基于吡啶基三联吡啶配体的锌(Ⅱ)和镉(Ⅱ)配合物的晶体结构和荧光性质
2022-09-16苑亚南王子璇王朝阳宋瑶瑶王庆伦
苑亚南 王子璇 王朝阳 宋瑶瑶 王庆伦 杨 春*,
(1河北工业大学化工学院,天津 300130)(2南开大学化学学院,天津 300071)(3南开大学先进能源材料化学教育部重点实验室,天津 300071)
0 Introduction
Terpyridine(terpy)ligands usually bind transition metal ions in a 2∶1 molar ratio to give pseudo-octahedral[M(terpy)2]complexes,offering a broad range of predictable thermodynamic stability:Cd(Ⅱ) < Zn(Ⅱ) < Fe(Ⅱ) <Ru(Ⅱ)[1-2].As tridentate ligands,terpyridine-derived compounds can form complexes[MⅡ(terpy)(L)n](L=Cl-,I-,NO3-,CH3COO-,P2O74-,H2O,etc.,n=1-3)[3-6]or[MⅡ(terpy)2][5-10](M=Zn,Cd),where the charge of the complexes has been omitted.Terpyridine-derived ligands usually display fluorescence in solution,while upon addition of Zn2+or Cd2+,the fluorescence may be quenched[2,4,9,11-13]or enhanced[4,7-9].However,there is rarely report on the fluorescent properties of[Zn(npyterpy)2]2+and[Cd(n-pyterpy)2]2+(n=2-4)(Scheme 1)and their mono-protonated and bis-protonated forms[6,10].
As one of the most widely used visible light responsive metastable-state photoacids(mPAH),mPAH1(Scheme 1)has weak ground state acidity(pKaGS=5.74 in water)in dark and strong metastable-state acidity(pKaMS=2.14 in water)under irradiation with visible light[14].It can exchange protons with pyridine group(pKa=5.23 in water)[15-16],acridine dye[17],and Bromo-Cresol Green[18],etc.Herein,the synthesis,crystal structure,and fluorescent properties of complexes[Zn(2-pyterpy)2](ClO4)2·0.25H2O(1)and[Cd(2-pyterpy)2]2(ClO4)4·2.33H2O·CH3OH(2)(2-pyterpy=4′-(2-pyridyl)-2,2′∶6′,2″-terpyridine)were reported,and the modulation of the fluorescence property of complex 1 in methanol solution with mPAH1 was also investigated.
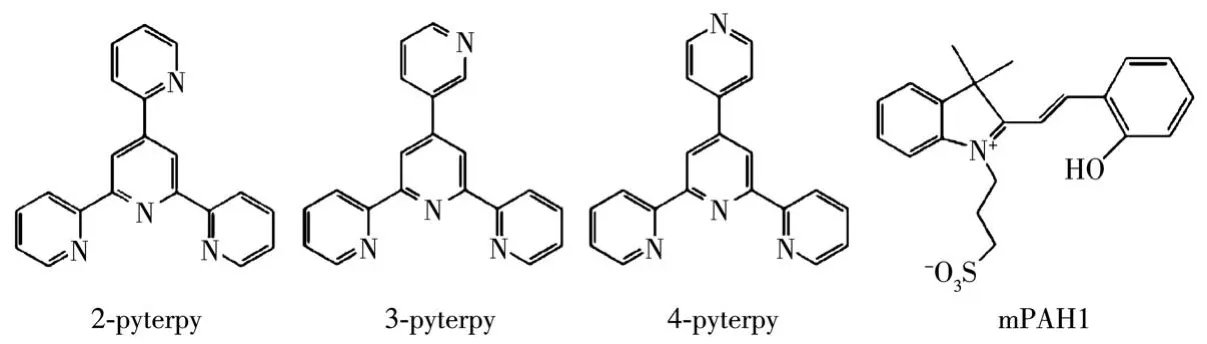
Scheme 1 Molecular structures of n-pyterpy(n=2-4)ligands and mPAH1
1 Experimental
1.1 Physical measurement
The C,H,and N elemental analyses were performed with a Perkin-Elmer 240C elemental analyzer.1H NMR spectra were obtained on a Bruker 400 MHz NMR spectrometer(Avance Neo).The IR spectra of KBr pellets were recorded with a Vector-22 Fourier FTIR spectrophotometer in a wavenumber range of 4 000-400 cm-1.The UV-Vis absorption spectra were recorded on a Varian Cary 300 Scan UV-Vis spectrophotometer.The fluorescence spectra were performed with a near-infrared spectrometer(FS920P,Edinburgh Instruments)with a 450 W xenon lamp as the steadystate excitation source,a double-excitation monochromator(1 800 mm-1),an emission monochromator(600 mm-1), and a semiconductor-cooled Hamamatsu RMP928 photomultiplier tube.Time-resolved fluorescence decay spectra were acquired on an Edinburgh FS5 spectrofluorometer.All spectral measurements were made at room temperature and the excitation wavelength was 365 nm.Powder X-ray diffraction(PXRD)data were recorded on a Rigaku D/max-2400 X-ray diffractometer with CuKαas the radiation source(λ=0.154 06 nm)at room temperature in a 2θrange of 5°-50°,with tube voltage of 40 kV and tube current of 40 mA.
1.2 Synthesis of 2-pyterpy,complexes 1 and 2,and mPAH1
Unless otherwise noted,all chemicals and starting materials for synthesis were of reagent grade and used without further purification.Caution!Although not encountered in our experiments,perchlorate salts are potentially explosive and should be handled with care and in small quantities.The ligand 2-pyterpy was prepared by a reported method[19-20].1H NMR(400 MHz,CDCl3):δ9.11(s,1H),8.71(dd,J=30.0,6.0 Hz,2H),7.98(d,J=76.3 Hz,2H),7.31(d,J=38.3 Hz,2H),1.25(s,1H),0.03(d,J=29.6 Hz,2H).
Complex 1 was solvothermally synthesized under autogenous pressure.A mixture of Zn(ClO4)2·6H2O(0.25 mmol,93.0 mg),2-pyterpy(0.5 mmol,156.1 mg),and CH3OH-H2O(1∶5,V/V,18 mL)mixed solvent was sealed in a Teflon-lined autoclave and heated to 120℃in 6 h.After being maintained for 48 h,the reaction vessel was cooled to room temperature over 12 h.Pure mauve crystals were then collected.Yield:96.37 mg(43%).Anal.Calcd.for C40H28.50Cl2N8O8.25Zn(%):C,54.01;H,3.18;N,12.60.Found(%):C,54.16;H,3.07;N,12.37.Main IR bands(KBr pellets,cm-1):3 000vw,1 604s,1 583s,1 470s,1 387m,1 089s,780m.
Complex 2 was synthesized in a similar method to complex 1.A mixture of Cd(NO3)2·4H2O(0.25 mmol,77.1 mg),NaClO4·H2O (0.5 mmol,70.2 mg),and 2-pyterpy(0.5 mmol,156.1 mg)and CH3OH-H2O(1∶5,V/V,18 mL)mixed solvent was sealed in a Teflon-lined autoclave and heated to 150℃in 6 h.After being maintained for 48 h,the reaction vessel was cooled to room temperature over 12 h.Light green crystals were then collected.Yield:252.6 mg(52%).Anal.Calcd.for C81H64.66Cl4N16O19.33Cd2(%):C,50.20;H,3.36;N,11.56.Found(%):C,50.32;H,3.25;N,11.77.Main IR bands(KBr pellets,cm-1):3 000w,1 610vs,1 583s,1 470s,1 390m,1 089vs,790s.
mPAH1 was prepared according to literature procedure[21].1H NMR(400 MHz,DMSO-d6,TMS):δ11.04(s,1H),8.60(d,J=16.4 Hz,1H),8.28(d,J=8.0 Hz,1H),8.02(d,J=7.2 Hz,1H),7.90-7.85(m,2H),7.65-7.59(m,2H),7.47(t,J=8.4 Hz,1H),7.04-6.97(m,2H),4.80(t,J=8.0 Hz,2H),2.65(t,J=6.4 Hz,2H),2.19-2.16(m,2H),1.77(s,6H).
1.3 Crystallographic measurements and structure determination
Diffraction data of complexes 1 and 2 were collected on a Bruker SMART 1000 CCD diffractometer equipped with graphite-monochromated CuKαradiation(λ=0.154 184 nm)and MoKαradiation(λ=0.071 073 nm),respectively.Intensities data were collected by theφ-ωscan technique for complexes 1 and 2.The structure was solved by the direct method using the SHELXS program of the SHELXTL package and refined by full-matrix least-squares methods with SHELXL.The nonhydrogen atoms were located in successive difference Fourier syntheses and refined with anisotropic thermal parameters onF2.Hydrogen atoms were constrained through the riding model in their position as determined by an analysis of the distances between heavy atoms.The parameters of crystal data collection and refinement of complexes 1 and 2 were given in Table 1.The selected bond distances and angles for complexes 1 and 2 were listed in Table 2.
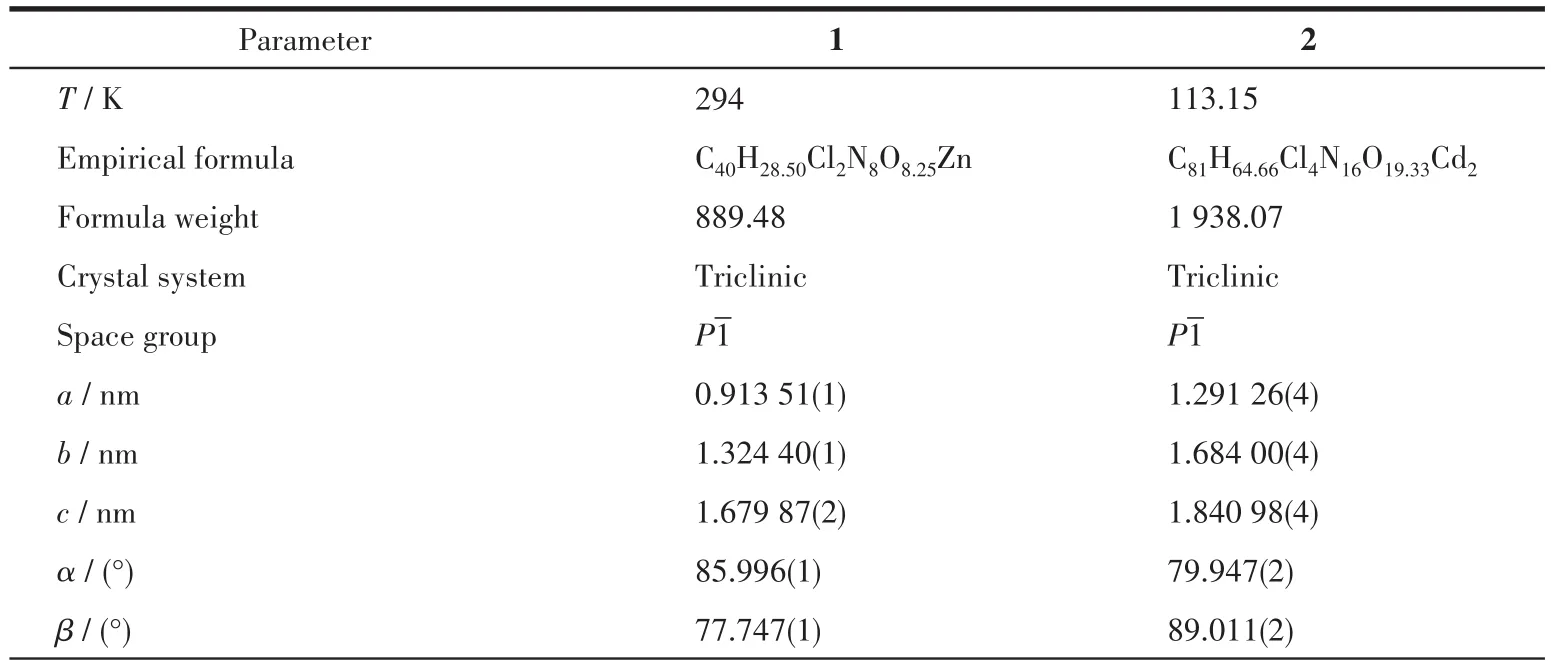
Table 1 Crystallographic data and structure refinements of complexes 1 and 2

Continued Table 1

Table 2 Selected bond lengths(nm)and bond angles(°)for complexes 1 and 2
CCDC:2149901,1;2149905,2.
2 Results and discussion
2.1 X-ray single-crystal structure analysis
As shown in Fig.1,complex 1 consists of one crystallographically independent[Zn(2-pyterpy)2]2+cation,two uncoordinated ClO4-counter anions,and 0.25 crystallized H2O solvent molecules.Each 2-pyterpy molecule behaves as a tridentate ligand and the Zn(Ⅱ)is coordinated by two 2-pyterpy molecules.In the distorted[ZnN6]octahedron,the Zn—N bonds fall in the normal range(0.220 7-0.207 4 nm)with an average Zn—N bond length of 0.215 4 nm,and thecis-andtrans-N—Zn—N angles are in a range of 75.01°-111.40°and 150.04°-172.62°,respectively,which are comparable to those in other[Zn(terpy)2]2+mononuclear complexes[5-6].The Zn…Zn distances between the adjacent ZnN6units are 0.913 5 nm.The N1,N2,N3,and Zn1 atoms as well as the N5,N6,N7,and Zn1 atoms lie almost in a plane,respectively,and the dihedral angle between the two planes is 92.9°.In complex 1,the two uncoordinated ClO4-counter anions have 1.5 completely disordered perchlorate anions and 0.5 completely ordered perchlorate anions.
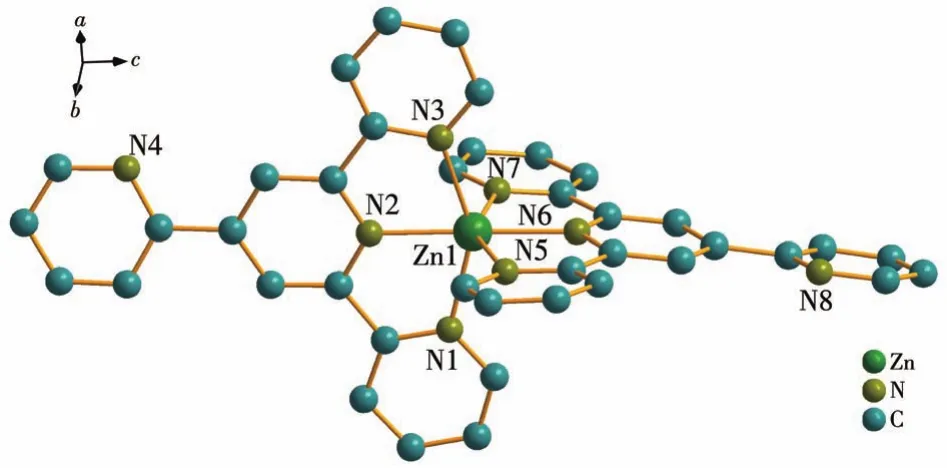
Fig.1 Crystal structure of complex 1
As shown in Fig.2,complex 2 consists of two crystallographically independent[Cd(2-pyterpy)2]2+cations,four uncoordinated ClO4-counter anions,2.33 crystallized H2O molecules,and one crystallized CH3OH molecule.In the distorted[CdN6]octahedron,the Cd—N bonds fall in the normal range(0.228 9-0.237 2 nm),and thecis-andtrans-N—Cd—N angles are in a range of 69.95°-120.39°and 140.11°-168.28°,respectively,which are comparable to those in other[Cd(terpy)2]2+mononuclear complexes[10].The N2,N3,N4,and Cd1 atoms as well as the N5,N6,N7,and Cd1 atoms lie almost in a plane,respectively,and the dihedral angle between the two planes is 95.2°.The Cd…Cd distances between the adjacent CdN6units are 0.954 0 nm.In complex 2,the four uncoordinated ClO4-counter anions have one completely disordered perchlorate anion and three completely ordered perchlorate anions.The experimental PXRD patterns of complexes 1 and 2 were in good agreement with the simulated patterns of complexes 1 and 2(Fig.3),respectively,confirming their phase purity.

Fig.2 Crystal structure of complex 2
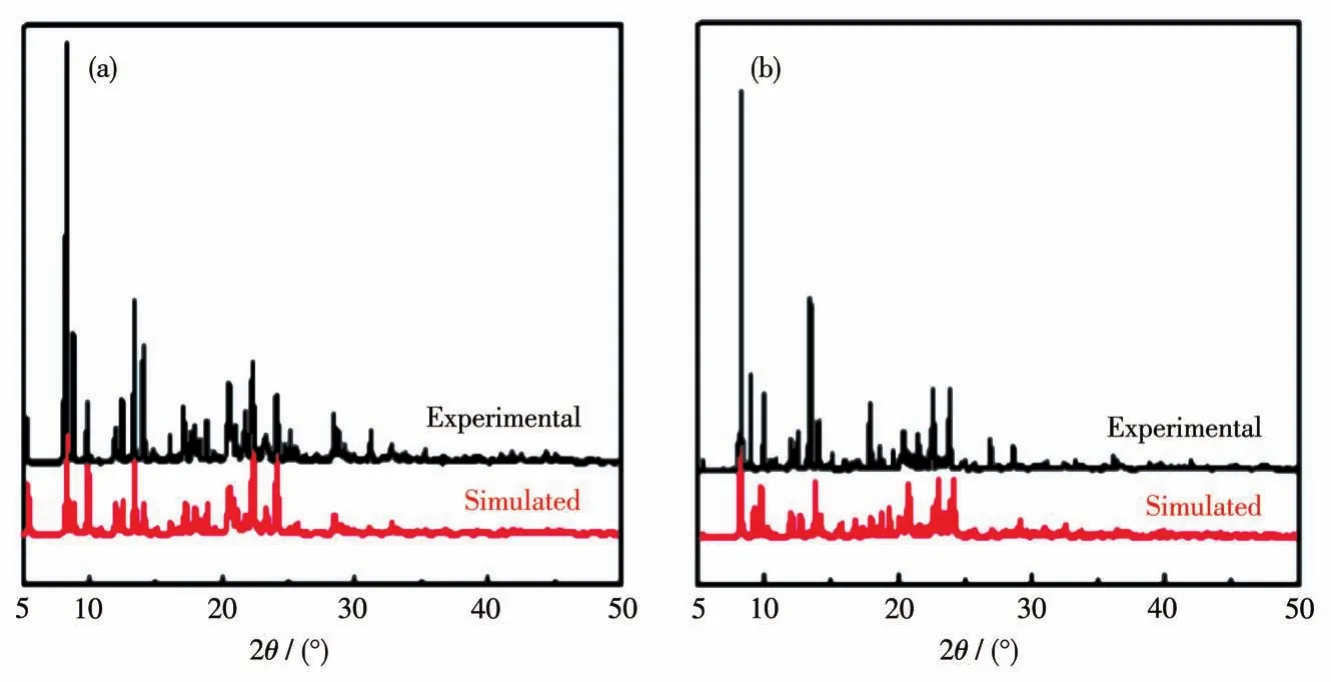
Fig.3 PXRD patterns of complexes 1(a)and 2(b)at room temperature
2.2 Fluorescence properties
Because Zn(Ⅱ)has a closed-shelld10electronic configuration,we anticipated that its coordination would alleviate photoinduced electron transfer(PET)and provide fluorescence“turn-on”[8,22-23].As shown in Fig.4,upon excitation at 405 nm,complexes 1 and 2 in solidstate showed broad emission bands centered at 539 and 547 nm,with the large Stocks shift of 134 and 142 nm,respectively.This is consistent with the terpybased emission[24].
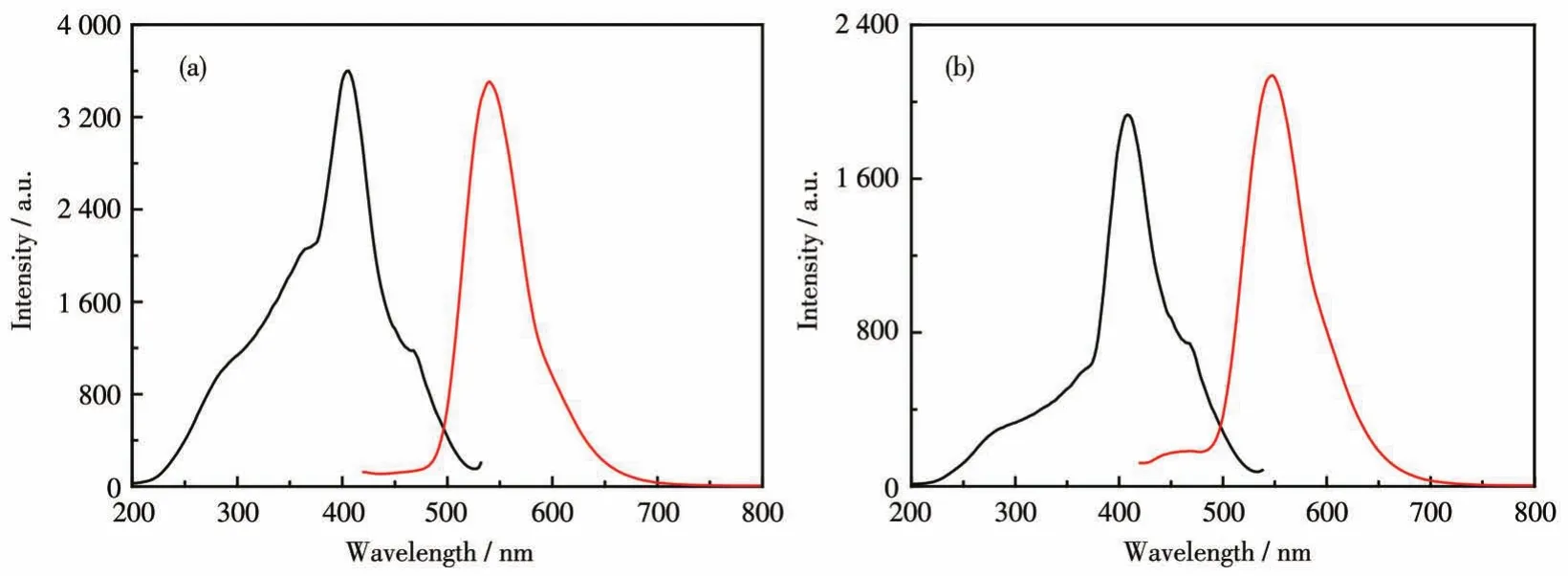
Fig.4 Excitation(black line)and emission spectra(red line)of complexes 1(a)and 2(b)in solid-state at room temperature
Based on the stability constant of the same class of complexes[1-2],the dissociation of complexes 1 and 2 in methanol solution has been ignored.As shown in Fig.5,the electronic and fluorescent spectra of complex 1 and mPAH1 were recorded.In the electronic spectra of the methanol solution of complex 1(80 μmol·L-1),the intense intraligandπ-π*andn-π*transition was observed at 260 and 340 nm(curve 1)[7,8,19,25-27].In the dark,the broad band centered at 439 nm(ε=2×104L·mol-1·cm-1)is assigned to theπ-π*transitions of protonated merocyanine form(HMC)of mPAH1(curve 3).Upon irradiation with 470 nm light,HMC was transformed into the cyclized spiropyran(SP)form bytranscisisomerization.The absorption band centered at 300 nm,which is assigned to SP,emerged accompanying the almost disappeared absorption peak at 439 nm for HMC with an isosbestic point at 310 nm(curve 2).
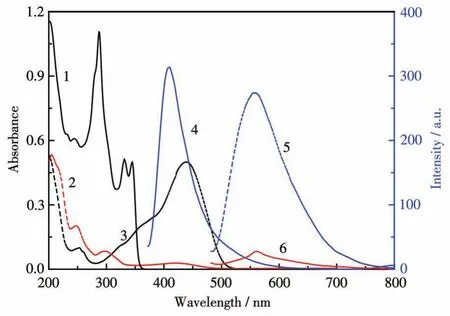
Fig.5 Absorption(curve 1)and emission spectra(curve 4,λex=357 nm)of complex 1(80 μmol·L-1),and the absorption spectra(before irradiation:curve 3;after irradiation:curve 2)and emission spectra(before irradiation:curve 5;after irradiation:curve 6,λex=467 nm)of mPAH1(80 μmol·L-1)
Excited with 357 nm,complex 1 emitted fluorescence at 408 nm in methanol solution(curve 4)[9].Compared with the solid state,the maximum excitation and emission wavelengths of complex 1 were blue-shifted by about 50 and 131 nm,respectively.The small Stokes shift(51 nm)implies there is little change in geometry or polarity between ground and excited states[12,28].Under excitation at 467 nm,before irradiation the methanol solution of mPAH1 had strong emission at 556 nm(curve 5),while after irradiation with 470 nm light the fluorescence was nearly quenched because of the formation of the spiral ring(SP form)and the contraction of theπ-conjugated system(curve 6).The sufficient overlap of the emission spectra of complex 1(curve 4)and the absorption spectra of mPAH1(curve 3)indicates that mPAH1 may quench the fluorescence of complex 1.In contrast,the overlap of the emission spectra of mPAH1(curve 5)and the absorption spectra of complex 1(curve 1)is nearly negligible,which indicates that complex 1 may not quench the fluorescence of mPAH1.
As shown in Fig.6a,upon addition of mPAH1 into the methanol solutions of complex 1(keep constant at 80 μmol·L-1),the fluorescence intensity at 408 nm gradually decreased,while that at 556 nm gradually increased.As shown in Fig.6b,the emission quenching constant(KSV)of 2.961×104L·mol-1(R2=0.983)was obtained by a linear least-squares fitting ofI0/IagainstcQaccording to the Stern-Volmer equation:I0/I=1+KSVcQ(1),whereI0is the fluorescence intensity of complex 1 in the absent of mPAH1,Iis the fluorescence intensity of complex 1 in the presence of mPAH1 at various concentrations,KSVis the Stern-Volmer quenching constant,cQis the concentration of the quencher,i.e.mPAH1.

Fig.6 (a)Fluorescence emission spectra of complex 1(80 μmol·L-1)with various concentrations of mPAH1(1-6:0,8,16,32,48,64 μmol·L-1)before irradiation(λex=357 nm);(b)Stern-Volmer curve of fluorescence quenching of complex 1 by mPAH1
The strong and broad absorption peak of mPAH1 overlaps with the emission peak of complex 1,suggesting that the inner filter effect(IFE)will take place when they are mixed together.To verify the fluorescence quenching is only caused by IFE,the fluorescence lifetimes of complex 1 in the absence and presence of mPAH1 were measured(Fig.7).The fluorescence lifetime decay curves of complex 1 were fitted according to the three-exponential function(Eq.2),and the nonlinear least-squares method was used to judge the size ofχ2.The closerχ2is to 1,the more accurate the fitting is.As shown in Table 3,there was no obvious change in the mean lifetimes(τm=B1τ1+B2τ2+B3τ3)of complex 1 before and after the addition of mPAH1,suggesting that the fluorescence quenching by fluorescence resonance energy transfer(FRET)is excluded[29].
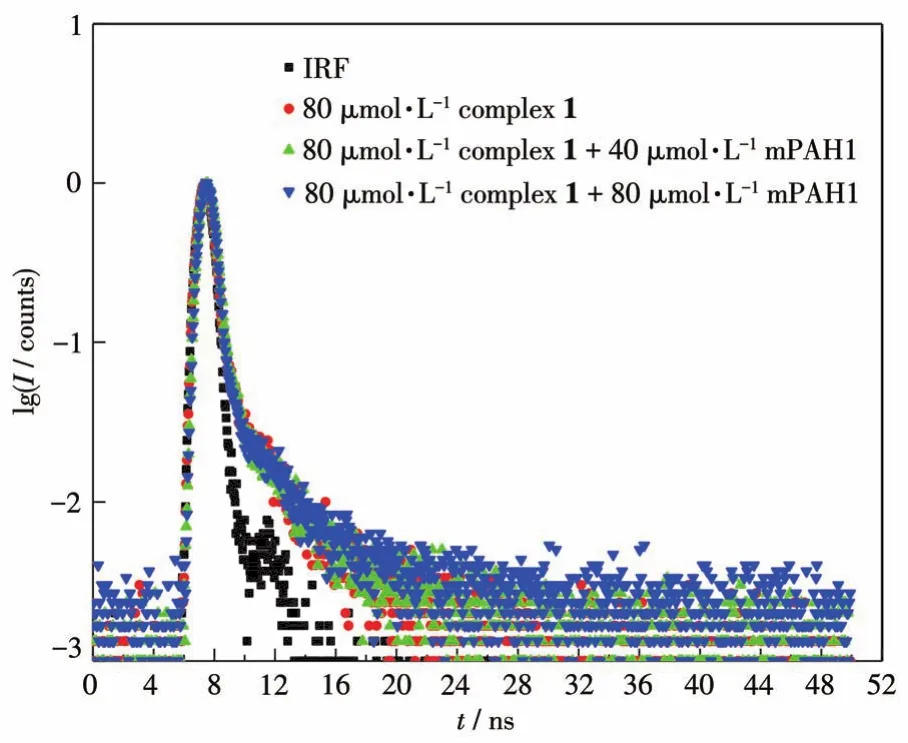
Fig.7 Fluorescence lifetime decay curves of complex 1 in the absence and presence of mPAH1
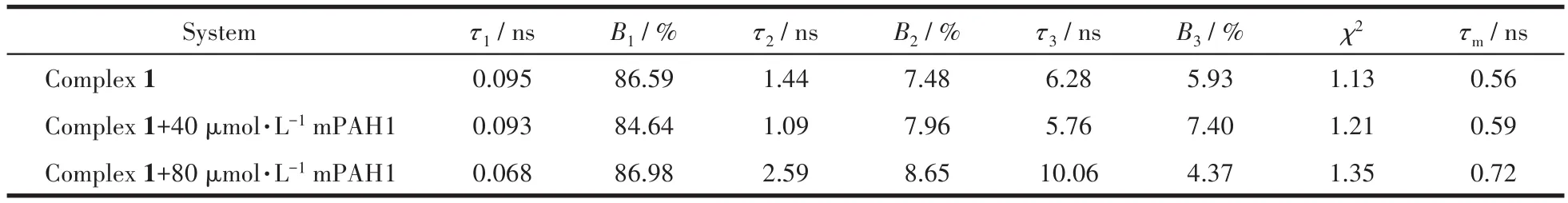
Table 3 Fluorescence lifetime decay parameters of complex 1(80 μmol·L-1)with gradual addition of mPAH1

Upon addition of complex 1 into the methanol solution of mPAH1,the fluorescence intensity at 408 and 556 nm both gradually increased(Fig.8a).The increase of fluorescence intensity at 408 nm is attributed to the increase of the concentration of complex 1 in a range of 0-112 μmol·L-1.Although the concentration of mPAH1 was constant,the fluorescence intensity at 556 nm,which is assigned to mPAH1,did not remain constant,and actually increased.After ruling out FRET and IFE,we investigated the effect of proton exchange between complex 1 and mPAH1.As shown in Fig.8b,upon addition of trifluoroacetic acid(TFA,pKa=-0.23 in water)into the methanol solution of complex 1,in addition to the enhancement of the fluores-cence intensity at 408 nm,a new fluorescence peak centered at 537 nm appeared.We attribute this new fluorescence peak to the pyridine salt formed by the protonation of complex 1.When the methanol solution of mPAH1 was titrated with complex 1,a portion of complex 1 received protons from mPAH1.The new fluorescence peak at 537 nm generated by the protonated complex 1 overlapped with that of mPAH1 at 556 nm,thus increasing the fluorescence intensity of the mixture at 556 nm.

Fig.8 (a)Emission spectra of mPAH1(40 μmol·L-1)with various concentrations of complex 1(1-8:0,32,40,48,64,80,96,112 μmol·L-1)before irradiation;(b)Emission spectra of complex 1(80 μmol·L-1)with various concentrations of trifluoroacetic acid(1-4:0,20,40,100 μmol·L-1)
3 Conclusions
Two luminescent complexes [Zn(2-pyterpy)2](ClO4)2·0.25H2O (1) and [Cd(2-pyterpy)2]2(ClO4)4·2.33H2O·CH3OH(2)were solvothermally synthesized and characterized by X-ray single crystal diffraction.Upon addition of mPAH1,the fluorescence at 408 nm of complex 1 was quenched by IFE.While upon addition of complex 1 into the methanol solution of mPAH1,the fluorescence at 556 nm of the mixture was enhanced by the protonation of complex 1.The combination of mPAH and complexes containing basic pendant groups may produce visible light responsive luminescent materials.
