Myological variation in the hindlimb of three raptorial birds in relation to foraging behavior
2022-10-03XinxinLiangMingjieLiuChenxiYingZihuiZhang
Xinxin Liang,Mingjie Liu,Chenxi Ying,Zihui Zhang
College of Life Sciences,Capital Normal University,Beijing,100048,China
Keywords:Architecture Birds of prey Functional morphology Grip force Pelvic muscles Talon closure
ABSTRACT Raptors share a common predatory lifestyle,but are different in food preferences and hunting behavior.The grip force and talons' grasping capabilities are fundamentally crucial for subduing and killing their prey to feed,but the abilities and differences to generate force are less known.In this study,the entire pelvic muscles were dissected with the muscle mass and fibre length measured and physiological cross-sectional area counted in the Common Kestrel (Falco tinnunculus),Eurasian Sparrowhawk (Accipiter nisus),and Long-eared Owl (Asio otus).Statistical tests were performed to explore the possible differences in architectural parameters among species.These species were same in distributing the greatest proportion of muscle mass to the shank region and the digital flexor functional group,allocating more than 60% muscle mass in relation to total single leg muscle mass to the same seven individual muscles including flexor digitorum longus(FDL),flexor hallucis longus(FHL),and tibialis cranialis(TC)which are three major muscles responsible for talon closure.Interspecies differentiations were most present in the shank and tarsus instead of other regions of the leg,which might reflect their difference in hunting mode and foot use.Greater force-generation capacity of FHL and some anatomical features suggest that digits 1 and 2 work together as an efficiently vise-like set,playing more critical role than digits 3-4 in foraging of diurnal raptors but to a different degree.In accordance with zygodactyl foot morphology,each digit of the Long-eared Owl plays a subequal role when hunting,evidenced by anatomical and architectural features.Because of its unique insertion to the base of the pygostyle,the striking numerical difference in the development of M.caudofemoralis was possibly related to raptors’flight behavior and feeding ecology.Concluded from anatomical and architectural aspects,the similarities and differences of the hindlimb musculature were correlated to common predatory lifestyle and different foraging behaviors in three raptor species.These results illustrated the underlying myological basis for the functional capacities of the leg muscles and may provide additional information useful in further biomechanical investigation and computer simulation.
1.Introduction
As top predators and scavengers in ecosystems,birds of prey provide critical ecosystem services in maintaining the stability,structure,and dynamics of food webs.They vary in lifestyles,such as diet,prey preference,hunting behavior,and killing mode;these differences bring substantial opportunities for studying several ecological and evolutionary questions(Sustaita,2008).Simultaneously,the samples of raptor species are rare and very precious because of their high conservation status,low population densities,slow life history,and difficulty in detection and access(McClure et al.,2018).
Raptors are composed of three orders: Accipitriformes,Falconiformes,and Strigiformes.Despite their phylogenetic differences,these birds share suites of features to correlate with their predatory lifestyle,such as elongated toes with sharp talons,a strong bill with a sharp edge and hooked end,and larger talons on digit 2 than digit 3 (Fowler et al.,2009).Unlike other birds,hindlimbs of raptors have roles far beyond that of just support and bipedal locomotion.Their legs are used to catch,hold and carry prey items,and for many species,they are the major killing devices aided by the bill (Sustaita,2008;Sustaita and Hertel,2010;Madan et al.,2017).Thus,extensive adaptive radiation in hindlimb and feet design(Goslow,1972;Ward et al.,2002;Csermely and Rossi,2006;Einoder and Richardson,2006,2007a,2007b;Fowler et al.,2009;Tsang and McDonald,2019;Tsang et al.,2019),as well as skull morphology(Hertel,1994,1995;Sun et al.,2018)among raptors is well known.For instance,Fowler et al.(2009)investigated the talon size variation among digits and found that these differences can be used to distinguish families of raptors and are related to different techniques of prey restraint and immobilization.The digital tendon locking mechanism (TLM) of birds locks the digits in a flexed position,so the foot can maintain a firm grasp.In birds of prey,the TLM is commonly located at the distal end of the digits (Einoder and Richardson,2006);quantitative studies suggested that its variation in size and structure has phylogenetic and functional implications (Einoder and Richardson,2006,2007a).In a recent comprehensive study combining three-dimensional geometric morphometrics,finite element modeling,and comparative phylogenetic methods,the results indicated that raptor talon shape and biomechanical performance are controlled by relative prey size (Tsang et al.,2019).Above-mentioned features are undoubtedly important for transmitting and maintaining grip forces of feet,but the abilities and differences to generate force are less known.
Muscle and skeleton working together affect movement and other functions (e.g.foraging behavior) of an animal.Regarding myological studies,previous works have paid much attention to the comparative anatomy of appendicular musculature to birds of prey,such as Fisher(1946) on New World vultures,Hudson (1948) on diurnal raptors,Volkov(2004)on true owls,and Mosto(2017a)on Falconidae.There were also a lot of descriptive reports on specific species,including but not limited toMilvago chimangoandTyto alba,which are necessary for electromyographic,biomechanical,and functional investigation (Mosto et al.,2013;Mosto,2017a,b).Researchers attempt to explain the differences in locomotion and behavior by qualitative information of myology,such as presence or absence,attachment sites,degree of development,and mass distribution.Nevertheless,the contractile ability and function of a whole muscle is also dependent on the architectural configuration (Gans,1982).For example,fiber length (FL) is proportional to maximum muscle excursion and contraction velocity,whereas the physiological cross-sectional area (PCSA) reflects a muscle's force-producing capacity (Gans,1982;Lieber and Fridén,2000).Available data suggested that architectural design of muscle reflects differential functional demands in light of ecology and evolution (e.g.,Yang et al.,2015;Bribiesca-Contreras et al.,2019).
Of raptors,irrespective of the prey type and size,the grip force and talons' grasping capabilities are fundamentally crucial for subduing and killing their prey to feed (Sustaita and Hertel,2010).Talon closure is mainly affected by two discrete mechanisms that function together in a potentially additive or alternative fashion:one involves contraction of M.flexor digitorum longus(FDL)and M.flexor hallucis longus(FHL)located on the posterior side of the tibiotarsus,and a second more indirect method results from the contraction of the M.tibialis cranialis (TC)(Conroy et al.,1997;Ward et al.,2002).Architectural analysis showed that hawks had significantly greater PCSA of FHL,owls were larger in FDL,and the PCSA of TC did not present difference;at the same time,in vivogrip force measurements using“hydraulic”perches found that owls produce greater forces than hawks (Ward et al.,2002).Similar but further comprehensive studies indicated that hallux accounted for the majority (roughly 49%) of the estimated grip forces in falcons and accipitrids,andin vivogrip forces was greater in accipiters (Sustaita,2008;Sustaita and Hertel,2010).Unfortunately,these studies only concerned a few leg muscles (three or nine muscles),and thus couldn't give more information on the similarity and variation among raptors.
In this study,comprehensive comparisons of qualitative anatomy and quantitative architectural investigations were performed on the entire pelvic muscles of the three predatory bird species: Eurasian Sparrowhawk (Accipiter nisus;ES),Common Kestrel (Falco tinnunculus;CK),and Long-eared Owl (Asio otus;LO).They are similar in body mass but are different in diet,hunting behavior,and killing methods.The Long-eared Owls are nocturnal,they prefer open farmland habitats,feed on almost entirely small rodents,detect prey by hearing and use harrier-like flight or perch-and-wait as hunting strategy,thrust the feet markedly downward just before contact with the prey (Goslow,1972;Marti,1974;Hayward and Garton,1988;Riegert et al.,2009).Common Kestrels live in open,grassy fields and farmlands,prefer to feed on small mammals,mostly voles,usually detect prey by windhovering flight (Videler et al.,1983),once the kestrel decides to capture its terrestrial prey,it performs a quick,fast glide,usually landing on the prey's body and seizes it with its foot.Soon after capture,the kestrel bites the prey to damage the central nervous system and thereby limit the possibility of escape (Csermely et al.,1998;Csermely and Bagni,2003).Whereas,Eurasian Sparrowhawks are avivores,feed on a variety of small bird species(Zawadzka and Zawadzki,2001),prefer open areas near forests or grasslands,tend to catch prey by brief periods of sustained chase (Barton and Houston,1994),attack prey from the horizontal direction with a marked forward thrust of the pelvis and rapid extension of the legs (Goslow,1971),and ultimately kill prey predominantly through suffocation resulting from thoracic compression and/or kneading action (Goslow,1971;Csermely et al.,1998).Herein,the muscular bases of these differences in modes of foraging and hunting among three raptorial species were investigated.Our primary aims were (1) to contribute a comprehensive dataset of mass,fibre length and physiological cross-sectional area (PCSA) for all muscles in the avian hindlimb,(2)to explore the possible differences on architectural parameters among species,and(3)to reveal similarities and differences in the pelvic muscles from anatomical and architectural aspects and their correlation with foraging behavior,which will be of further help in biomechanical modelling and ecological analysis of fossil species.
2.Materials and methods
Bird carcasses were obtained from the International Fund for Animal Welfare Beijing Raptor Rescue Center (IFAW BRRC).The qualitative anatomical comparison was based on 12 individuals (four for each species).Seven kestrels,three sparrowhawks,and four owls were used for quantitative architectural study;their mean body weights were 188±27 g,206±15 g,227±45 g,respectively.These birds were kept frozen at-18°C after death and defrosted before dissection.Individual muscles of one leg were identified,systematically dissected,and measured,and the myological nomenclature follows that of Vanden Berge and Zweers(1993).
Muscles were divided into three regional groups by their location in the hindlimb segments(hip,thigh,and shank)according to where most of the mass was distributed,and eight functional groups (hip flexors/extensors,knee flexors/extensors,ankle flexors/extensors,and digital flexors/extensors),based on their primary function on the femur,tibiotarsus,tarsometatarsus,and digits following Wang et al.(2021) (Appendix Table S1).Because each muscle group was composed of different muscles,before analyses,the percentage of the mass of each separate muscle with respect to the total limb MM was calculated,and then the sum of them was calculated to result in one value for each specimen to compare.
Reduced major axis regressions were performed using SMATR(Standardized Major Axis Tests and Routines) (v.2.0) software (Falster et al.,2006) to evaluate the slopes andY-intercepts of log-log linear relationships between body mass and muscle mass.All measurements were log10 transformed.Isometric growth was considered when scaling exponent (slope) was 1;confidence intervals above or below isometry,andp<0.05 were considered to be positively or negatively allometric,respectively.In order to compare the relative fiber lengths in muscles of different sizes,give information about the configuration and shape of the muscle,Architectural Index(AI)which is proportional to the velocity of contraction,was adopted and calculated as the ratio of muscle fiber length to muscle belly length(Sharir et al.,2006).In the same way,MM and PCSA were divided by body mass and body mass2/3to assess relative differences among species.Possible differences of architectural parameters were statistically analyzed in SPSS v.22.2 software.All data were tested for the normality and uniformity using Shapiro-Wilk Test (S-W)and Homogeneity Variances Test (H-V),respectively.If thepvalues of S-W and H-V Tests were greater than 0.05,statistical analysis was performed using a one-way analysis of variance(ANOVA).If thepvalues of S-W and/or H-V Tests were less than 0.05,statistical analysis was performed using Kruskal-WallisHnonparametric test.Pairwise comparisons using Dunn's post hoc test with Bonferroni adjustment were then conducted for any dependent parameters for which the Kruskal-Wallis test is significant.
3.Results
Anatomical dissection identified 35,35,and 33 individual muscles in the kestrel,sparrowhawk,and owl,respectively.M.caudofemoralis pars pelvica,M.flexor cruris lateralis,M.abductor digiti II and M.extensor proprius digiti III were absent in the kestrel.M.caudofemoralis pars pelvica,M.flexor cruris lateralis,M.plantaris,and M.extensor proprius digiti III were absent in the sparrowhawk.M.caudofemoralis pars pelvica,M.flexor cruris lateralis,M.fibularis longus,M.plantaris,M.ambiens,and M.abductor digiti II were absent in the owl(Appendix Table S1).Sesamoids were found in 13 muscles in the owl,but present only in one individual muscle (M.femorotibialis pars intermedius and lateralis FTL+I) in the sparrowhawk and three muscles (FTL+I,FDL,FHL) in the kestrel (Appendix Table S1).Obvious differences were also noticed for the insertion tendon of the major digital flexors between diurnal and nocturnal species.First is the relationship between their insertion tendon of FDL and FHL,and in the kestrel and sparrowhawk,the tendon of FHL split into two branches,one to the hallux,another one to digit 2,which fuses with that of the FDL.In the owl,FHL inserts on the hallux only,the insertion tendon of FDL divides into two branches in the proximal part of the tarsus;the outer branch goes to digit 4 while the inner one breaks into two supplying to digits 2 and 3.The second difference is the presence of a fibrous vinculum connecting the insertion tendons of FPPD2 and FPD2 in the owl that do not exist in the kestrel and sparrowhawk.The third was the peculiarity of intrinsic foot muscles:ABD2 and the sesamoid in EBD4 were found only in the sparrowhawk and the owl,respectively.
The quantitative analysis did not show a significant difference in the body weight of the study species.The total muscle mass of all pelvic muscles in one single leg of the kestrel is 9.61 ± 3.23 g,accounting for 5.03±0.9%(mean±SD)of body mass.The corresponding data for the sparrowhawk are 12.50±1.12 g and 6.1±0.2%and for the owl,13.03±3.50 g and 5.7±0.5%.The relative development of leg musculature is similar for the three species withp-values >0.05.The total hindlimb muscle mass exhibited positive allometry with body mass(slope=1.89,1.45 <95%confidence interval <2.45,R2=0.82,p<0.001).
The spatial distribution of muscle mass is shown in Fig.1.In general,muscles occupied in shank region took the greatest proportion of the total limb muscle mass in the studied species,followed in sequence by the thigh and hip regions.Percentage of shank muscles accounted for 48%of total MM in the kestrel,51% in the sparrowhawk,and 56% in the owl.Regarding the functional muscle group,the digital flexor group was the most massive for all species among eight functional muscle groups,representing 24% of total limb MM in the kestrel,27% in the sparrowhawk,and 30%in the owl,and the least massive group was the digital extensors (Fig.2).Flexors of the hip joint constituted a lower proportion than corresponding extensors,as same for the knee joint;but for the ankle joint,the flexors are slightly larger than extensors.
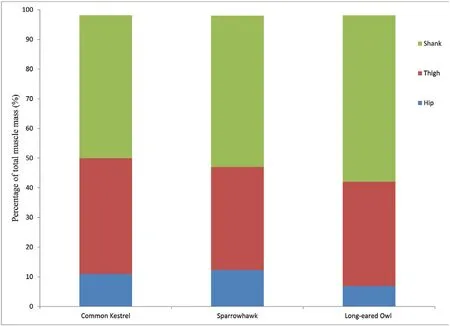
Fig.1.Muscle mass distribution among segments in three birds of prey.
The three study species share 29 pelvic muscles.Two foot intrinsic muscles,the ADD2 and EBD4,were very rudimentary in the sparrowhawk and kestrel,respectively,and were excluded from quantitative analysis.For the remaining 27 muscles,13 muscles are situated in the hip and thigh region,11 in the shank,and 3 in the tarsus.More interestingly,FDL,FHL,PTL+I,TC,GAS,IF,PIF are the seven largest muscles in these species (Fig.3),and they formed the bulk of the leg and accounted for 60% of the total hindlimb MM in the kestrel,63% in the sparrowhawk,and 66%in the owl.However,FHL was the largest,accounting for 12.8%and 16.2%of the total MM in the kestrel and sparrowhawk.FDL was the greatest in the owl taking 16.1%of the total MM.Intrinsic foot muscles were relatively insignificant,and those controlling the hallux(including EHL and FHB)were more developed than digits 2,3,4.
Their brother who is taller than they are, stands in the swing; he has one arm round the rope, to steady himself; in one hand he holds a little bowl, and in the other a clay pipe; he is blowing bubbles
The MM,PCSA and FL of the hindlimb muscles in the three study species are indicated in Appendix Table S2.Muscles with longer FL were usually located in the proximal limb,in particular IC,FCM,CF,IF,and IL.Architectural Index (AI) was higher in hip and tarsus regions,whereas muscles in the thigh and shank regions had lower values (Fig.4).Additionally,the owl had the lowest AIs in the muscles of shank and thigh regions with six shank individual muscles presenting significant difference (Fig.4;Appendix Table S3).Similar results of statistic tests were also found in the analysis of the PCSA and MM.Although very small,two of the three intrinsic foot muscles,located in the tarsus,EHL and FHB were different in the MM and PCSA with each other and were the greatest in the sparrowhawk,followed by the kestrel and the owl the last;meanwhile,the difference between the sparrowhawk and the owl was significant (Appendix Tables S2-S3).Seven and eight out of the 11 muscles in the shank region showed significant difference in the MM and PCSA,respectively (Appendix Table S3).When considering the eight digital flexor muscles as a whole,their total PCSA was not significantly different between each other,but was the smallest in the kestrel(3.56±1.00 cm2);the PCSA of the flexors controlling the hallux accounted for 55% and 63% of the PCSA of the total digital flexors in the kestrel and sparrowhawk,respectively.Regarding individual muscles of the shank region,FHL exhibits significantly better development in the kestrel and sparrowhawk than in the owl;even within diurnal species,this muscle mass is obviously greater in the sparrowhawk than in the kestrel.The owl was quiet different in possessing the largest FDL which was significantly different from that of the two diurnal species,and well-developed FPD2 and FPD3 which markedly differ from the kestrel(both MM and PCSA).In sum for the owl,the total mass of all flexor muscles controlling toes 2-4 was more than three times larger than that of the hallux (Fig.5);further analysis found that proximal-inserted flexors of digit 2 were the largest (Fig.6).Mass differences of digital flexor muscles between fore toes and hind toe,and among individual fore digits were not too large between diurnal species.Muscles located in the hip and thigh region showed the least variances,with less than half of them(4 of 13 muscles)detected with significant difference(Appendix Tables S2-S3).
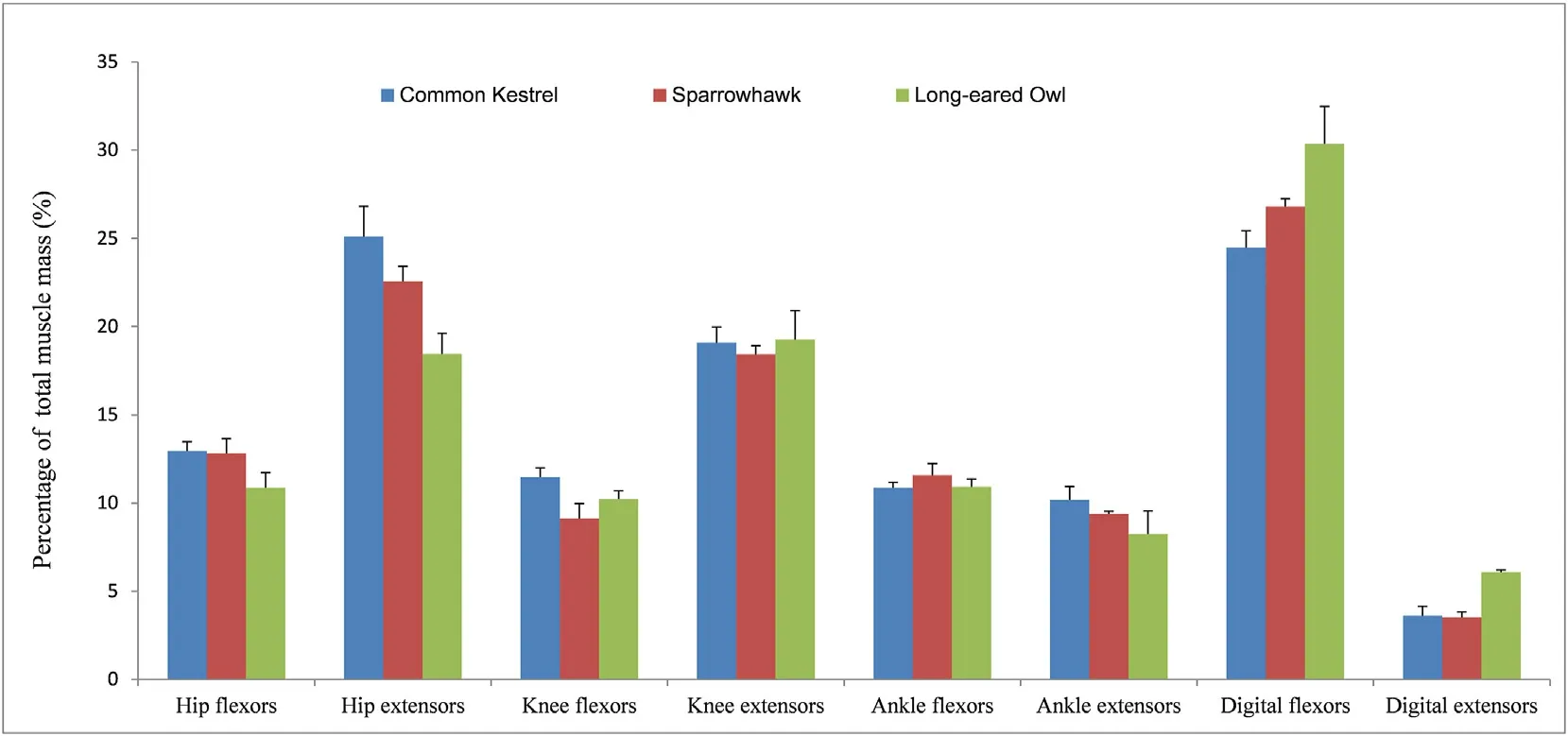
Fig.2.Distribution of functional group muscle mass in three birds of prey.Data are expressed as Mean ± SD.
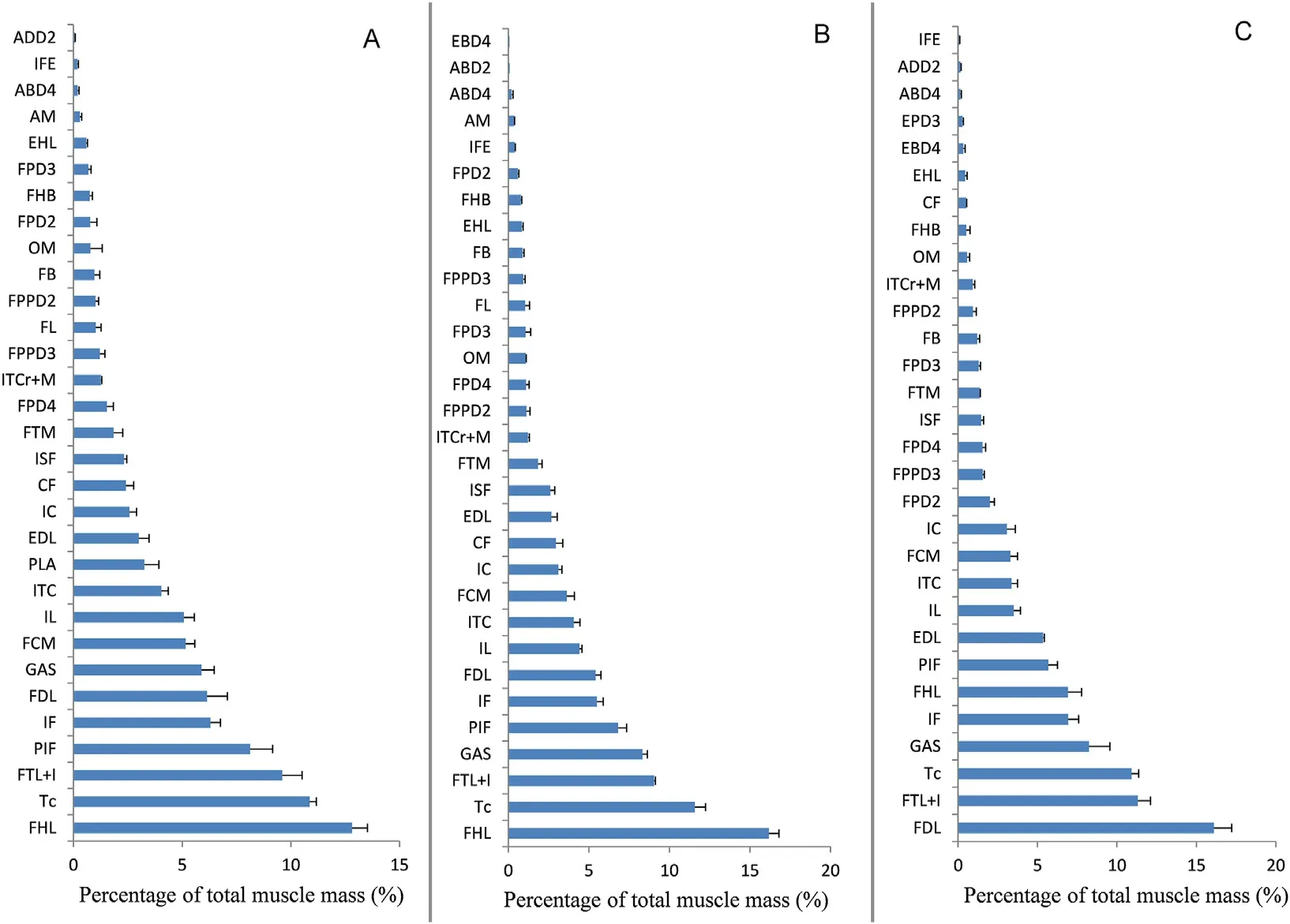
Fig.3.Mean muscle mass (±SD) as a proportion of total hindlimb muscle mass in three birds of prey (A: Common Kestrel;B,Eurasian Sparrowhawk;C: Longeared Owl).
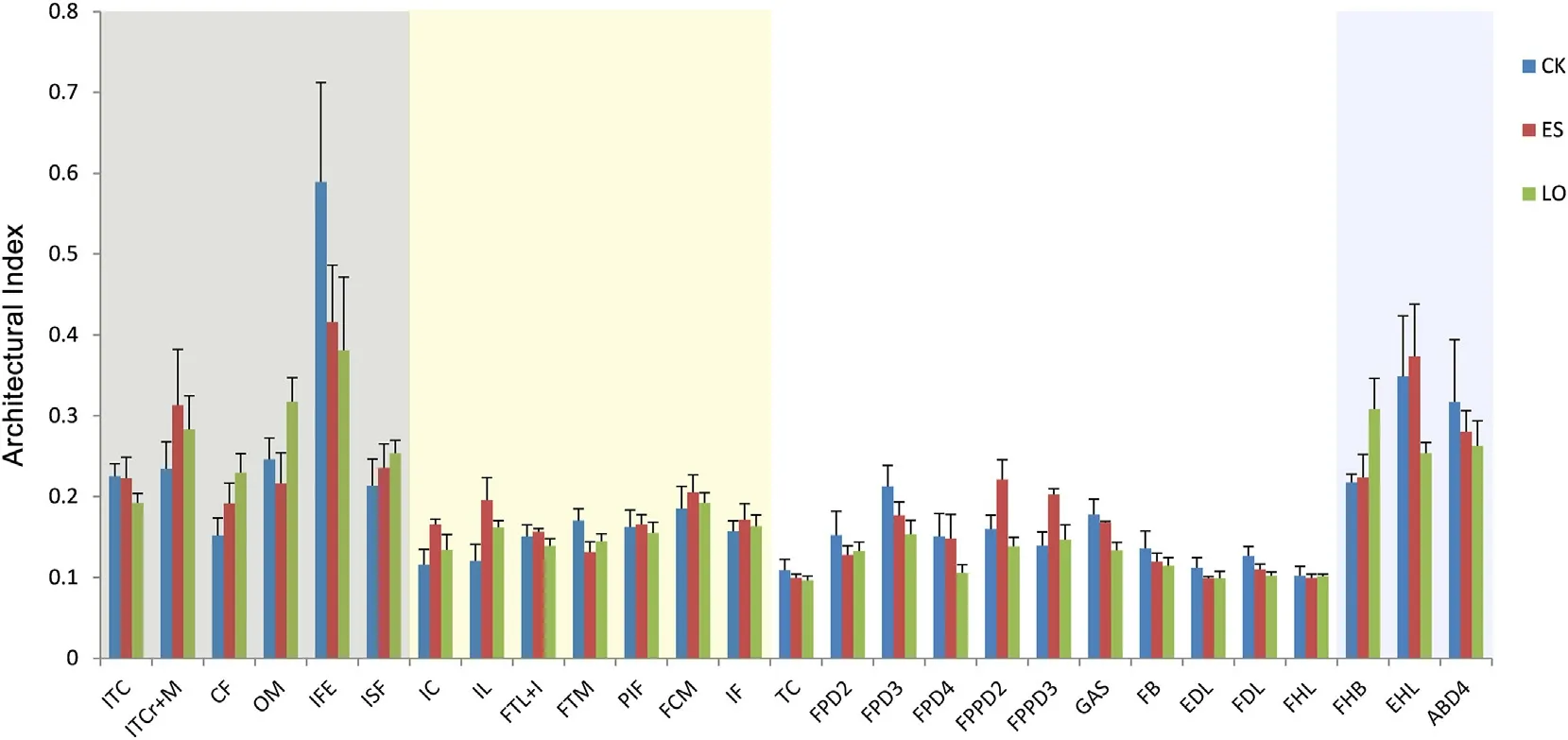
Fig.4.Architectural Index for individual muscles in three birds of prey (shading colors represent different regions: light grey for the hip,light yellow for the thigh,white for the shank,and light purple for the tarsus).CK: Common Kestrel;ES: Eurasian Sparrowhawk;LO: Long-eared Owl.
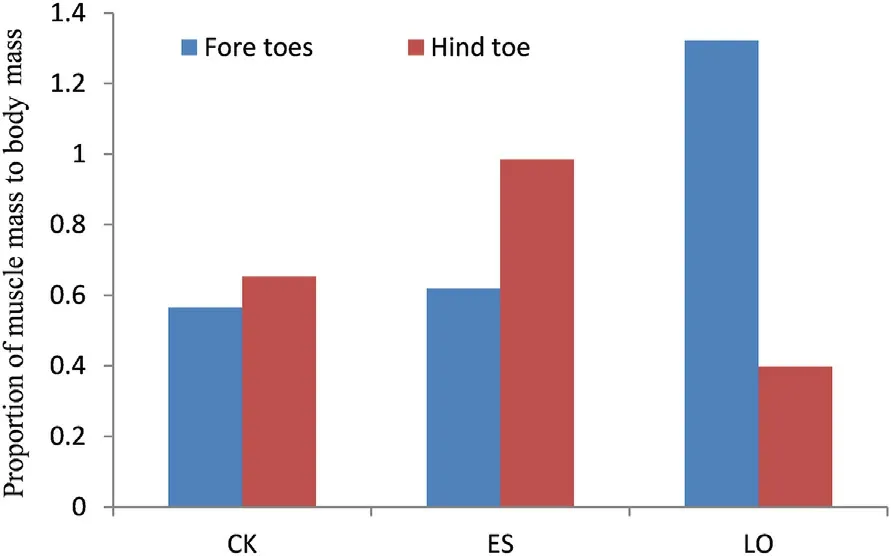
Fig.5.Percentage of mass of flexor muscles controlling the hind toe and fore toes in relative to body mass.CK: Common Kestrel;ES: Eurasian Sparrowhawk;LO: Long-eared Owl.

Fig.6.Percentage of mass of proximal-inserted flexor muscles controlling fore toes in relative to body mass.
4.Discussion
It has been suggested that there is probably more adaptive modification in hindlimb musculature than in any other region of the body(Jollie,1977).And it was expected that raptors would allocate more MM to the digital flexors because they use feet for seizing and killing prey.Toe flexors are mainly composed of five proximally inserted flexors(FPD2,FPPD2,FPD3,FPPD3,and FPD4)and two distally inserted flexors(FHL and FDL).Our results conformed to the expectation,and indicated that FHL was the greatest in diurnal raptors,the kestrel and sparrowhawk,while FDL was the largest in the nocturnal owl.Both the tendons of FDL and FHL insert on the distal ungual phalanges of digits II-IV and the hallux,respectively.Simulation analysis demonstrated that the elimination of all but the distally inserted flexors would not change the performance or force requirement of the grasp when applied to grasping and carrying tasks,furthermore suggested that FHL and FDL are determinants in grasping or carrying (Backus et al.,2015).Compared with fore toes,the hallux has the conspicuous potential in force generation inferred by the fact that flexor muscles controlling this digit(FHL and FHB)took the majority(more than 50%)of the total PCSA of eight digital flexors in the kestrel and sparrowhawk.Sustaita(2008) obtained a similar result,and suggested in a later work that the total grip force tended to be greater in accipiters than in falcons,consistent with the former’greater reliance on gripping for dispatching prey with their feet(Sustaita and Hertel,2010).The tendency was also revealed by our analysis that the total PCSA of eight digital flexors was greater in the sparrowhawk;although not significant,the difference between the kestrel and sparrowhawk was speculated to be associated with their different hunting behavior.Relatively weak grip force in the kestrel,together with smaller and subequally sized talons on each digit than accipitrids and owls(Fowler et al.,2009),cause kestrel incapable of holding prey more firmly by its foot that has been confirmed by experimental test(Csermely and Gaibani,1998),and in line with the hunting behavior observed in the kestrel(Csermely et al.,1998).As in other falcons,the kestrel might have greater bite force capacities than similar sized accipiters(Sustaita,2008;Sustaita and Hertel,2010),and by using powerful bites to damage preys' central nervous system thereby reducing the possibility of the prey escaping (Csermely et al.,1998).For individual digital flexor muscles,anatomical observation found that,as in other Accipitridae andFalcospecies (Hudson,1948),the insertion tendon of FHL in the kestrel and sparrowhawk split into two branches,one to the hallux,another one fusing with that of the insertion tendon of FDL to digit 2;this pattern of insertion tendon suggested that FHL can control digits 1 and 2 simultaneously,and thus might aid the grasping behavior of digit 2.Based on the fact and assumption,diurnal raptors are possibly equipped with an efficiently vise-like set by the opposing digits 1 and 2 that play more important role than digits 3-4 in foraging.This functional set of digits 1 and 2 has greater force generation capacity in the sparrowhawk,together with its hypertrophied talons on digits 1 and 2 (Fowler et al.,2009),give the species strong guarantee and high success to restrain struggling prey by their foot as the primary device,without the aid of bites.Whereas in the owl,it not only allocated the greatest PCSA and MM to FDL which was more than three times than those in the kestrel and sparrowhawk (Appendix Table S2),but also exhibited distinct pattern in terms of the insertion tendon of FDL in giving rise to two branches in the proximal part of the tarsus,with the outer branch goes to digit 4,the inner one breaks into two at the distal tarsus supplying to digits 2 and 3.We speculated that such a pattern favors the owl to transmit more grasping force to digit 4 compared with the kestrel and sparrowhawk whose tendon of FDL divides into three branches at the base of toes supplying three fore digits.These features might strengthen the role of digit 4 to owl's hunting.The assumption is consistent with other external morphological characteristics,such as greater claw size and highest claw-toe ratio in digit 4(Fowler et al.,2009;Wang and Zhang,2020).Toe arrangement is zygodactylous in the owl,composed of two opposing sets(digit 3 with digit 4,digit 1 with digit 2).In another opposing set formed by the hallux and digit 2,although force production ability of the hallux was suggested to be significantly low(Fig.5),the whole effect of the vice-like set was promised by strengthening that of the digit 2(Fig.6).Meanwhile,the proximal-inserted digital flexors of each digit all had larger MM proportion in relative to body mass in the owl than in diurnal raptors,and were the largest for digit 2 in the owl(Fig.6).From the view of architectural design and force production capacity,in addition to the zygodactyl feet,we speculate that,unlike kestrel and sparrowhawk,the two opposing sets or in other words,each digit of the owl plays subequal role in hunting.
It was also interesting that besides FDL and FHL,these species are the same in distributing more muscle mass and PCSA to PTL+I,TC,GAS,IF,PIF.The highly developed TC and IF should be a convergent reflection to predatory habits of these species.Because IF and TC act as powerful flexor of the shank and tarsometatarsus,respectively.It is reasonable that great force-generation potential of the two muscles is favor of the indirect mechanisms of talons closure and the action of holding prey close to the body at the time of carrying(Ward et al.,2002).Unlike our results,in a study of a facultative scavenging Polyborinae species,Milvago chimango,the gastrocnemius (GAS),neither FDL nor FHL,was found to have the greatest mass,followed by the femorotibial group,PIF,FHL,TC,IF,ITC;furthermore,FDL was less developed in this species and only 30% of the FHL(Mosto et al.,2013);these features may be a reflection of a different lifestyle such as diet and have functional implications even within the birds of prey.
Interestingly,unlike other pelvic muscles,M.caudofemoralis (CF)take origin from the femur to the base of the pygostyle,which is no longer a crucial element in powering the limb in birds(Cracraft,1971;Vanden Berge and Zweers,1993).Accordingly,its pygostyle insertion gives the muscle specific and complicated functions in ventilation (Baumel et al.,1990) and aerodynamic function (Fisher,1946;Gatesy and Dial,1993;Mosto et al.,2020).The ability of a flying sparrowhawk who usually capture avian preys in flight presupposes itself to be either very fast,deftly maneuverable,or both(Goslow,1971;Barton and Houston,1994).Previous studies have long been aware that broad wings with a more rounded tip,long tail,shorter small intestine,and heavier pectoral MM in the sparrowhawk give it maneuverability (Barton and Houston,1994;Swaddle and Lockwood,1998).In the study,we noticed the greatest development of CF in the sparrowhawk,and the significant difference in the MM and PCSA between it and the owl.We presumed the conspicuous difference should reflect discrepancy on tail use and control during flight,based on the fact that contraction of M.caudofemoralis may result in depression or side-to-side movement of the tail which will cause flight surface change (Fisher,1957;Baumel et al.,1990;Gatesy and Dial,1993).A larger CF in the sparrowhawk is possible to cause more flexible tail movement and shape change which will enhance agility of bird's flight performance.Because tail is controlled by several pairs of muscle,one cannot know to what extent the tail surface changes by the action of CF by the present data,in the same way,one cannot rule out the role of this muscle in controlling tail.Tail use and its fine adjustment were proposed to be necessary for soaring flight (Fisher,1946).It is well known that accipiters soar in the air frequently and when soaring,it can spread out its tail feathers to create additional lift and stability.However,owls never use soaring flight to any extent(Barton and Houston,1994).CF is essential in abduction of the rectrices to form a fan-shaped tail,thus the significant difference in MM and PCSA of CF between the sparrowhawk and owl is possibly related to the their flight style and even feeding ecology,and need further investigation.Similar to accipitrids,falcons are familiar by their hovering flight,and at that moment their tails are often fanned (Videler et al.,1983;Rosen and Hedenstrom,2002).Available data on the tail myology of raptorial species revealed significant difference on total and/or individual muscle mass,further demonstrated the association between tail myology(including CF)and flight behavior(Lo Coco et al.,2020;Mosto et al.,2020).
Individually fine control of the extension,flexion,adduction,or abduction of toes is attributed to the function of intrinsic muscles.To have the ability to position the digits at wider angles would provide larger grip area once the prey is captured(Tsang and McDonald,2019).In view of muscle function and muscle control,abduction of digits 2 and 4 contributed mainly to dexterous feet (Fisher,1946).The presence of ABD2,together with the highest force-generating capacity of ABD4 in the sparrowhawk suggested by the present study,were thought to form the muscular basis for broadening digits’range of motion and more flexible feet which were probably suitable for catching highly mobile avian preys.Three species all possess EHL,FHB,ADD2,EBD4,and ABD4,while EHL and FHB are the greatest in the sparrowhawk,the weakest in the owl.As a result,the action of the two muscles on the digit is expected to be significantly different,which was assumed to reflect how important a role the hallux plays in prey capture and handling.For the sparrowhawk,the strongest flex forces of FHL and FHB revealed by this study,together with other morphological traits such as larger tubercle pads,larger TLM,and hypertrophied larger talon size in digit 1 (Einoder and Richardson,2007a;Fowler et al.,2009),would probably be capable of providing a stronger lock and sufficient grip force when hunting that feet can be used as the primary killing and puncture device (Einoder and Richardson,2006).Architectural Index of muscles (Fig.4) was higher in the tarsus region than shank region,indicating relatively long fiber length for intrinsic foot muscles.Judged from architectural aspects only,the work range and velocity of contraction of long-fibered muscles are great and high (Sharir et al.,2006),giving these intrinsic muscles advantage in speed instead of force production.Likewise,AIs of the muscles in the shank region were the lowest in the owl reflecting its relatively low speed in activity.
Consistent with previous studies,the present work supported the statement that qualitative and quantitative traits of hindlimb muscles have functional and phylogenetic signals(e.g.Carril et al.,2014;Hertel et al.,2015).When muscles were grouped by their primary function,our results,as expected,showed that the digital flexor group was the largest among the eight functional groups,and flexors of the ankle exceeded those of the opposing functional group.These features coincide with the mechanism of digit closure,which involves contraction of the flexor muscles and M.tibialis cranialis(Ward et al.,2002).They should be the basic myological adaptation and convergence for producing a strong grip for the diurnal and nocturnal raptors.The development of functional muscle groups can also reflect the habit difference (Wang et al.,2021),and is variable among ecological groups.For instance,Cabot's Tragopan(Tragopan caboti),as one of the terrestrial species,is characterized by the largest knee extensor group,supporting the idea that knee extension is primarily responsible for the terrestrial movement (Wang et al.,2021).Hip and ankle extensors constituted the first two greatest functional groups in ostriches,and thus were most important for powering their high-speed cursorial locomotion (Smith et al.,2006).One might think the differences are reasonable between raptors and terrestrial (and cursorial)species because they are quite different in foot use.Compared with Monk Parakeet (Myiopsitta monachus),one of the Psittaciformes,which is similar to raptors in their foot use and arboreal habits;their hindlimbs take the high manipulative ability for feeding and arboreal locomotion,and thus were posed with a high necessity for a strong grip.From the original data of Carril et al.(2014),it was found that the greatest proportion of pelvic muscle was for hip and knee extensors,while digital flexors accounted for a relatively high proportion(16%)as expected,but not as well-developed as they are in raptors.In addition to grasping,digital flexors are proposed to be more critical during perching(Backus et al.,2015);a relatively high proportion of this muscle group in the parrot might be a myological adaptation to arboreal habits,except for food handling.A similar situation was also found in Cabot's Tragopan,which nests in trees and is more arboreal than other pheasants,possessing a relatively high percentage of digital flexors(11%)(Wang et al.,2021),but in ostrich,this muscle group is smaller with the corresponding percentage of 5% (Smith et al.,2006).Even within diurnal raptors,different uses of the hindlimbs (e.g.,prey capturing,food preference,habitat use,and locomotion) can be reflected in muscle group development.Falconidae,for example,is composed of three subfamilies,and Polyborinae prefer scavenging carrion and wal on the ground searching for food;their leg muscles are featured by the predominance of the extensors of the hip(and the knee)and less massive digital flexors(20% inMilvago chimango) (Mosto et al.,2013;Mosto,2017a,b).But in Falconinae and Herpetotherinae who must hold prey,the digital flexor group comprised the greatest proportion of the hindlimb myology.In another diurnal raptorial assemblage,the Accipitriformes,Hertel et al.(2015)reported that the average percentage of mass allocated to digital flexors in three vultures was about 14%smaller than in two raptors.
In general,muscles of the fore-and hind-limbs present a proximal to distal mass reduction in birds,which can minimize the moment of inertia during locomotion (Smith et al.,2006;Yang et al.,2015;Bribiesca--Contreras et al.,2019).But this was not the fact in the studied species,and the relatively high percentage of shank muscle may indicate a less terrestrially adapted capacity for them.On the other hand,high muscle mass distribution in the shank region can be attributed to well-development of digital flexors and TC,and consequently reflect myological adaptation to predatory lifestyle.
Anatomical and architectural studies on the appendicular muscles of birds form the base for investigating muscle-tendon function during locomotion and feeding,and play important role in biomechanical reconstructions of extinct animals (Bates and Falkingham,2018).In the present study,we reported a comprehensive dataset of MM,FL and PCSA for all muscles in the hindlimb of three raptorial species.The spatial and functional distribution of muscle mass within the hindlimb has also been established.As expect,digital flexor muscle group accounted for the greatest proportion of the total hindlimb muscle mass,and for individual muscles,FDL,FHL,TC,and IF were among the seven largest muscles.The spatial distribution of muscle was unusual in allocating the largest mass to the shank region,not the proximal leg,mainly due to having massive flexor muscles situated in the lower leg.These features were shared by three species and were speculated to be the similarity between diurnal and nocturnal raptors due to their predatory lifestyle.Due to difficulties in obtaining raptors carcasses and thus small sample size and the limited species,more works are needed across a larger sample of individuals and species to verify our conclusions.Marked differences in MM,PCSA and AI along their leg musculature were detected,and most of them were found in the distal part (shank and tarsus regions).Architectural differences reflected the distinction in force-generation capacity among species and might be related to different foraging behavior.More attentions were also paid to the disparity in CF and its functional role in flight,considering close relationship between flight mode and foraging environment.Apart from fiber arrangement,many factors contribute to muscle function including fiber type,moment arm,synergistic actions of muscle groups,etc.More data from different aspects,such as muscle activity,kinematics,and histochemistry are needed to better understand the functional role of these muscles during various hunting behaviors.
Authors’ contribution
ZZ conceived and designed this study.XL performed the experiments,XL and ML carried out data analysis.ZZ,XL and CY contributed to the writing and improvement of the manuscript.All authors read and approved the final manuscript.
Ethics statement
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
We are grateful to International Fund for Animal Welfare Beijing Raptor Rescue Center(IFAW BRRC)for kindly providing materials,three reviewers for their valuable comments that greatly improve our manuscript,and Enago for language editing service.This work was supported by the National Natural Science Foundation of China (Grant No.31970411).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.avrs.2022.100053.
杂志排行
Avian Research的其它文章
- Comparative analysis of the intestinal tract microbiota and feeding habits of five sympatric flycatchers
- Seasonal variations in gonad morphology and hypothalamic GnRH-I and GnIH in Eurasian Tree Sparrow,a multi-brooded passerine
- Within-brood body size and immunological differences in Blue Tit(Cyanistes caeruleus) nestlings relative to ectoparasitism
- Factors affecting post-release survival and dispersal of reintroduced Crested Ibis (Nipponia nippon) in Tongchuan City,China
- Long-term monitoring data reveal effects of age,population density,and environmental aspects on hatching success of Common Cranes (Grus grus)
- Using citizen science data to improve regional bird species list:A case study in Shaanxi,China
