n=3. *P<0.05, **P<0.01 vs mimic or inhibitor control group.
3 miR-889-3p调节滋养层细胞的迁移和侵袭
为了进一步分析miR-889-3p在GDM发生中的作用,通伤口划痕实验和Transwell实验评估miR-889-3p对滋养层细胞迁移和侵袭的影响。与模拟物对照组相比,miR-889-3p模拟组迁移和侵袭的细胞数量显著减少(<0.05),见图3A、B;而与抑制物对照组相比,miR-889-3p抑制物组迁移和侵袭的细胞数量显著增加(<0.05),见图3C、D。

Figure 3. miR-889-3p regulated trophoblast migration and invasion. A and B: wound scratch assay and Transwell assay to detect the migration and invasion of HTR-8/SVneo cells 48 h after miR-889-3p mimic transfection (scale bar=50 μm); C and D: wound scratch assay and Transwell assay to detect the migration and invasion of HTR-8/SVneo cells 48h after miR-889-3p inhibitor transfection (scale bar=50 μm). Mean±SD. n=3. *P<0.05, **P<0.01 vs mimic or inhibitor control group.
4 miR-889-3p与vimentin相互作用并促进其乙酰化水平
通过生物信息学分析确定,人类vimentin在其3'-UTR内具有一个保守的miR-889-3p结合位点(图4A)。在vimentin-WT转染的HTR-8/SVneo细胞中,miR-889-3p模拟物的施用抑制了萤光素酶活性,而在vimentin-MUT转染的细胞中,miR-889-3p模拟物对萤光素酶活性的抑制作用被消除(图4B)。据报道,vimentin在K120处被乙酰化,该位点的去乙酰化与肝细胞癌细胞迁移能力增强有关[12]。因此,我们进一步检查了miR-889-3p对vimentin乙酰化的影响。结果显示,miR-889-3p模拟物显著提高vimentin乙酰化水平,并下调了vimentin和Sirt1(一种使vimentin去乙酰化的脱乙酰酶)的表达(图4C)。

Figure 4. miR-889-3p interacted with vimentin and inhibited its acetylation. A: the putative binding site between miR-889-3p and vimentin; B. vimentin-WT or vimentin-MUT was co-incubated with HTR-8/SVneo cells transfected with miR-889-3p mimic or mimic control, and luciferase activity was measured; C: Western blot to detect the protein levels of acetylated vimentin (Ac-vimentin), vimentin and Sirt1 in HTR-8/SVneo cells 48 h after miR-889-3p mimic transfection. Mean±SD. n=3. **P<0.01 vs mimic control group.
5 vimentin是miR-889-3p的功能靶点
为了分析vimentin是否是miR-889-3p的功能靶标,我们进行了靶基因补偿实验。与对照组相比,miR-889-3p模拟物组细胞增殖、迁移和侵袭显著抑制(<0.05);当vimentin在转染miR-889-3p模拟物的细胞中过表达时,细胞增殖、迁移和侵袭能力强于单独转染miR-889-3p模拟物的细胞(<0.05),见图5A~C。与对照组相比,miR-889-3p抑制物组细胞增殖、迁移和侵袭显著增加(<0.05);当vimentin在转染miR-889-3p抑制物的细胞中被敲减时,细胞增殖、迁移和侵袭能力弱于单独转染miR-889-3p抑制物的细胞(<0.05),见图5D~F。

Figure 5. Vimentin was a functional target of miR-889-3p. A, B and C: miRNA mock control and pcDNA3.1 control vectors were co-transfected into HTR-8/SVneo cells as controls, while pcDNA3.1 control vector or vimentin-pcDNA3.1 was co-transfected with miR-889-3p mimic; D, E and F: miRNA inhibitor control and scrambled siRNA control vectors were co-transfected into HTR-8/SVneo cells as controls, while scrambled siRNA control or vimentin siRNA (si-vimentin) was co-transfected with miR-889-3p inhibitor. Cell viability was detected by MTT assay (A and D), and cell migration and invasion were detected by wound scratch assay (B and E) and Transwell assay (C and F, scale bar=50 μm). Mean±SD. n=3. *P<0.05, **P<0.01 vs mimic control group; #P<0.05, ##P<0.01 vs mimic or inhibitor control group.
讨论
GDM对胎儿和母亲都有严重后果,其影响是长期的[3]。妊娠期患有GDM的妇女剖宫产和妊娠高血压综合征的发生率增加,并且在以后的生活中更容易患肥胖症、糖尿病和其他代谢性疾病[13]。现在普遍认为胎盘在GDM的发病机制中起关键作用。胎盘是连接母亲和胎儿的唯一接口,它不仅是参与母亲和胎儿之间物质交换和循环的重要器官,而且还具有重要的内分泌功能[14]。妊娠后,胎盘合成胎盘催乳素、性激素和母体肾上腺皮质激素,可拮抗胰岛素的功能,使孕妇靶组织对胰岛素的敏感性降低,从而导致GDM的发生[15]。因此,胎盘已成为研究GDM发病机制的重要器官。胎盘特异性miRNA可能有助于GDM的发展。本研究发现,与正常对照组相比,GDM患者胎盘组织中miR-889-3p的表达显著降低。先前研究在GDM大鼠胎盘中发现,miR-889-3p表达也减少[16]。这些数据表明miR-889-3p的减少可能与GDM有关。
滋养层细胞是滋养层的衍生物,完成胎盘的内分泌、交换、侵袭和植入过程。为了研究滋养层细胞的功能,研究者建立了不同的细胞系[17]。例如,JAR、JEG-3、BeWo和HTR-8/SVneo。但值得注意的是,JAR、JEG-3和BeWo细胞系是绒毛膜癌的癌细胞,而HTR-8/SVneo最初是在妊娠早期由绒毛外植体生成的。因此,我们在体外实验选择HTR-8/SVneo细胞用于检测miR-889-3p对细胞生物学行为的影响,结果显示miR-889-3p过表达抑制细胞生长、迁移和侵袭,而miR-889-3p敲减促进细胞迁移和侵袭。对胎盘细胞生物学行为的研究表明,GDM孕妇胎盘中滋养层细胞的增殖增加[18]。CYP1B1在GDM女性的胎盘中高度表达,并促进绒毛外滋养层活动,包括增殖、迁移和侵袭[11]。由此推测,抑制miR-889-3p可通过促进绒毛外滋养层的活动参与GDM的发生过程。
通常,miRNA通过调节其靶基因的表达来执行功能。本研究证实了vimentin的3'-UTR具有miR-889-3p响应元件,这是不同物种间高度保守的结构域。双萤光素酶报告基因实验结果表明,miR-889-3p反向调节滋养层细胞中的vimentin表达,并且miR-889-3p模拟物显著增加vimentin乙酰化。据报道,vimentin在K120处被乙酰化,该位点的去乙酰化与肝细胞癌细胞迁移能力增强有关[12]。恢复vimentin表达可以挽救miR-889-3p过表达对滋养层细胞的抑制作用;减少vimentin表达可能会减弱miR-889-3p敲减对滋养层细胞的促进作用。这些数据进一步证实vimentin是miR-889-3p的功能靶标。vimentin通过非经典转录非依赖性机制促进结直肠癌细胞生长[19]。miR-320的过表达通过下调vimentin表达来抑制胃癌细胞的体外增殖、侵袭和迁移以及体内肿瘤生长[20]。所有这些事实表明,miR-889-3p通过反向调节vimentin表达来调节滋养层细胞的活性。
总之,本研究表明胎盘组织中miR-889-3p的下调参与GDM病理过程。miR-889-3p通过上调vimentin乙酰化来减弱滋养层细胞的活性。
[1]孙贺, 马晓丹, 刘爽, 等. 血清丝氨酸蛋白酶抑制剂B1水平与妊娠期糖尿病发生风险的相关性研究[J]. 中华糖尿病杂志, 2020, 12(7):469-473.
Sun H, Ma XD, Liu S, et al. Correlation between serum serpin B1 levels and the risk of gestational diabetes mellitus[J]. Chin J Diabetes, 2020, 12(7):469-473.
[2] Novakovic B, Mansell T, Saffery R. Micromanaging human placental function: differential microRNA expression in feto-placental endothelial cells of gestational diabetes pregnancies[J]. Clin Sci, 2019, 133(2):315-319.
[3] Heidari Z, Mahmoudzadeh-Sagheb H, Narouei M, et al. Effects of gestational diabetes mellitus on stereological parameters and extravillous trophoblast cells of placenta compared to the control group[J]. J Obstet Gynaecol, 2019, 39(7):928-933.
[4] Herman HG, Dekalo A, Jubran L, et al. Obstetric outcomes and placental findings in gestational diabetes patients according to maternal prepregnancy weight and weight gain[J]. J Matern Fetal Neonatal Med, 2019, 32(10):1682-1687.
[5] Gillet V, Ouellet A, Stepanov Y, et al. miRNA profiles in extracellular vesicles from serum early in pregnancies complicated by gestational diabetes mellitus[J]. J Clin Endocrinol Metab, 2019, 104(11):5157-5169.
[6] Strutz J, Cvitic S, Hackl H, et al. Gestational diabetes alters microRNA signatures in human feto-placental endothelial cells depending on fetal sex[J]. Clin Sci, 2018, 132(22):2437-2449.
[7] Shah KB, Chernausek SD, Teague AM, et al. Maternal diabetes alters microRNA expression in fetal exosomes, human umbilical vein endothelial cells and placenta[J]. Pediatr Res, 2021, 89(5):1157-1163.
[8] Zhu Q, Li Y, Li L, et al. MicroRNA-889-3p restrains the proliferation and epithelial-mesenchymal transformation of lung cancer cells via down-regulation of Homeodomain-interacting protein kinase 1[J]. Bioengineered, 2021, 12(2):10945-10958.
[9] Mary S, Kulkarni MJ, Mehendale SS, et al. Differential accumulation of vimentin fragments in preeclamptic placenta[J]. Cytoskeleton, 2017, 74(11):420-425.
[10] Chung WK, Erion K, Florez JC, et al. Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)[J]. Diabetologia, 2020, 63(9):1671-1693.
[11] Wu Z, Mao W, Yang Z, et al. Knockdown of CYP1B1 suppresses the behavior of the extravillous trophoblast cell line HTR-8/SVneo under hyperglycemic condition[J]. J Matern Fetal Neonatal Med, 2021, 34(4):500-511.
[12] Guo D, Song X, Guo T, et al. Vimentin acetylation is involved in SIRT5-mediated hepatocellular carcinoma migration[J]. Am J Cancer Res, 2018, 8(12):2453-2466.
[13] 陈海燕, 魏瑗, 刘晓红, 等. 妊娠期糖尿病对双胎妊娠孕妇母婴结局的影响:Meta分析[J]. 中华糖尿病杂志, 2020, 12(9):702-709.
Chen HY, Wei Y, Liu XH, et al. Effects of gestational diabetes mellitus status on maternal and fetal outcomes among women with twin pregnancy: a Meta-analysis[J]. Chin J Diabetes, 2020, 12(9):702-709.
[14] Qiao L, Lee S, Nguyen A, et al. Regulatory effects of brown adipose tissue thermogenesis on maternal metabolic adaptation, placental efficiency, and fetal growth in mice[J]. Am J Physiol Endocrinol Metab, 2018, 315(6):E1224-E1231.
[15] Hu D, Li J, Zhuang Y, et al. Adrenocorticotropic hormone: an expansion of our current understanding of the treatment for nephrotic syndrome[J]. Steroids, 2021, 176:108930.
[16] Zhang L, Zeng M, Tang F, et al. Circ-PNPT1 contributes to gestational diabetes mellitus (GDM) by regulating the function of trophoblast cells through miR-889-3p/PAK1 axis[J]. Diabetol Metab Syndr, 2021, 13(1):58.
[17] Phipps E, Prasanna D, Brima W, et al. Preeclampsia: updates in pathogenesis, definitions, and guidelines[J]. Clin J Am Soc Nephrol, 2016, 11(6):1102-1113.
[18] Nguyen-Ngo C, Jayabalan N, Haghvirdizadeh P, et al. Role of adipose tissue in regulating fetal growth in gestational diabetes mellitus[J]. Placenta, 2020, 102:39-48.
[19] Yang Y, Zhang J, Chen X, et al. LncRNA FTX sponges miR-215 and inhibits phosphorylation of vimentin for promoting colorectal cancer progression[J]. Gene Ther, 2018, 25(5):321-330.
[20] Zhu Y, Zhang Y, Sui Z, et al. USP14 de-ubiquitinates vimentin and miR-320a modulates USP14 and vimentin to contribute to malignancy in gastric cancer cells[J]. Oncotarget, 2017, 8(30):48725-48736.
miR-889-3p attenuates invasive function of placental trophoblast cells in gestational diabetes mellitus by promoting vimentin acetylation
YANG Wei-hui△, HE Xin, DING Dan-ni
[,(),410000,]
To explore the mechanism of microRNA-889-3p (miR-889-3p) regulating the invasion function of placental trophoblastic cells in gestational diabetes mellitus (GDM).From January to December 2020, 30 parturient women with GDM and 30 parturient women with normal glucose tolerance were enrolled in this study. The expression levels of miR-889-3p in maternal placenta, human cancer cell lines, human trophoblast HTR-8/SVneo cells and human umlibical vein endothelial cells (HUVEC) were detected by qRT-PCR. Cell culture, cell transfection, MTT assay, EdU assay, wound scratch assay, and Transwell analysis were carried out to determine the effects of silencing and overexpression of miR-889-3p on the HTR-8/SVneo cells. The binding relationship between miR-889-3p and vimentin was determined by bioinformatics analysis. The effects of miR-889-3p overexpression on dual-luciferase activity and vimentin acetylation were analyzed by dual-luciferase reporter assay and Western blotting, respectively.Relative miR-889-3p expression level in HTR-8/SVneo cells was higher than that in cancer cells and HUVEC, and was lower in the GDM placentas than that in the normal placentas (<0.05). miR-889-3p overexpression inhibited the growth, migration and invasion of HTR-8/SVneo cells (<0.05), while miR-889-3p knockdown promoted cell growth, migration and invasion (<0.05). Vimentin was identified as a direct target of miR-889-3p by bioinformatics analysis. In the HTR-8/SVneo cells transfected with vimentin-WT, administration of miR-889-3p mimic significantly inhibited luciferase activity and increased vimentin acetylation level (<0.05). Up-regulation of vimentin partially antagonized the inhibitory effect of miR-889-3p overexpression on cell growth, migration and invasion (<0.05), while enhancement of cell growth, migration and invasion induced by miR-889-3p knockdown was attenuated by down-regulation of vimentin (<0.05).Down-regulation of miR-889-3p in placental tissue is involved in the pathological process of GDM. miR-889-3p attenuates the invasive function of trophoblast cells by up-regulating vimentin acetylation.
MicroRNA-889-3p; Vimentin; Gestational diabetes mellitus; Placenta; Cell invasion
1000-4718(2022)09-1625-09
2022-04-08
2022-06-27
13874920040; E-mail: yangweihui198@163.com
R714.256; R363.2
A
10.3969/j.issn.1000-4718.2022.09.012
[基金项目]湖南省卫生计生委科研课题(No. 20190118)
(责任编辑:林白霜,罗森)
