杂化化合物(C7H11N2)2[CdCl4]·0.5H2O的晶体结构、光谱表征和光学性质
2022-09-16FatmaGarciAxelKleinHammoudaChebbiMohamedFaouziZid
Fatma Garci Axel Klein Hammouda Chebbi*,,3 Mohamed Faouzi Zid
(1University of Tunis El Manar,Faculty of Sciences of Tunis,Laboratory of Materials,Crystal Chemistry and Applied Thermodynamics,Tunis,Tunisia)(2University of Cologne,Faculty of Mathematics and Natural Sciences,Department of Chemistry,Institute for Inorganic Chemistry,Cologne,Germany)(3University of Tunis,Preparatory Institute for Engineering Studies of Tunis,Tunis,Tunisia)
0 Introduction
Halocadmates(Ⅱ)have received a lot of attention because of their remarkable structural,thermal,catalytic,and electrical properties[1-4].The flexibility of Cd(Ⅱ),due to thed10configuration[5],allows for a wide range of coordination numbers in part with unusual structural variations.They range from simple tetrahedral anions[CdX4-n(H2O)n]x-(n=0-2,x=2-0)with halides and water as ligands to polymeric halidocadmate anions such as∞[CdXn]-(n=1-3)forming 1D chains or 2D layers[6].The use of different organic ligands and their cations has previously allowed us to design and synthesize halidocadmate polymeric compounds[6-8].The features of the organic cations on the packing interactions affect the expected crystal organization,hydrogen-bond interactions between the polar protonated amine or pyridine groups of the organic cations,and chloride of the inorganic anions,therefore affect also the specific properties[6-8].In the frame of organic-inorganic hybrid perovskites,the impact of the organic cation on dimensionality has been broadly studied[9-11].In order to investigate the effect of functional groups on pyridinium cations on the structure and physical properties of pyridinium cadmates,we have recently reported the 1D perovskite(2-amino-4-methylpyridinium)trichlorocadmate(Ⅱ)(C6H9N2)1∞[CdCl3][12].Herein,we report on our attempts to use 4-(dimethylamino)pyridinium as a cation in the synthesis of a new pyridinium chlorocadmate which has led to the compound bis(4-N,N-dimethylaminopyridinium)tetrachlorocadmate(Ⅱ)hemihydrate(C7H11N2)2[CdCl4]·0.5H2O.We studied the crystal structure using single-crystal X-ray diffraction,which was able to support the phase purity by powder X-ray diffraction(PXRD)and the hydrogen bonding network by Hirshfeld surface analysis,and investigated the IR and UV-Vis spectroscopic properties of the title compound.
1 Experimental
1.1 Instrumentation
The infrared spectrum was recorded using a KBr pellet on a Thermo Scientific FT-IR spectrometer(type Nicolet 6700),fitted with a DRIFTS-cell,in a range of 4 000-400 cm-1.UV-Vis,emission,and excitation spectra were recorded on a Double Edinburgh Instruments FLSP920/FSP920 spectrometer setup in the solid state.Diffuse reflectance measurements were performed on a Perkin Elmer Lambda 900 UV-Vis-NIR spectrophotometer in a range of 250-800 nm at room temperature.The PXRD pattern was recorded at room temperature using a Bruker D8 Advance diffractometer operating with CuKαradiation(λ=0.154 06 nm)in a wide 2θrange of 5°-80°.The working voltage was 40 kV,and the working current was 40 mA.
1.2 Synthesis of(C7H11N2)2[CdCl4]·0.5H2O
To a solution of 0.244 g(2 mmol)4-(dimethylamino)pyridine in 10 mL MeOH,1 mL of 36% HCl were added.Then,10 mL of a MeOH solution of CdCl2·H2O(0.200 g,1 mmol)containing 1 mL of 36% HCl was added.After 2 h of stirring at room temperature,the mixture was filtered and left to evaporate.Slow evaporation produced colorless crystals after three weeks.Yield:393 mg(0.77 mmol,77% based on CdCl2·H2O).Anal.Calcd.for(C7H11N2)2[CdCl4]·0.5H2O(%):C,33.06;H,4.36;N,11.02;Cd,22.10;Cl,27.88;Found(%):C,33.02;H,4.33;N,11.07;Cd,22.10;Cl,27.86.The PXRD pattern of the title compound was in good agreement with the calculated(Fig.1),confirming the phase purity of the synthesized product.In the experimental pattern,some reflections were very dominant over the others,e.g.those at around 2θ=12°,17°,22°,and 29°.We assume that this is due to a layered structure.

Fig.1 Experimental and calculated PXRD patterns of the compound
1.3 Single crystal X-ray diffraction
Under a polarizing microscope,a good quality single crystal was selected with a parallelepiped shape of 0.35 mm×0.24 mm×0.17 mm,which was chosen for measurement on an automatic four-circle Enraf-Nonius diffractometer.A search was performed by means of 25 reflections using the CAD4-Express program[13],the radiation source used corresponds to theKαradiation of molybdenum(λ=0.071 07 nm).The unit cell dimensions were measured and the data collection was performed.Absorption corrections were carried out usingψ-scans[14].
The structure was solved using direct methods and refined by full-matrix least-squares refinement onF2using the WinGX software package[15].Crystal data,data collection,and structure refinement details are summarized in Table 1.The water molecule was modeled by an anisotropic ADP.Thus,the hydrogen atoms on H2O were not located.The composition and the density in the cif file were corrected for the two missing H atoms.All remaining H atoms were positioned geometrically and refined using the riding model(N—H(ring)was set to 0.086 nm usingUiso(H)=1.2Ueq(N),C—H(aromatic)was set to 0.093 nm withUiso(H)=1.2Ueq(C)and C—H(methyl)was set to 0.096 nm withUiso(H)=1.5Ueq(C)).

Table 1 Crystal data and structure refinement parameters for(C7H11N2)2[CdCl4]·0.5H2O
CCDC:2136319.
2 Results and discussion
2.1 Single crystal X-ray analysis
The title compound (C7H11N2)2[CdCl4]·0.5H2O crystallizes in the triclinicP1ˉspace group.The asymmetric unit includes two 4-(dimethylamino)pyridinium cations,an isolated[CdCl4]2-anion and half a water molecule(Fig.2).
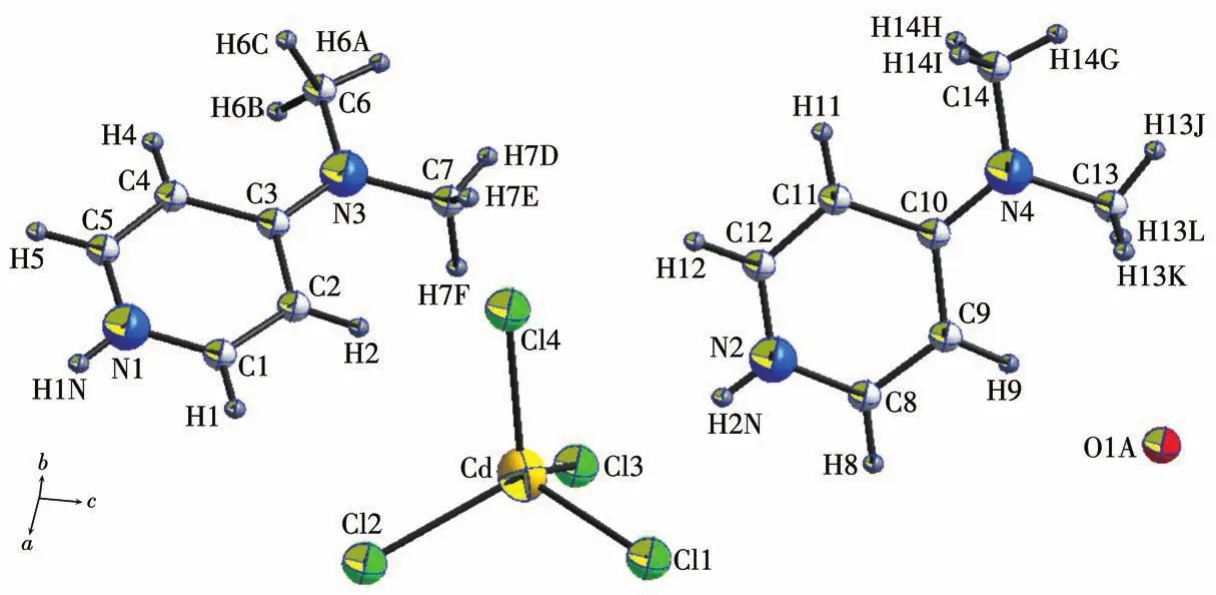
Fig.2 Asymmetric unit of(C7H11N2)2[CdCl4]·0.5H2O with a numbering scheme
The coordination geometry of the four chlorides around Cd(Ⅱ) is distorted tetrahedral.The Cd—Cl bonds are in a range of 0.243 92(18)-0.247 61(16)nm.Most of the Cl—Cd—Cl angles deviate only marginally from the ideal 109°,(Cl2—Cd—Cl4 110.75(6)°;Cl3—Cd—Cl1 110.47(6)°;Cl3—Cd—Cl4 110.38(6)°;Cl3—Cd—Cl2 110.36(7)°;Cl4—Cd—Cl1 109.94(6)°)while Cl2—Cd—Cl1 with 104.82(6)°is slightly more acute.The calculated average values of the different distances and angles distortion indices(DIs)are 0.004 8 for Cd—Cl and 0.001 4 for Cl—Cd—Cl.These values are comparable to those previously reported[5,12,16-18].The C1—N1—C5(120.7(6)°)and C8—N2—C12(119.9(5)°)bond angles in the pyridinium cations are wider than those in the neutral pyridine(114.15(5)°)[19],which is in line with the protonation on the pyridine N atom.
The crystal structure of the title compound(Fig.3)shows alternating organic and inorganic layers parallel to the(001)plane and located atx=n+1/2(n∈Z)[18].Overlapping rows of coplanar pyridinium rings held together byπ-stacking interactions with the centroidcentroid distances of 0.368 5(17),0.375 0(14),and 0.376 6(17)nm(Fig.4),are complemented by rows of tetrachloridocadmate anions and water molecules.These layers form a 3D architecture through N—H…Cl,N—H…O,C—H…Cl,and C—H…O hydrogen bonds(Fig.3,Table 2).

Fig.3 View of(C7H11N2)2[CdCl4]·0.5H2O on the bc plane
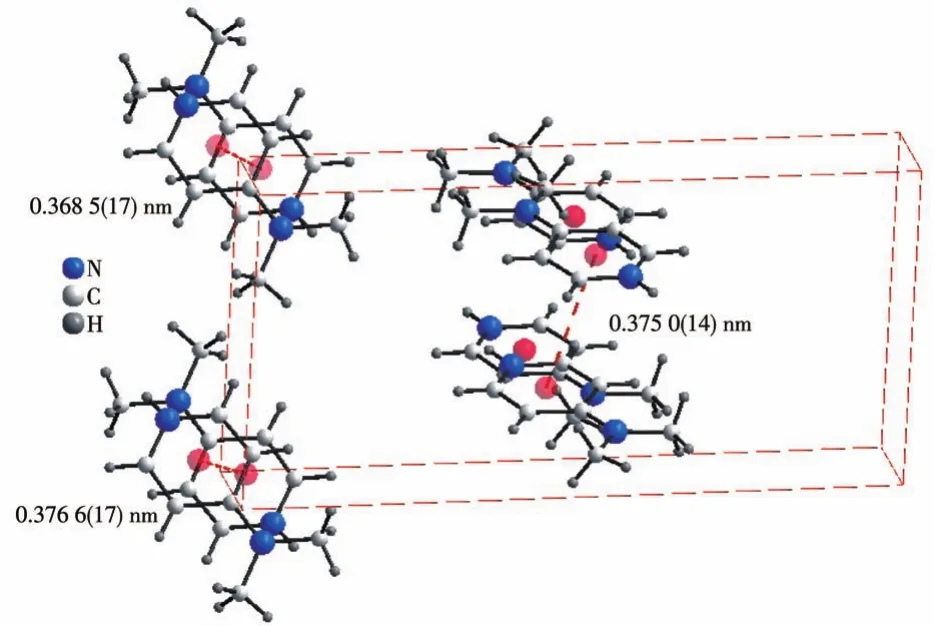
Fig.4 π-stacking interactions between the neighboring aromatic organic cations in(C7H11N2)2[CdCl4]·0.5H2O
2.2 Hirshfeld surface analysis
CrystalExplorer 3.1 software[20]was employed to generate the Hirshfeld surface thus assessing the contributions of the different intermolecular interactions in the structure.The Hirshfeld surface volume and surface area are calculated to be 0.518 96 nm3and 0.463 72 nm2.Thednormmapping of Hirshfeld(Fig.5)are shown in blue,red,and white color schemes.The red region(dnormis negative)is due to intermolecular N—H…Cl,C—H…Cl,N—H…O,andC—H…O hydrogen bonds the white colored regions represent the contacts which are equal to the van der Waals radii(dnorm=0),and the blue spots(dnormis positive)show the contacts longer than the sum of van der Waals radii[21].The 2D fingerprint of the Hirshfeld surface(Fig.6)shows the relative contribution to various intermolecular contacts.The H…Cl/Cl…H interaction contributes 36.9% of the total Hirshfeld surface,the H…H interactions have a significant contribution amount of 32.3%,and the contribution of C…H/H…C interaction to 7.5%.From this we conclude that these van der Waals forces exert an important influence on the stabilization of the packing in the crystal structure,other visible spots in the surfaces refer basically to O…H/H…O,N…H/H…N,Cl…O/O…Cl,C…C.

Fig.5 Hirshfeld surface of(C7H11N2)2[CdCl4]·0.5H2O mapped with dnorm
2.3 IR spectroscopy
The IR spectrum of(C7H11N2)2[CdCl4]·0.5H2O(Fig.7)was assigned based on comparison with the spectra of 4-(dimethylamino)pyridinium in dimercurate(C7H11N2)2[Hg2Cl6](C7H11N2=4-(dimethylamino)pyridini-um)[22], the mixed cadmate/mercurate (C6H9N2)2(Hg0.75Cd0.25)Cl4(C6H9N2=2-amino-4-methylpyridinium)[23],3-hydroxy 2-nitropyridine[24],and the Li salt[Li(4-dimethylaminopyridine)]Cl[25].

Fig.7 Infrared spectrum of(C7H11N2)2[CdCl4]·0.5H2O
The band at 3 258 cm-1can be assigned to theν(N—H)stretching vibration of the pyridinium cations in the title compound.The bands at 3 127-3 100 cm-1are ascribed to asymmetric and symmetric stretching of the NCH3groups(νasandνs)[22].Overtones of deformation modes were observed between 2 000 and 1 980 cm-1.The N—H bending observed at 1 650 cm-1,and the bands at 1 565 and 1 445 cm-1are attributed to the C=C and C=N stretching modes of the pyridine ring[23].The sharp peak at 1 394 cm-1is assigned to the symmetric C=C(aromatic)stretching[24].The absorption vibration bands corresponding to the C—N group are identified at about 1 217 cm-1.These bands observed in the wavelength range of 1 071-750 cm-1come from C—N,C—C stretching vibrations and symmetrical C—N stretching vibrations[25].
2.4 Optical band gap determination from diffuse reflectance spectra
The diffuse reflectance spectrum(Fig.8)shows two bands with maxima at 286 nm(4.33 eV)and 345 nm(3.59 eV)which are assigned to theπ-π*excitations of 4-(dimethylamino)pyridinium cations[22,25-26].In EtOH solution,the absorptions of 4-dimethylmaminopyridinium at 213 nm(5.82 eV)and 278 nm(4.46 eV)were reported for the compound(C7H11N2)[Cl2Hg(μ-Cl2)HgCl2][22],thus the broad feature in the reflectance spectrum peaking at 345 nm does not correspond isolated pyridinium cations.

Fig.8 Diffuse reflectance spectrum of(C7H11N2)2[CdCl4]·0.5H2O
The optical band gapEgwas calculated using the Kubelka-Munk function[27]:

whereRdis the diffuse reflectance.The absorption coefficientαis a function of the photon energy(hν)[28-29]:

WhereBis a constant andnis the index,which takes different values depending on the mechanism of interband transitions,withn=1/2 orn=2 corresponding to direct or indirect transitions,respectively[29].
The band gap energies were calculated from the plots of(αhν)1/2vshν(Fig.9,top)or(αhν)2vshν(Fig.9b,bottom).Extrapolation of the linear portion to thex-axis gave theEgof 3.596 and 3.684 eV,respectively.In keeping with the lowestπ-π*transition at 3.59 eV(Fig.8),the character is thus that of a direct semiconductor.These values are quite high and the corresponding high gap between the HOMO and the LUMO indicates relatively high stability in terms of energy and a high chemical hardness[22,30].The behavior as a semiconductor material,is in line with previous reports[22,25,31]and we assume that the extensiveπ-stacking of the pyridinium cations is the underlying structure in line with the observation of the 345 nm maximum in the reflectance spectrum,while for isolated(dissolved)cations there is no such absorption.
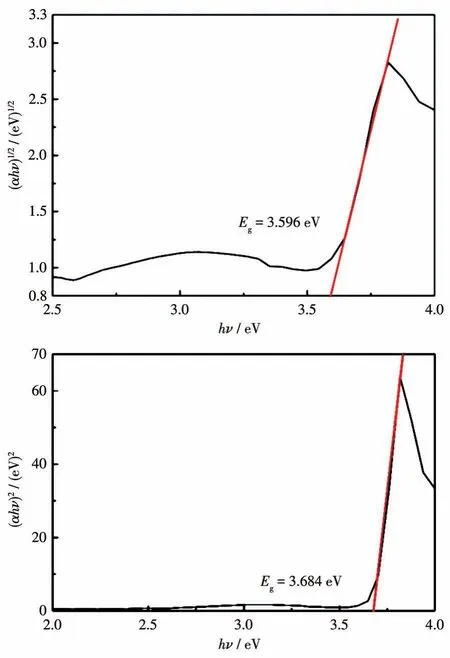
Fig.9 Plots of(αhν)1/2(top)and(αhν)2(bottom)vs hν for(C7H11N2)2[CdCl4]·0.5H2O
Because of lacking suitable equipment,we were not able to experimentally study the semiconducting behavior of the title compound.We will do this in future studies.Quite generally,such UV semiconductors(wide bandgap semiconductors)are interesting as photocatalysts[29]or UV detectors and sensors[32-34]in the form of nanoparticles,nanowires,or thin films[32,34].Compared to the established binary and ternary metal oxides,nitrides,and carbides in this field,the hybrid organic-inorganic materials offer the benefit of easy synthesis,simple processability for the manufacturing of thin films,and high stability[9,11,35].
2.5 Photoluminescence properties
The solid-state photoluminescence spectrum at room temperature showed an emission maximum at 319 nm and upon excitation at 562 nm(Fig.10).The spectrum of the 4-(dimethylamino)pyridine molecule is very similar[26]and we assign the emission to fluorescence originating fromπ-π*excited states of the aromatic cation.Remarkably,the maximum of the excitation spectrum at 319 nm(3.88 eV)coincides roughly with the minimum between the two maxima at 286 and 345 nm in the diffuse reflectance spectrum which lies atca.325 nm(3.81 eV)(Fig.8).

Fig.10 Emission and excitation spectra of(C7H11N2)2[CdCl4]·0.5H2O:emission at λex=319 nm(black),excitation at λem=562 nm(red)
3 Conclusions
In summary,the compound(C7H11N2)2[CdCl4]·0.5H2O (C7H11N2=4-(dimethylamino)pyridinium)was synthesized by slow evaporation at room temperature from HCl-containing MeOH solutions in a yield of 77%.The crystal structure was solved in the triclinic space groupP1ˉ.The crystal structure shows multiple N—H…O,N—H…Cl,C—H…O,andC—H…Cl hydrogen bonds andπ-πinteractions.Hirshfeld surface analysis confirms the hydrogen bonding andπstacking but also reveals that H…Cl/Cl…H,H…H,and C…H/H…C van der Waals interactions constitute about 77% of the total Hirshfeld surface.IR spectroscopy confirms the presence of the different groups in the structure.UV-Vis diffuse reflectance measurement showed maxima at 286 nm(4.33 eV)and 345 nm(3.59 eV),the Tauc plot analysis gave a value of 3.596 eV for a direct semiconductor in line with the long-wavelength absorption of the material.Fluorescence at 562 nm upon excitation at 319 nm(3.89 eV)is assigned to excitedπ-π*states.Comparison of the reflectance data and the luminescence behavior let us conclude that the photophysics is dominated by the 4-(dimethylamino)pyridinium cations and a closer inspection shows that extensiveπ-stacking of these cations leads to pro-nounced layers in the structure,which are the underlying structural units for the semiconductor behavior.Thus,we have not obtained a semiconducting 1D or 2D material similar to the organic-inorganic hybrid perovskites in which the inorganic halidometalate largely determines the electronic properties,as we initially hoped.Instead,we obtained a structure with isolated[CdCl4]2-anions separated by stacks of the pyridinium cations.These stacks are very probably the lead structures for the semiconducting properties.In future studies,we will investigate them experimentally in detail.
Acknowledgments:We thank the director of XStruct Laboratory,Department of Chemistry,Ghent University,Krijgslaan 281-S3,B-9000 Ghent,Belgium,Prof.Dr.Kristof Van Hecke,and Dr.Marina Saab for the spectroscopic measurement.
Disclosure statement:No potential conflict of interest was reported by the author(s).
