Oncologic aspects of the decision-making process for surgical approach for colorectal liver metastases progressing during chemotherapy
2022-10-11RaphaelAraujoCamilaCarvalhoCarlosMaedaJeanMichelMilaniDiogoBuganoPedroHenriquedeMoraesMarceloLinhares
Raphael L C Araujo, Camila G C Y Carvalho, Carlos T Maeda, Jean Michel Milani, Diogo G Bugano, Pedro Henrique Z de Moraes, Marcelo M Linhares
Raphael L C Araujo, Carlos T Maeda, Jean Michel Milani, Marcelo M Linhares, Department of Surgery, Universidade Federal de São Paulo, São Paulo 04024-002, Brazil
Raphael L C Araujo, Diogo G Bugano, Pedro Henrique Z de Moraes, Department of Oncology, Ηospital Israelita Albert Einstein, São Paulo 05652-900, Brazil
Raphael L C Araujo, Camila G C Y Carvalho, Department of Surgical Oncology, Ηospital e Maternidade Brasil Rede D'Or São Luiz, Santo André 09030-590, São Paulo, Brazil
Abstract Colorectal cancer represents the third most diagnosed malignancy in the world. The liver is the main site of metastatic disease, affected in 30% of patients with newly diagnosed disease. Complete resection is considered the only potentially curative treatment for colorectal liver metastasis (CRLM), with a 5-year survival rate ranging from 35% to 58%. However, up to 80% of patients have initially unresectable disease, due to extrahepatic disease or bilobar multiple liver nodules. The availability of increasingly effective systemic chemotherapy has contributed to converting patients with initially unresectable liver metastases to resectable disease, improving long-term outcomes, and accessing tumor biology. In recent years, response to preoperative systemic chemotherapy before liver resection has been established as a major prognostic factor. Some studies have demonstrated that patients with regression of hepatic metastases while on chemotherapy have improved outcomes when compared to patients with stabilization or progression of the disease. Even if disease progression during chemotherapy represents an independent negative prognostic factor, some patients may still benefit from surgery, given the role of this modality as the main treatment with curative intent for patients with CRLM. In selected cases, based on size, the number of lesions, and tumor markers, surgery may be offered despite the less favorable prognosis and as an option for non-chemo responders.
Key Words: Colorectal liver metastases; Oncology; Disease progression; Surgery; Liver resection; Ηepatectomy
lNTRODUCTlON
Colorectal cancer (CRC) represents the third most diagnosed malignancy and the second cause of cancer-related death in the world, with an estimated incidence of 1931590 new cases in 2020[1]. Approximately 30% of patients will present metastases at diagnosis, and 10% to 20% of stage 1-3 diseases will progress to local or distant metastases[2]. Half of the patients with metastatic disease will have liver metastases, which are unresectable in up to 80% of cases due to extrahepatic disease or bilobar multiple liver nodules[2].
Patients with initially resectable colorectal liver metastasis (CRLM) but with either high tumor burden or bad prognostic factors usually go to upfront chemotherapy and then surgery. Complete resection is considered the only potentially curative treatment for CRLM, with 5-year survival rates ranging from 35% to 58%[3]. However, part of these patients will progress during pre-operative chemotherapy, and for this group, the role of resection of CRLM remains controversial and with large discrepancies in the literature. This minireview article aims to address oncologic aspects that drive the decision-making process, in a multidisciplinary manner, to offer surgery for patients with CRLM who are progressing during chemotherapy. Despite the scarcity of literature on the subject, we believe that this specific patient population deserves more individualized evaluation because their inherent condition of progression during systemic chemotherapy has kept them from being included in most of the trials with curative-intent treatment.
LlVER RESECTlON FOR CRLM
The mainstream curative-intent treatment of CRLM is complete surgical resection. Although metastasectomy has never been tested in a randomized controlled trial, studies have demonstrated long-term survival and cure after this approach[4]. The standard recommended surgical treatment for CRLM is complete macroscopic resection with negative margins (R0 resection). However, complete removal of the macroscopic tumor without safe margins (R1 resection) may be accepted in vascular proximity or multi-nodularity cases. The use of increasingly effective chemotherapy has changed long-term outcomes after R1 resection, with survival similar to that of R0 resection[5].
In 1999, Fonget al[6] described the most used Clinical Risk Score (CRS) to predict recurrence after hepatic resection for metastatic CRLM. It was based on five independent prognostic factors: Positive nodal status of the primary tumor, the disease-free interval from identification of the primary tumor to the discovery of liver metastases of < 12 mo, number of metastatic tumors > 1, preoperative carcinoembryonic antigen (CEA) level > 200 ng/mL, and size of the largest tumor > 5 cm. Patients with scores of 0, 1, or 2 had more favorable outcomes compared with scores of 3, 4, or 5[6]. This CRS works as a practical clinical tool helping to select patients for upfront surgery or systemic therapy according to the estimated risks.
Despite the definition of resectability varying from center to center, metastases are usually considered resectable if they can be completely removed (R0 resection) while leaving an adequate functional parenchyma volume[7]. Usually, resectable lesions are those that can be completed removed with a remnant liver representing at least two contiguous segments, granting the patency of inflow and outflow structures, and sparing at least 20% of total liver volume, for healthy and unexposed livers to chemotherapy, or at least 30% for patients who underwent previous chemotherapy[8]. However, up to 70%-80% of patients with CRLM are not initial candidates for hepatic resection[9].
Several strategies have been introduced to the clinical practice to increase the number of patients eligible for curative hepatic resection, including neoadjuvant chemotherapy, two-stage hepatectomies, and portal vein embolization. In 2004, Adamet al[10] reported postoperative 5-year survival of patients submitted to conversion therapy is 33% after rescue surgery[10]. This outcome remains a work in progress and has been increasing with the advent of more modern systemic therapy such as triplet therapies and monoclonal antibodies.
PERlOPERATlVE CHEMOTHERAPY lN lNlTlALLY RESECTABLE PATlENTS
Despite patients undergoing surgical curative-intent treatment, R0 Liver resection, nearly 50%-65% of patients submitted to surgery will relapse within 5 years[11]. Therefore, the use of perioperative systemic chemotherapy has increased over the last decades as an effort to improve long-term outcomes.
Regardless of being associated with an objective response rate of 50%-65%, the survival benefit of perioperative chemotherapy remains controversial[12]. The EPOC clinical trial randomized patients with initially resectable CRLM into preoperative chemotherapy (FOLFOX4) or surgery alone. While no benefit in overall survival (OS) was demonstrated, preoperative chemotherapy significantly increased progression-free survival (PFS) in eligible patients and those with resected CRLM[13]. Based on those findings, the addition of systemic chemotherapy to surgical resection has become the standard of care for CRLM in many centers.
A comparison between perioperative and postoperative chemotherapy after potentially curative hepatic resection for metastatic CRC was conducted at the Memorial Sloan-Kettering Cancer Center. Both OS and recurrence-free survival (RFS) were similar between the groups when adjusted for clinicalpathological factors and CRSs. Therefore, the authors concluded that the timing of additional chemotherapy for resected CRLM was not associated with outcomes[14].
Corroborating those findings, a systematic review, and meta-analysis of chemotherapy for patients with CRLM who underwent curative hepatic resection showed that regardless of timing and based on nonrandomized and randomized data, patients submitted to hepatic resection for CRLM should receive additional chemotherapy, given that this strategy relative increases RFS and OS in 29 and 23%, respectively[15]. Recently, a randomized controlled trial examining the use of adjuvant chemotherapy (modified infusional fluorouracil, leucovorin, and oxaliplatin-mFOLFOX6) in patients with liver-only metastatic CRC was published. Kanemitsuet al[16], after a median follow-up of 59.2 mo, demonstrated that adjuvant chemotherapy improved 5-years disease-free survival when compared to hepatectomy alone (49.8%vs38.7%, CI: 0.41-0.92;P= 0.006). No significant differences in 5-year OS were detected, 71.2% (95%CI: 61.7-78.8) with adjuvant chemotherapy and 83.1% (95%CI: 74.9-88.9) with hepatectomy alone. Nonetheless, this trial was not designed to detect a difference in OS as a primary endpoint, and indeed, it has not a long enough follow-up to detect this difference, so improvements in OS could not be demonstrated[16].
The benefit of adding new systemic therapies to improve outcomes in patients with resectable CRLM has been tested. The New EPOC was a phase III trial that included patients with resectable exon-2 RAS wild-type metastatic CRC, randomly assigned to receive perioperative chemotherapy, doublet oxaliplatin-based therapy, with or without cetuximab. The incorporation of cetuximab not only correlated with significantly inferior PFS but also with a trend towards decreased OS. Although the addition of cetuximab to chemotherapy may improve outcomes in patients with initially inoperable metastatic disease, its use preoperatively in resectable patients confers a significant disadvantage and should not be a routine[17].
It seems that chemotherapy should be incorporated into the treatment of resectable CRLM, increasing PFS, and possibly OS. However, the best timing for additional chemotherapy remains unclear. Delivering chemotherapy preoperatively may be used as a means of testing tumor biology in vivo, identifying patients who will benefit most from surgery. Recently, response to neoadjuvant chemotherapy has been established as a major prognostic factor once patients with disease stabilization or progression while on chemotherapy seem to have worse outcomes than responders[18]. Other benefits of initial chemotherapy may be the earlier treatment of micrometastatic disease and cytoreduction of the hepatic disease, facilitating surgical resection. On the other hand, oxaliplatin or irinotecan-based neoadjuvant chemotherapy can increase the rates of perioperative morbidity and cause liver toxicity.
Considering symptomatic synchronous tumors, it is suggested to direct the treatment to the primary tumor first, with resection and/or deviation, followed by systemic chemotherapy. For asymptomatic patients with synchronous tumors and those with metachronous hepatic disease, the timing of additional chemotherapy should be guided by the CRS of recurrence, as proposed by Fonget al[6]. For potentially resectable patients with a low risk of recurrence (0-2), initial surgery rather than neoadjuvant che-motherapy could be chosen, followed by postoperative chemotherapy. For patients with a high risk of recurrence (3-5), neoadjuvant chemotherapy is the preferred approach[3]. Pre-operative chemotherapy, on the other hand, is an important resource for liver parenchyma sparing in patients who require extended hepatectomy, regardless of whether they have a high or low CRS. Perhaps this action prevents postoperative liver dysfunction and increases the chances of a preserved clinical performance when undergoing postoperative chemotherapy or re-hepatectomy when indicated.
PERlOPERATlVE CHEMOTHERAPY lN lNlTlALLY UNRESECTABLE PATlENTS
For patients with initially unresectable or critically located colorectal liver metastases, upfront chemotherapy represents an appropriate option as conversion therapy. However, the likelihood of downstaging a patient to the point of resectability seems to be below, on the order of 5% to 15%, even in the hands of aggressive surgeons[19].
A regime leading to high response rates and a large tumor shrinkage is recommended. Although there are uncertainties surrounding the best combination to use, it seems that for RAS wild-type disease a cytotoxic doublet in association with an anti-epidermal growth factor receptor (EGFR) offers the best benefit-risk/ratio. For patients with RAS-mutant disease, the preference is for a cytotoxic doublet plus bevacizumab or FOLFOXIRI plus bevacizumab[20].
A meta-analysis assessing the effect of cetuximab and panitumumab in patients with liver-limited initially unresectable CRLM showed that the addition of anti-EGFR increased the R0 resection rate by 60% and reduced the risk of progression by 32%[21]. Considering non-liver limited disease, the CRYSTAL trial demonstrated that FOLFIRI plus anti-EGFR as first-line treatment was beneficial when compared to FOLFIRI alone, especially for the subgroup of wild-type K-RAS[22]. The FOLFIRI plus anti-EGFRvsFOLFIRI plus anti-vascular endothelial growth factor (VEGF) for the non-liver limited disease was addressed in the FIRE-3 trial and despite neither difference in objective response nor PFS being identified, FOLFIRI plus anti-EGFR achieve longer OS for patients with wild-type KRAS (33vs25 mo,P= 0.017)[23,24]. However, in a posthoc analysis of this study population, after a centralized analysis of radiological response, FOLFIRI plus anti-EGFR demonstrated better response outcomes than FOLFIRI plus anti-VGFR[23,24]. Furthermore, Tejparet al[25] investigated the primary tumor locations, whether right-sided (from the appendix to the transverse colon) or left-sided (from the splenic flexure to the rectum), in patients with wild-type RAS from both CRYSTAL and FIRE-3[25]. The data suggested that adding anti-EGFR to patients with wild-type RAS right-sided tumors had no benefit; contrary, the data showed that patients with left-sided tumors had better objective response rates, PFS and OS, which seems to be useful for this subgroup of patients, particularly those with symptomatic primary tumors or high tumor burden of CRLM.
Regarding anti-VGFR action, Xuet al[26] demonstrated in a systematic review and metanalysis that Bevacizumab-based combination therapies for patients with advanced mCRC show significant higher objective response rates [risk ratios (RR): 1.40], PFS [hazard ratio (HR): 0.64], and OS (HR: 0.82) values when compared than monotherapy. Regrettably, combined anti-VGEF therapies also increase the risk of grade 3/4 treatment-related toxicity (RR: 1.27) when compared to monotherapy[26]. Among the anti-VEGF combined therapies, capecitabine use is associated with a higher risk of grade 3/4 adverse effects (RR: 1.89vs1.12) than IFL[26].
EVALUATlON OF RESPONSE TO PREOPERATlVE CHEMOTHERAPY
The Response Evaluation Criteria in Solid Tumors is the recommended method of assessing objective response to preoperative chemotherapy in most clinical trials. The total tumor burden is evaluated by selecting up to five target lesions and calculating the average diameter change based on imaging studies. A reduction of at least 30% is classified as a response and an increase of at least 20% as progression[27].
ROLE OF SURGERY lN PATlENTS PROGRESSlNG WHlLE ON CHEMOTHERAPY
The role of surgery in patients with CRLM progressing while on systemic chemotherapy remains controversial. A summary of the major publications addressing this subject is represented in Table 1.

Table 1 Study characteristics according to the type of preoperative chemotherapy, type of response, overall and disease-free survivals of patients who underwent curative-intent treatment hepatectomies for colorectal liver metastases
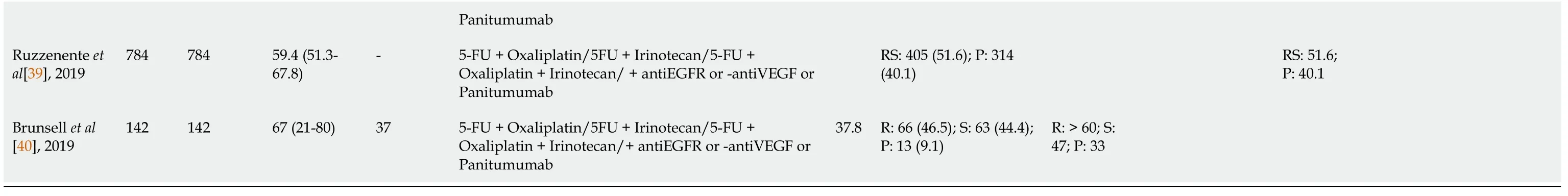
1Total per study.2Median (range) or mean plus standard deviation as described by the authors.FU: Follow-up; 5-FU: 5-fluorouracil; R: Disease response group; S: Stable disease group; P: Progression disease group; RS: Response and stable disease group; O: Overall; OS: Overall Survival; DFS: Disease-Free Survival; MdG: Modified de Gramont; CA: Chemotherapy alone group; CC: Chemotherapy plus cetuximab group; G1: Resection after first-line chemotherapy group; G2: Resection after second-line chemotherapy group.
Allenet al[28] evaluated patients with synchronous colorectal liver metastases treated between January 1995 and January 2000. Patients who received preoperative chemotherapy, as a group, had similar OS compared to those submitted to surgery upfront. However, the subgroup of patients with diseases that did not progress while on chemotherapy showed significantly improved survival[28].
Similar results were demonstrated by Adamet al[29] in a retrospective analysis of 131 patients submitted to liver resection for CRLM after systemic chemotherapy. In this group, patients could achieve long-term survival after hepatic resection if the disease was controlled by chemotherapy before surgery. However, tumor progression before the operation conferred a poor outcome, even after potentially curative surgery[29].
Neumann et al[2] evaluated 160 patients exposed to preoperative chemotherapy, followed by liver resection for CRLM. Factors associated with poor outcomes were noncurative resection, CEA levels > 200 ng/dL, tumor grading, size of largest tumor > 5cm, and the number of metastases. Controversially, tumor progression while on chemotherapy did not influence long-term survival[2]. Those findings are supported by a retrospective study by Gallagher et al[30], that found no difference in survival among the three response groups after chemotherapy[30].
A retrospective analysis of patients with hepatic resection of CRLM following second-line chemotherapy was conducted by Brouquet et al[31] The regime proved to be feasible and associated with modest survival benefits, representing a viable option in patients with advanced CRLM[31]. Similarly, Adam et al[9] found that selected patients submitted to hepatic resection of CRLM after second-line preoperative chemotherapy could have comparable outcomes to patients resected after firstline chemotherapy. In this scenario, independent predictive factors of worse prognosis were positive primary lymph nodes, extrahepatic disease, tumor progression on second-line therapy, and R2 resection[9].
For patients with extensive bilobar disease, selection based on response to pre-hepatectomy chemotherapy seems to be extremely important before planning a two-stage hepatectomy (TSH). Giuliante et al[7] found that tumor progression while on preoperative chemotherapy significantly increased the risk of failure to complete the second stage. However, for patients who completed the TSH, long-term outcomes were similar to those reported for patients following a single-stage hepatectomy[7]. In this context, Jouffret et al[32] showed that resectable hepatic disease progression in the future remnant liver after portal vein embolization should not be considered a contraindication for second stage hepatectomy[32]. Vigano et al[33] reported a series of 128 patients with disease response or stabilization while on preoperative chemotherapy. Early progression of the disease between the end of chemotherapy and liver resection was reported in approximately 15% of patients and was associated with extremely poor survival[33].
Additionally, caution is necessary for patients in the setting of preoperative use of Anti-VGEF since they have a higher risk of treatment-related complications such as hemorrhage, hypertension, neutropenia, stroke, GI perforation, fistula formation and wound healing complications[34]. Thus, it has been recommended an interval of at least 6 wk between the last dose of bevacizumab and elective surgery to mitigate the risk of complications. Nevertheless, its postoperative use should be delayed at least 6 to 8 wk after surgery[34].
CONCLUSlON
Complete surgical resection remains the only potentially curative treatment for colorectal liver metastases. In this context, several strategies have been introduced to the clinical practice to increase the number of patients eligible for curative hepatic resection, including preoperative chemotherapy, portal vein embolization, two-stage hepatectomies, and association of ablative techniques. In recent years, response to preoperative systemic chemotherapy before liver resection has been established as a major prognostic factor. It seems that progression while on chemotherapy confers a worse prognosis than disease response or stabilization[28,29].
Although the role of surgery in patients progressing while on chemotherapy remains controversial, some patients may still benefit from surgery in this scenario, given the role of this modality as the mainstream curative-intent treatment for patients with CRLM. In selected cases, based on size, the number of lesions, and tumor markers, surgery may be offered despite the less favorable prognosis and as an option for non-chemo responders.
FOOTNOTES
Author contributions:Araujo RLC contributed to the study conception, data preparation, data interpretation, and writing; Carvalho CGCY contributed to the data preparation, data interpretation, and writing; Maeda CT, Milani JM contributed to the data acquisition, data preparation, and writing; Bugano DG, de Moraes PHZ and Linhares MM contributed to the data interpretation, and critical writing of the paper.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Brazil
ORClD number:Raphael L C Araujo 0000-0002-7834-5944; Camila G C Y Carvalho 0000-0003-2661-103X; Carlos T Maeda 0000-0002-0824-7599; Jean Michel Milani 0000-0002-8604-8042; Diogo G Bugano 0000-0001-5284-1555; Pedro Ηenrique Z de Moraes 0000-0001-7221-7821; Marcelo M Linhares 0000-0001-9562-0058.
Corresponding Author's Membership in Professional Societies:Society for Surgery of the Alimentary Tract; American Ηepato-Pancreato-Biliary Association; International Ηepato-Pancreato-Biliary Association; International Laparoscopic Liver Society.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Prediction factors for ischemia of closed-loop small intestinal obstruction
- Successful treatment of acute symptomatic extensive portal venous system thrombosis by 7-day systemic thrombolysis
- Retrorectal mucinous adenocarcinoma arising from a tailgut cyst: A case report and review of literature
- Minimally invasive endoscopic repair of rectovaginal fistula
- Laparoscopic appendectomy, stump closure and endoloops: A meta-analysis
- Effect of cardiac output - guided hemodynamic management on acute lung injury in pediatric living donor liver transplantation
