Analysis of Chemical Modification Mechanism and Rheological Properties of Polyphosphoric Acid Modified Asphalt
2022-10-08WANGLanPEIKeLIChao
WANG Lan, PEI Ke, LI Chao*
(1. School of Civil Engineering, Inner Mongolia University of Technology, Hohhot 010051, China; 2. Inner Mongolia Autonomous Region Key Laboratory of Civil Engineering Structure and Mechanics, Hohhot 010051, China)
Abstract: In order to study the chemical modification mechanism and rheological properties of polyphosphoric acid (PPA) -modified asphalt, asphalt modified with different PPA contents were characterized by four-component test, atomic force microscopy (AFM) and Fourier transform infrared spectroscopy (FTIR).In the test, changes in asphalt chemical composition and colloidal structure were analyzed for different PPA contents, and infrared spectra were fitted with a Gaussian function. The reaction mechanism of PPA and matrix asphalt was also discussed. Based on dynamic shear rheometer (DSR) test and bending beam rheometer (BBR)test, rheological index G*/sinδ and S/m were used to evaluate the modification effect of PPA on asphalt. The results show that, with an increase in PPA content, both large and small honeycomb structures increased in the three-dimensional topography seen in the atomic force microscope (AFM). In a certain space range, some of the micelles in the asphalt are connected each other to form interlocking skeleton structures, and locally form dense spatial network structures. The added PPA does not chemically react with the functional groups in the functional-group area of the infrared spectra (3 100-2 750 cm-1, 1 800-1 330 cm-1), and the structure is very stable. However, there is an obvious new absorption peak below 1 330 cm-1 in the fingerprint area, that is, the chemical reaction between PPA and the matrix asphalt generates a new compound, inorganic phosphate. Infrared spectra of PPA-modified asphalt with different contents were fitted by a Gaussian function, which makes up for the limitation that the absorption intensity information of each superimposed functional group cannot be obtained directly from the original infrared spectra. Results of this qualitative analysis were further verified by quantitative analysis. The addition of PPA can effectively improve the high and low-temperature performance of asphalt, and the lower the temperature is in the negative temperature zone, the more obvious the improvement is.When PPA content is more than 1%, the improvement of asphalt low-temperature performance is not obvious.
Key words: polyphosphoric acid-modified asphalt; dynamic shear rheometer (DSR) test; Fourier transform infrared spectroscopy (FTIR); chemical properties
1 Introduction
Polyphosphoric acid (PPA) is a low-cost asphalt chemical modifier with good modification effects that has been used in modification of asphalt in other countries[1-4]. In recent years, the use of scanning electron microscopy (SEM)[5-7], Fourier transform infrared spectroscopy (FTIR)[8-10], fluorescence microscopy(FM)[11-13], differential scanning calorimetry (DSC)[14-16],and other techniques to study the microstructure of modified asphalt have become common methods for investigating the modification mechanism at the microscopic level. With growing research on PPA-modified asphalt, these techniques have become important tools for study of chemical structural changes and microscopic morphology.
J F Masson[17]studied the microstructure of PPA-modified asphalt with nuclear magnetic resonance(NMR) and gel permeation chromatography, and proposed a salification reaction and esterification between PPA and the chemical components of matrix asphalt,resulting in saturates aromatization, aromatics cyclization, and resins ionization of the chemical components of matrix asphalt. Pereira G Det al[18]studied the joint modification effects of PPA and ethylene-methyl acrylate-glycidyl methacrylate terpolymer (EMGMA) by FTIR, and found that a chemical reaction occurs between PPA and EMGMA to form a three-dimensional polymer network. B Liuet al[19]used FTIR and FM to study the modification mechanism of PPA-modified asphalt with different contents. By comparing the infrared spectra of matrix asphalt and PPA-modified asphalt,it was found that no new absorption peak was formed,PPA was evenly distributed throughout the asphalt, and PPA-modified asphalt was a physical modification process. Henglong Zhanget al[20]investigated the effects of PPA content on different asphalts variants using chemical composition analysis and AFM. These researchers found that after adding PPA, the resins in the asphalt are transformed into asphaltenes, which leads to the obvious association of dispersed phase in asphalt,and the stiffness of asphalt increases significantly. E R Douradoet al[21]analyzed the three-dimensional surface morphology of PPA-modified asphalt with AFM, and found that adding PPA to the asphalt can increase the elastic modulus and apparent viscosity of the asphalt.Analyzing the chemical composition and structure of asphalt self-healing microcapsules by SEM and FTIR,Jiupeng Zhanget al[22]compared the characteristic functional groups and chemical bonds to verify its rationality. M Sarnowskiet al[23]studied the chemical reaction mechanism between PPA and matrix asphalt, and found that dispersion of asphaltenes was enhanced after PPA modification. G Yadollahiet al[24]have proposed that PPA reacts mainly with asphaltenes. Other investigators have hypothesized that PPA reacts with other light components such as saturate and aromatic components[25]. F Zhanget alnoted that although PPA reacts with some components of asphalt, the absorption peak caused by the formation of new compounds could not be detected in the infrared spectra due to overlapping with other peaks[26]. In summary, there is no consensus about the chemical mechanism of PPA either in China or other countries; thus, further study of the chemical modification mechanism of PPA is needed[27,28].
In this paper, four-component analysis, AFM and FTIR were used to explore the chemical composition and colloidal structural changes of asphalt with different contents of PPA. Gaussian function was also used to fit infrared spectra to reveal the chemical modification mechanism of PPA-modified asphalt. Finally, dynamic shear rheometer (DSR) test and bending beam rheometer (BBR) test were used to investigate the modified effect of asphalt with different PPA contents.
2 Experimental
2.1 Raw materials
The matrix asphalt used for analysis was Panjin 90# asphalt provided by China Panjin North Asphalt Co., Ltd. We used industrial-grade polyphosphoric acid (H6P4O13) with a phosphoric acid (H3PO4) content of 116%. In this experiment, the PPA content was 0%, 0.5%, 1.0%, 1.5% (the content of PPA is the mass percentage of modifier in matrix asphalt); the corresponding number of PPA-modified asphalt binder was PPA0%, PPA0.5%, PPA1.0% and PPA1.5%. The matrix asphalt was preheated to 150-160 ℃, and then mixed with a certain content of PPA at a speed of 7 000 rpm for 30 min in high-speed shear machine. The temperature was kept constant during this process. The prepared PPA-modified asphalt was placed in the oven;tests were carried out after bubbles disappeared from the asphalt.
2.2 Four-component test
A four-component test of PPA-modified asphalt was carried out by solvent precipitation and chromatographic column methods. Based on the selective dissolution of asphalt in different organic solvents and the selective adsorption of different adsorbents, the asphalt was separated into four components with similar chemical properties, namely asphaltenes (As), saturates (S),aromatics (Ar) and resins (R). The influence of different PPA contents on the chemical components of matrix asphalt was then analyzed.
2.3 Atomic force microscopy (AFM) test
We tested asphalt samples of matrix asphalt(PPA0%) and asphalt variants modified with PPA0.5%,PPA1% and PPA1.5%. Tests were carried out at room temperature (about 24 ℃). There are three main test modes of AFM, namely contact mode, tapping mode,and non-contact mode. Among these options, tapping mode is the most suitable for soft cohesive samples. As a viscoelastic material, asphalt has a certain viscosity at room temperature. Therefore, the mode used in this study was tapping mode with a scanning rate of 1 Hz.The scanning area was 15 μm×15 μm, with a resolution of 512×512. The probe used in the test was a SNL-10 silicon nitride probe with a frequency of 65 kHz and an elastic constant of 0.35 N/m.
2.4 FTIR test
An EQUINOX 55 FTIR was used in this experiment. The spectral information was scanned in the wavenumber range of 400-4 000 cm-1with a resolution of 4 cm-1. The modified asphalt samples of matrix asphalt(PPA0%) and PPA0.5%, PPA1% and PPA1.5% were mixed in a solution containing 5% trichloroethylene,which was coated on the potassium bromide sheet to form a film. The mixed solution was irradiated by the spotlight until it volatilized, and then the test was carried out.
2.5 Dynamic shear rheometer (DSR) test
In this study, the DiscoveryHR-1 dynamic shear rheometer was used to scan the temperature of PPA0%,PPA0.5%, PPA1% and PPA1.5% modified asphalt samples. The temperature scanning range was 28-82 ℃, the step size was 6 ℃, the scanning frequency was 10 rad/s, and the target value of control stress was selected for the test.
2.6 Bending beam rheometer (BBR) test
The modified asphalt samples of PPA0%,PPA0.5%, PPA1% and PPA1.5% were tested by TEBBR bending beam rheometer. The size of the beam specimens was 101.6 mm (length) ×12.7 mm (width)×6.4 mm (thickness). The test temperature was -12,-18, -24 and -30 ℃, and the loading time was 60 s.
3 Results and discussion
3.1 Four-component analysis
By carrying out a four-component test on PPA-modified asphalt with different contents, we were able to assess changes in asphaltenes (As), saturates(S), aromatics (Ar), and resins (R) components of the asphalt with variation in PPA content (Fig.1).

Fig.1 The relationship between different asphalt components and PPA content
It can be seen from Fig.1 that the addition of PPA has a significant impact on the asphalt components.When the content of PPA increases from 0% to 1.5%,the content of asphaltene increases by 4.3% and resins decrease by 5%. There is no significant change in the content of aromatics or saturates.
According to modern colloidal theory, changes in asphalt composition affect changes in colloidal structure[29-31]. On the one hand, with an increase in asphaltenes content, namely the increase in the colloidal core, the colloidal core absorbs more oil and resins,forming more micelles around the colloidal core. With an increase in asphaltenes content, the numbers of micelles in asphalt increase. In a certain space range, an increase in micelle number means that the distance between micelles decreases. Some micelles are connected to each other to form interlocking skeleton structures,increasing the force between the micelles. Macroscopically, the viscosity of asphalt increases. On the other hand, as asphaltenes content increases, the molecular weight of asphaltenes also increases, resulting in the ability of asphaltenes to absorb oil and resins increases,and the larger volume of the micelle formed. Simultaneously, the concentration of micelles in the asphalt becomes larger, and the attractive force between the micelles is increased, so that the distance between the micelles is further reduced, forming locally dense spatial network structures. The joint effect of these two aspects changes the colloidal structure of asphalt from sol structure to sol-gel structure, which makes PPA-modified asphalt more stable.
3.2 Atomic force microscopy (AFM) test analysis
By observing changes in honeycomb structures in the three-dimensional topography, the changes of colloidal structures in the four component analysis of asphalt can be explained. The three-dimensional morphology of matrix asphalt (PPA0%) and PPA0.5%,PPA1% and PPA1.5% seen by AFM test is shown in Fig.2.
It can be seen from Fig.2 that with an increase in PPA content, the surface of the modified asphalt becomes rougher and more rugged, and the height and size of the peak area increase significantly and become denser. From the perspective of colloidal and crystallographic theory, on the one hand, with an increase in PPA content, asphaltenes content increases while colloidal content decreases, which means that the number of colloidal cores in the colloidal structure increases. When the temperature decreases, the colloidal core will act as the crystal nucleus. According to the nucleus-growth theory[32,33], an increase in the number of crystal nuclei means an increase in the number of crystallized wax, that is, an increase in honeycomb structure. In addition, an increase of the number of crystal nuclei will also increase the probability of agglomeration between crystal nuclei. On the other hand,as asphaltenes content increases, the molecular weight increases. The larger the molecular weight, the greater the number of molecules that move together, and the increase in frictional resistance between molecules in motion will slow down the rate of molecular diffusion.Similarly, when the temperature drops quickly, the molecules will not have time to relax to the lowest energy position. The phase transition can occur in only a small area, and the size of honeycomb structures will decrease. Therefore, with an increase in PPA content, both large and small honeycomb structures in PPA-modified asphalt increase, and PPA-modified asphalt becomes somewhat more stable.
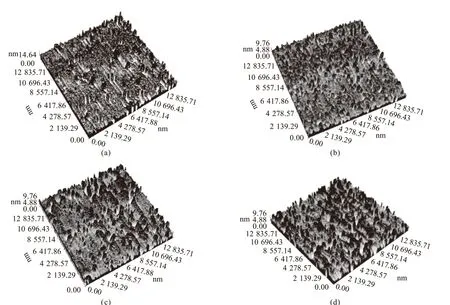
Fig.2 AFM three-dimensional topography: (a) matrix asphalt (PPA0%); (b) PPA0.5%; (c) PPA1%; (d) PPA1.5%
3.3 IR spectrum analysis
FTIR analysis was carried out on the matrix asphalt (PPA0%) and asphalt modified with PPA0.5%,PPA1% and PPA1.5%. The infrared spectra are shown in Fig.3.
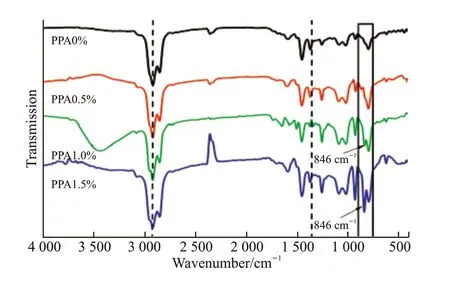
Fig.3 Comparison of infrared spectra of different content in PPA modified asphalt
Fig.3 shows the infrared spectra of asphalt modified with different contents of PPA. According to the characteristics of IR spectra and molecular structure,the infrared spectra can be roughly divided into functional group region and fingerprint region. The functional group region (4 000-1 330 cm-1) is the characteristic vibration frequency part of chemical bond and group. The absorption spectra in this region mainly reflects the vibration of the characteristic group in the molecule. The identification of the group is carried out chiefly in this spectral region. The absorption spectra of the fingerprint region (1 330-400 cm-1) is more complex, but it can reflect subtle changes in molecular structure. In this region, each compound has a different band position, intensity, and shape, whose use is equivalent to that of a human fingerprint and is used to verify the reliability of organic compounds. There are some characteristic absorption bands in the fingerprint area that are helpful for the identification of functional groups.
It can be seen from Fig.3 that in the range of 3 100-2 750 cm-1, asphalt with and without PPA(PPA0% and PPA0.5%, PPA1% and PPA1.5%) all show obvious C-H stretching vibration absorption peaks; but there is no significant change in this pattern with an increase in PPA content. At 2 924 and 2 852 cm-1, the peaks correspond to the asymmetric and symmetric stretching vibrations of -CH2-. However, on the left side of 2 924 cm-1(about 2 960 cm-1),there is a subtle shoulder peak, which corresponds to the asymmetric stretching vibration of -CH3, indicating that the content of aliphatic carbon in PPA-modified asphalt with different contents (PPA0% and PPA0.5%,PPA1% and PPA1.5%) is higher. The fact that the asymmetric and symmetric stretching vibration of-CH2- is stronger than the corresponding asymmetric stretching vibration of -CH3indicates that the aliphatic carbon in PPA-modified asphalt with different contents(PPA0% and PPA0.5%, PPA1% and PPA1.5%) is composed mainly of long chains and relatively fewer side chains. The structure in this range is relatively stable;chemical reactivity is very weak; there is strong chemical reaction inertia; and PPA does not react with the functional groups in this spectral region. In the range of 1 330-1 800 cm-1, the PPA-modified asphalt with different contents (PPA0% and PPA0.5%, PPA1% and PPA1.5%) show obvious C=O and C=C stretching vibration absorption peaks in the functional group area,but there is no significant change with an increase in PPA content. The band at 1 710 cm-1, signifies carbonyl (C=O) stretching vibration of aldehyde, ketone,and acid. The band at 1 599 cm-1is the C=C stretching vibration on the aromatic ring. The bands at 1 458 and 1 375 cm-1represent in-plane bending vibration of CH2- and CH3-. Most of the functional groups are composed of cycloparaffins in this range, and the structure is very stable, indicating that the chemical reactivity is very weak, there is strong chemical reaction inertia, and PPA does not react with the functional groups in this region. Compared with matrix asphalt(PPA0%), the asphalt modified with 0.5%, 1% and 1.5% PPA content shows obvious new absorption peaks below 1 330 cm-1in the fingerprint area, mainly the out-of-plane bending vibration absorption peaks of non-saturated CH2- and CH3-. For example, at 1 261,1 099, 1 027, 802, 872 and 937 cm-1, there are bending vibrations of oxygen-containing functional groups-OH or -COOH. With increase in PPA content, the bending vibration of the oxygen-containing functional group -OH or -COOH is enhanced at 937 cm-1, which is caused by the increasing numbers of the oxygen-containing functional group -OH in PPA. After adding the modifier PPA, a new absorption peak appears in the fingerprint area at 846 cm-1. The main functional group of the new absorption peak is inorganic phosphate, perhaps because P-OH stretching in H2PO4and monohydric phosphate becomes the anti-symmetric stretching of P-O-C in phosphate ester (RO)3P=O. It shows that when the polyphosphoric acid modifier is added, a new chemical reaction occurs with the matrix asphalt to form a new compound, possibly inorganic phosphate. Related studies have proposed that after adding the modifier PPA, the alcohol in the asphalt reacts with PPA, the hydroxyl -OH in the alcohol is easily phosphorylated, and the product is phosphate ester[34,35]. With an increase in PPA content, on the one hand, the degree of esterification will increase in the asphalt, indicating that when hydroxyl -OH is dehydrated, the macromolecules produced by condensation with PPA increase. The content and molecular weight of asphaltenes increase. The ability of asphaltenes to absorb both oil and resins is enhanced, and the volume of micelles formed is larger. On the other hand, the long-chain hydrocarbon components in the asphalt will also increase, and a dense spatial network structure can be formed locally. Thus, the PPA-modified asphalt becomes somewhat more stable. These findings are consistent with the results of the four-component test and AFM analysis.
Infrared spectra can provide some reference value in qualitative analysis. Due to the complexity of the asphalt structure, however, the spectral peaks of different functional groups are superimposed in the same spectral region. Because of this complexity, the degree of spectral overlap and the absorption intensity information of each superimposed functional group cannot be obtained from the original infrared spectra. Therefore,it is necessary to separate the superimposed spectral peaks before analyzing chemical products[36]. In order to further resolve the problem, the Gaussian function is used to fit the infrared-spectra peaks of PPA-modified asphalt with different contents, so as to analyze the changes of functional groups of each component in the asphalt with an increase in PPA content. From the infrared spectra of PPA-modified asphalt with different contents in Fig.3, the Gaussian function was used to fit the superimposed peaks in two ranges (2 750-3 050 cm-1, 760-910 cm-1). Among these ranges, the 2 750-3 050 cm-1band is the functional group area,and 760-910 cm-1is the fingerprint area. Using Origin to perform spectra fitting, we obtained the results shown in Fig.4 and Fig.5. The infrared spectra parameters obtained by calculating the peak area of the fitting curve are shown in Tables 1 and 2.

Fig.4 Fitting infrared spectra curve of PPA-modified asphalt with different contents in the range of 2 750-3 100 cm-1: (a) Modified asphalt of PPA0%; (b) Modified asphalt of PPA0.5%; (c) Modified asphalt of PPA1%; (d) Modified asphalt of PPA1.5%
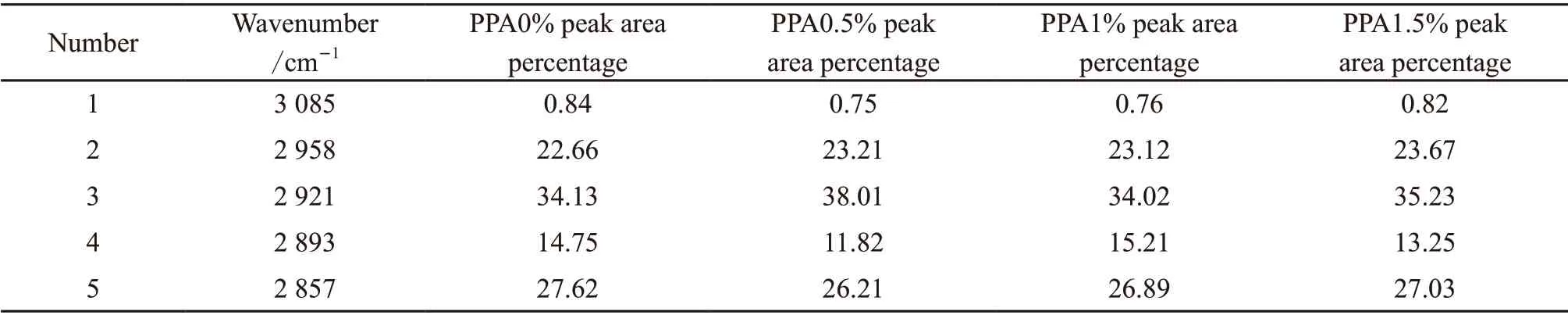
Table 1 The percentage of each peak area of PPA modified asphalt with different content in the range of 2 750-3 100 cm-1
As can be seen from Fig.4 and Table 1, the superimposed peaks originally located at 2 750-3 100 cm-1can be separated into five peaks at 2 857, 2 893, 2 921,2 958, and 3 085 cm-1. The related coefficient of the fitting is greater than 0.99, indicating that the fitting effect is good. After fitting the original superimposed peaks, we found that with an increase in PPA content,the number, position, relative strength and shape of the five infrared absorption peaks fitted by Gaussian function did not change in the 2 750-3 100 cm-1range;this indicates that addition of PPA does not react with the functional groups in the range of 2 750-3 100 cm-1.These results indicate that structure in this range is relatively stable, chemical reactivity is very weak,and there is strong chemical inertia, all of which is consistent with the conclusions of qualitative IR analysis. The fitting results indicate that the area percentage of each absorption peak in the range of 2 750-3 100 cm-1does not change significantly with increase in PPA content. Once again, these results deomonstarate that the addition of PPA does not chemically react with functional groups in the range of 2 750-3 100 cm-1. The structure is relatively stable in this range,chemical reactivity is very weak, and there is strong chemical inertia.

Fig.5 Infrared spectrum fitting curve of PPA modified asphalt with different contents in the range of 760-910 cm-1: (a) Modified asphalt of PPA0%; (b) Modified asphalt of PPA0.5%; (c) Modified asphalt of PPA1%; (d) Modified asphalt of PPA1.5%
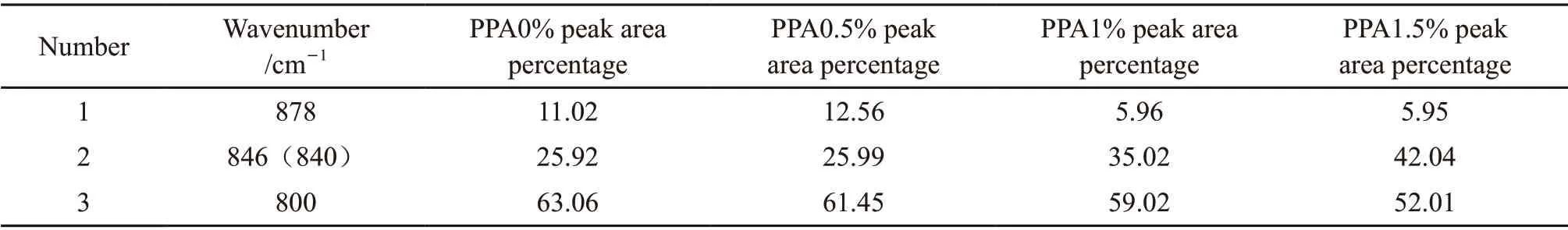
Table 2 The percentage of each peak area of PPA modified asphalt with different contents in the range of 760-910 cm-1
Fig.5 and Table 2 show that the original superposed peak at 760-910 cm-1can be separated into three peaks at 800, 841 (846) and 878 cm-1. The related coefficient of the fitting is greater than 0.99, which shows that the fitting effect is good. After fitting the original superimposed peaks, we found that with an increase in PPA content, the number, position, relative strength,and shape of the two peaks at 800 and 878 cm-1does not change. However, the position of the absorption peak at 841 cm-1shifts to 846 cm-1, and its relative intensity also becomes larger, indicating that the addition of PPA results in phosphorylation of the asphalt.This occurs because there are many oxygen-containing compounds and functional groups in asphalt, and they are the main functional groups of chemical reactions;again, these findings are consistent with the qualitative IR analysis. The fitting results indicate that with an increase in PPA content, the area percentages of the three infrared-spectra absorption peaks change in the range of 760-910 cm-1. The area percentage of the absorption peak at 846 cm-1shows a particularly notable increase with increase in PPA content, indicating that when the PPA modifier is added, a new chemical reaction occurs with the matrix asphalt to form a new compound, possibly inorganic phosphate.
3.4 Dynamic shear rheometer (DSR) test analysis
Compared with dynamic shear modulus (G*) and phase angle (δ), the rutting factorG*/sinδcan comprehensively evaluate the high-temperature stability of asphalt[37]. The higherG*/sinδ, the better the high-temperature stability and the stronger rutting resistance of asphalt[38]. Through dynamic shear rheometer test, the relationship curve betweenG*/sinδand temperature of PPA modified asphalt with different contents was obtained, as shown in Fig.6.
It can be seen from the figure that the rutting parameterG*/sinδvalues of PPA modified asphalt with different contents (PPA0%, PPA0.5%, PPA1% and PPA1.5%) all show a decreasing trend with the increase of temperature. High temperature stability becomes worse and the rutting resistance becomes weaker with the increase of temperature; Under the same temperature condition, with the increase of PPA content, the rutting parameterG*/sinδincreases, indicating that with the increase of PPA content, the high-temperature stability of PPA modified asphalt becomes better and the rutting resistance ability is enhanced.
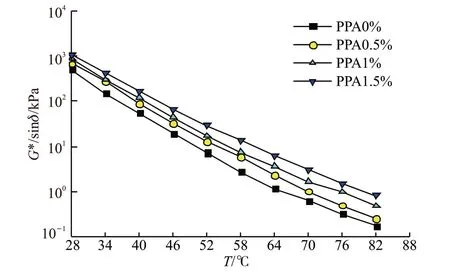
Fig.6 The relationship curve between G*/sinδ and temperature of PPA modified asphalt with different contents
3.5 Bending beam rheometer (BBR) test analysis

Fig.7 The relationship curve between S/m and temperature of PPA modified asphalt with different contents
In this study, the rheological indexS/m(the ratio of creep stiffness modulus (S) and creep rate (m)) was used to evaluate the performance at low temperature.The smaller theS/mvalue is, the better the low-temperature performance of asphalt is. The bending beam rheometer (BBR) test of PPA modified asphalt with different contents were carried out at different temperatures, and theS/mvalue at 60 s was calculated, as shown in Fig. 7.
As can be seen from the figure, within the range of test temperature (-12 ℃--30 ℃), theS/mvalue of PPA-modified asphalt with different contents (PPA0%,PPA0.5%, PPA1% and PPA1.5%) showed the same change rule with different temperature. That is, with the decrease of temperature, theS/mvalue shows an upward trend. In the negative temperature zone, with the decrease of temperature, the low-temperature flexibility of asphalt becomes worse, the stress relaxation performance also becomes worse, and its low-temperature performance decreases. Under the same temperature condition, theS/mvalue of PPA modified asphalt generally shows a decreasing trend with the increase of PPA content. Moreover, the lower the temperature, the more obvious the trend is. PPA improves the low-temperature performance of asphalt in the negative temperature area, and the lower the temperature, the more obvious the improvement is. However, when PPA content is more than 1%, the improvement of asphalt low-temperature performance is not obvious.
4 Conclusions
a) With an increase in PPA content, the number of micelles and molecular weight of asphaltenes in asphalt increase, and both large and small honeycomb structures increase. Within a certain space range, some micelles connect with each other to form interlocking skeleton structures, forming dense spatial network structures in some regions.
b) There is no significant difference in the absorption spectra of asphalt modified with different PPA contents (PPA0% and PPA0.5%, PPA1% and PPA1.5%)in the functional group area (3 100-2 750 cm-1, 1 800-1 330 cm-1), indicating that the structure of this area is very stable, and PPA does not chemically react with the functional groups in this spectral region.
c) Compared with matrix asphalt (PPA0%), asphalt modificed with 0.5%, 1% and 1.5% PPA content shows obvious new absorption peaks below 1 330 cm-1in the fingerprint area. At the new absorption peak 846 cm-1, the main functional group is inorganic phosphate,perhaps due to the fact that P-OH stretches in H2PO4and monohydric phosphate becomes anti-symmetric stretching of P-O-C in phosphate ester (RO)3P=O.Thus, the chemical reaction between PPA and matrix asphalt generates a new compound, inorganic phosphate.
d) The infrared spectra of PPA-modified asphalt with different contents are well fitted by Gaussian function, which makes up for the limitation that the absorption intensity information of each superimposed functional group cannot be obtained directly from the original infrared spectra. The qualitative analysis results are further verified by quantitative analysis.
e) With the increase of PPA content, the rutting factorG*/sinδincreases, and theS/mvalue decreases,the performance at high and low temperatures is improved obviously. In the negative temperature zone,the lower the temperature, the more obvious the improvement. When PPA content is more than 1%, the improvement of asphalt low-temperature performance is not obvious.
杂志排行
Journal of Wuhan University of Technology(Materials Science Edition)的其它文章
- Preparation and Performance of Graphene Oxide Modified Polyurethane Thermal Conductive Insulating Adhesive
- Effect of Polymer Network Morphology on the Performance of Polymer Dispersed Liquid Crystal (PDLC) Composite Films
- Preparation of Controllable Cross-Linking Polyethylene Foaming Materials and Their Properties
- Enhanced Thermoelectric and Mechanical Properties of p-type Bi0.5Sb1.5Te3 Bulk Alloys by Composite Electroless Plating with Ni&Cu
- Fatigue Behavior of a Dissimilar Aluminum Alloy Welding Joint With and Without Natural Defect
- Characteristic of Near-surface Microstructure and Its Effects on the Torsion Performance of Cold Drawn Pearlitic Steel Wires for Bridge Cables
