基于3-羧基-5-氨基-1,2,4-三唑的Cd(Ⅱ)配合物的合成、晶体结构及催化性能
2022-09-16高学智朱小双田晓燕
高学智 宋 欢 李 冰*, 王 睿 朱小双 田晓燕
(1宁夏大学煤炭高效利用与绿色化工国家重点实验室,银川 750021)(2宁夏大学化学化工学院,银川 750021)
0 Introduction
In recent years,energetic materials have attracted more and more attention because of their increasing applications in military and civilian applications[1-3].The traditional energetic compounds,such as 2,4,6-trinitrotoluene(TNT)[4],1,3,5-trinitro-1,3,5-triazine(RDX)[5],1,3,5,7-tetranitro-1,3,5,7tetrazoctane(HMX)[6],pentaerythritol tetranitrate(PETN)[7],2,4,6-triamino-1,3,5-trinitrobenzene(TATB)[8]and hexanitrohexaazaisowurtzitane(CL-20)[9]have good detonation performance.However,in the course of manufacture,transport,and storage of explosives,accidental detonations are easily triggered by stimuli and lead to injury and destruction of property[10].Therefore,it is very important to design new energetic materials with good energy properties,good thermal stability,and low sensitivity.
Nitrogen-rich energetic salts are environmentally friendly high-energy-density materials(HEDMs)that have attracted considerable interest due to the lower vapor pressures,higher heats of formation,and enhanced thermal stabilities[11].1,2,4-Triazole has multiple N-coordination sites and relatively small volume,which not only reduces steric hindrance but also increases spatial density.The introduction of oxygenrich groups on the heterocyclic ring,such as carboxyl and ketone can improve the oxygen balance of the system and increase the coordination modes[12-13].Thus,as one derivative of triazole,3-carboxyl-5-amino-1,2,4-triazole(H2atzc)can be considered a good candidate for constructing HEDMs with multiple coordination modes and binding sites[14].
Ammonium perchlorate(AP)is a key inorganic oxidizer for rocket technologies which have been widely used as the main component of solid rocket propellant[15].The thermal decomposition performance of AP has a great influence on the combustion characteristics of solid propellants[16].Most metal oxides and inorganic salts were inert catalysts that do not provide energy and even lose a part of the heat,reducing the performance of the propellant during the combustion process[17-18].However,the energetic complexes can provide a diverse structure and greater heat of formation,which may improve combustion performance[19].Therefore,the energetic complexes can be applied as an additive to the combustion catalytic of propellants.
In this work,a new energetic complex[Cd(Hatzc)2(H2O)](LH1)was designed and synthesized.The topics of energetic properties,thermal stability,safety characteristics,and thermal decomposition effect were studied.What′s more,LH1 exhibited a good catalytic effect on the thermal decomposition of AP which has potential application in propellants.
1 Experimental
1.1 Chemicals and methods
All chemicals of reagent grade were purchased and used without purification.Elemental analyses were performed on a Vario EL Ⅲ analyzer.The FT-IR spectra were recorded on a BEQ VZNDX 550 infrared spectrometer(KBr)from 400 to 4 000 cm-1.13C NMR spectra were recorded on a Bruker Avance Ⅲ100 MHz spectrometer.Chemical shifts(in parts per million)were calibrated with dimethyl sulfoxide (DMSO).Differential scanning calorimetry(DSC)and thermogravimetric(TG)analysis were carried out on a TA Instruments NETZSCH STA 449C simultaneous TGA at a heating rate of 10℃·min-1under hydrostatic air.
1.2 X-ray crystallography
The crystal suitable for X-ray diffraction measurements was obtained by slow evaporation of the ethanol solution containing H2atzc and Cd(CH3COO)2·2H2O at room temperature.The crystal with a size of 0.32 mm×0.21 mm×0.11 mm was selected for structural determination.The single-crystal X-ray experiment was carried out on a Bruker Smart Apex Ⅱ CCD diffractometer using graphite-monochromatized MoKαradiation(λ=0.071 073 nm).The structures were determined by the direct method using SHELXS[20]and refined by means of full-matrix least-squares procedures onF2with the programs SHELXL-2014[21].All non-hydrogen atoms were modified with anisotropic displacement parameters.All H atoms were positioned geometrically and were refined as riding with isotropic displacement parameters.A summary of the crystallography data and structure refinements for LH1 is given in Table 1.The selected bond lengths,bond angles,and hydrogen bonding geometry for complex LH1 are listed in Table 2 and 3.

Table 1 Crystallographic data and structure refinements for complex LH1

Table 2 Selected bond lengths(nm)and angles(°)for complex LH1

Table 3 Hydrogen bond parameters for complex LH1
CCDC:2100672.
1.3 Synthesis of complex LH1
H2atzc(2.6 mg,0.02 mmol)was dissolved in a mixture of NaOH(1 mL,1 mol·L-1),C2H5OH(5 mL),and water(5 mL),and adjusted to pH 6.0 by adding HCl solution(1.0 mol·L-1).Then,Cd(CH3COO)2·2H2O(13.0 mg,0.05 mmol)was dissolved in water(5 mL)at room temperature and the solution was mixed with the solution of H2atzc.The reaction mixture was stirred for 30 min.It was then allowed to stand at room temperature for three weeks,whereupon colorless block-shaped crystals of LH1 were formed in a 48% yield based on H2atzc.Anal.Calcd.for CdC6H8N8O5(%):C,18.74;H,2.10;N,29.14.Found(%):C,18.85;H,2.31;N,28.97.IR(cm-1,KBr):3 455s,3 310s,1 668s,1 543w,1 479m,1 294s,678s.13C NMR(100 MHz,DMSO-d6):δ139.8,136.3,119.6.
1.4 Sensitivity test
The mechanical sensitivities(impact(IS)and friction(FS))of the energetic materials were determined using the standard BAM method[22].IS was determined by a Fall Hammer Apparatus.The complex(20 mg)was compacted to a copper cap under the press of 39.2 MPa and was hit by a 2 kg drop hammer,and the calculated value of H50 represents the drop height of 50% initiation probability.FS of LH1 was measured by applying a Julius Peter′s machine using a 20 mg sample.
2 Results and discussion
2.1 IR spectrum of LH1
Fig.1 shows the FT-IR spectrum of complex LH1.The broad peak with high intensity at 3 455 cm-1corresponds to the O—H stretching vibration of the coordinated water molecule[23-24].The absorption peaks at 1 543 and 3 310 cm-1correspond toδ(N—H)andν(N—H)of the amino group.The intense absorption band at 1 294 cm-1in LH1 corresponds to the C—N stretching vibration.The absorption peaks at 1 668 and 1 479 cm-1show symmetric(νs(COO-))and antisymmetric(νas(COO-))vibrational absorption peaks of the carboxyl group,respectively,which indicates the oxygen atom in the carboxyl group has been coordinated to the metal ion[25].
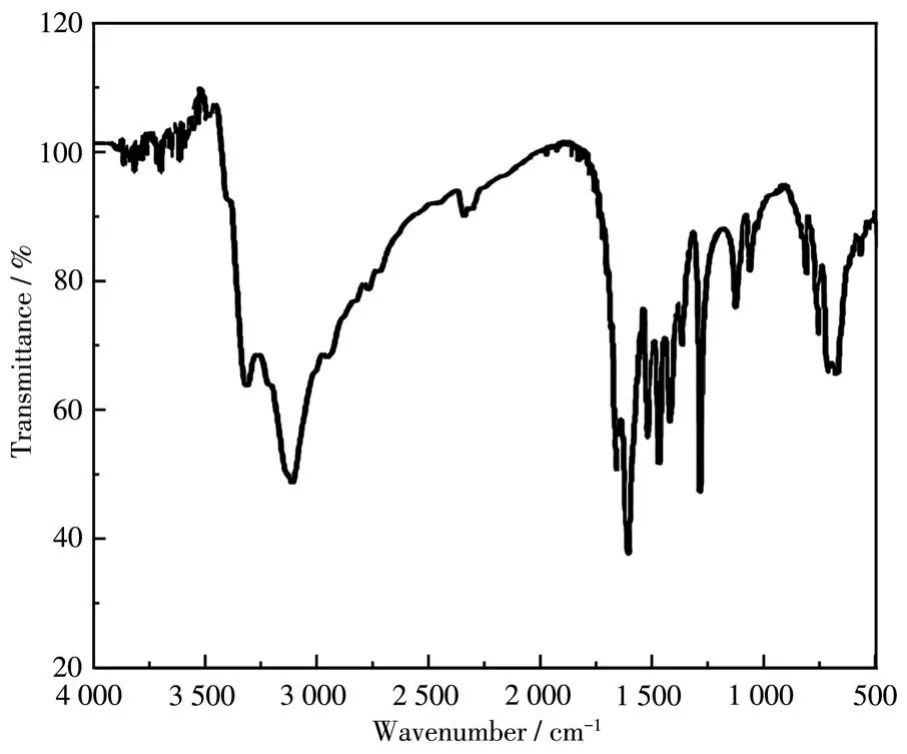
Fig.1 FT-IR spectrum of complex LH1
2.2 Crystal structure of complex LH1
Single crystal X-ray diffraction analysis shows that LH1 crystallizes in the monoclinic space groupP21/n.The lattice unit of compound LH1 consists of one Cd(Ⅱ)ion,two Hatzc-,and one coordinated water molecule.The Cd(Ⅱ)is seven-coordinated by two N atoms(N5 and N8)from two H2atzc ligands,four O atoms(O1,O2a,O4,O1a)from three H2atzc ligands,and one O atom(O6)from coordinated water presenting a twisted single-capped trigonal structure(Fig.2).The Cd—O bond length ranges from 0.237 4(2)to 0.256 61(18)nm and the Cd—N bond length ranges from 0.224 6(2)to 0.229 7(2)nm.As shown in Fig.3,Hatzc-ligand adopts a bridging mode linking the adjacent Cd1 and Cd1aions to generate a 1D chain.Further,the 1D chains form a 2D pattern through N7—H7…O2c,N11—H11B…N13d,and N10—H10…O3d.Finally,these plains are cross-linked by O6—H6A…O3ato create a 3D supramolecular network structure(Fig.4).

Fig.2 Coordination environment of LH1:ellipsoid diagram with 50% probability level

Fig.3 One-dimensional structure diagram of LH1

Fig.4 Crystal cell frame of complex LH1
2.3 Thermal stability
To investigate the thermal behavior of LH1,TGDSC was performed.As shown in Fig.5,the first weight loss of 4.8% from 140 to 180℃with a loss of a coordination water molecule(Calcd.4.7%).In a range of 180 to 720℃,the complex roughly underwent two weight loss processes,which are mainly the collapse of the main framework with an exothermic peak at 229℃and then releasing all the ligands completely from 229 to 720℃.The final product of CdO with a residue mass percentage of 32.7% is in good agreement with the calculated value of 33.3%.There were two main peaks in the DSC curve of LH1.The endothermic peak matched with the dehydration behavior(Tp=164℃),while the exothermic peak was at 229℃.

Fig.5 TG and DSC curves of complex LH1
2.4 Energetic properties
Density functional theory(DFT)was used to compute the energy of detonation(ΔHdet)[26],which was estimated by using a linear correlation equations(ΔHdet=1.127ΔEdet+0.046,r=0.968)[27].All the calculations were completed in the Gaussian 09 programs.For LH1,the formation of metal ions was supposed as cadmium oxide.Water,nitrogen,and carbon were assumed to be the final products of the decomposition of the organic part of the framework[28].
The detonation reaction for LH1 is described by Eq.1:

Detonation performance of the related energetic material LH1 here was evaluated by the Empirical Kamlet-Jacob formula,as

whereDis detonation velocity(km·s-1),pis detonation pressure(GPa),Φis characteristic value of explosive,Nis moles of detonation gases per gram of explosive,Mis the average molecular weight of the gases,Qis the chemical energy of detonation(kJ·g-1),andρis the density of materials(g·cm-3).
As shown in Table 4,the heat of detonation (ΔHdet)of LH1 was calculated as 16.51 kJ·g-1,which was even much larger than those for the conventional explosives and some energetic complexes.What′s more,LH1 had good energetic properties(D=10.4 km·s-1andp=55.2 GPa)which make LH1 a promising high-energy solid propellant.

Table 4 Physicochemical properties of LH1 and relevant energetic materials
Continued Table 4
The sensitivity experiments for LH1 were explored to test the safety of applying energetic materials.The IS was greater than 40 J and the FS exceeded 360 N,which indicates that LH1 was less sensitive to impact and friction.It is probably due to the fact that the tight connection between the nitrogen-rich ligand and metallic nodes constructs a solid and insensitive 3D framework[31].Furthermore,the H-bonding interaction between the water molecule and the ligand in LH1 is responsible for the lower friction sensitivity[2].
2.5 Effects on thermal decomposition of AP
To check the effects on the thermal decomposition behavior of AP,complex LH1 was mixed with AP at a mass ratio of 1∶3 to prepare the target sample.The study was investigated by DSC measurements with a heating rate of 10℃·min-1in nitrogen atmosphere with Al2O3as references.The total sample mass used was about 0.8 mg for all runs.
Fig.6 shows the DSC curves of AP and mixtures of AP with LH1.The thermal decomposition of AP possessed three peaks:one endothermic peak and two exothermic peaks.It can be seen that the phase transition(from cubic to orthorhombic system)process of AP reached an endothermic peak at 245℃[32].The exothermic peaks at 310 and 393℃correspond to the lowtemperature decomposition(LTD)process and hightemperature decomposition(HTD)process,respectively.The heat release of the exothermic process was 0.18 and 0.76 kJ·g-1,respectively[33].
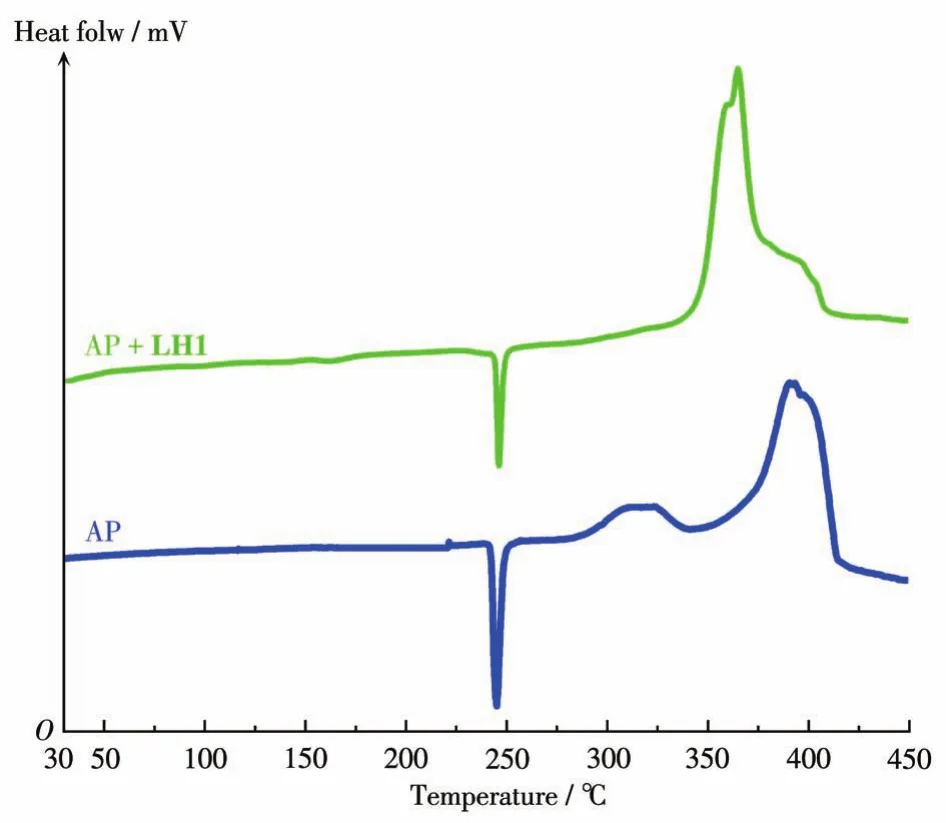
Fig.6 DSC curves for AP and the mixture of AP with LH1 at a heating rate of 15℃·min-1
After adding the mixture of AP with LH1,there were no significant impacts on the phase transition of AP,but a noticeable change can be seen in the exothermic phases.The exothermic process at 245-420℃for pure AP became narrow to 322-360℃with the exothermic peaks at 355℃,indicating that AP decomposed in a short time with the presence of LH1 at the same heating rate.In addition,the decomposition heat increased dramatically to 1.22 kJ·g-1for AP with LH1,significantly higher than the corresponding heat value for pure AP.Obviously,AP was decomposed completely in a shorter time and released much heat in the presence of LH1,which might contribute to the catalytic effects of AP[19].
2.6 Non-isothermal kinetics analysis
Kissinger′s method[34]was employed to determine the apparent activation energy(Ek,kJ·mol-1)and the pre-exponential factor(A,s-1).The Kissinger equation(Eq.5)is as follows:

whereTpis the peak temperature(K),Ris the gas constant(J·mol-1·K-1)andβis the linear heating rate(K·min-1).
Non-isothermal kinetic analyses of AP with LH1 were determined by DSC tests.Based on the exothermic peak temperatures measured at four different heating rates of 5,10,15,and 20 K·min-1.TheEavalue was calculated as 112.90 kJ·mol-1for AP with LH1.Compared with pure AP,the activation energy significantly decreased.Therefore,LH1 provides a certain amount of energy and has a good catalytic effect on AP decomposition(Table 5).

Table 5 Kinetic parameters of thermal decomposition for AP and AP with LH1
We compared LH1 with other energetic materials with H2atzc as ligand,Zn(atzc)2(H2O)[29]and[Mn(atzc)2(H2O)2]·2H2O[29].Zn(atzc)2(H2O)forms a fivecoordination mode showing a distorted tetragonal pyramidal geometry,[Mn(atzc)2(H2O)2]·2H2O forms a sixcoordination mode,displaying a slightly distorted octahedral geometry,while LH1 forms a seven-coordination mode,which displays a twisted single-capped trigonal structure.It can be found that the electronic configuration of the central ion has a strong influence on the structure[35-36].
In this essay,the activation energy of pure AP was 142.02 kJ·mol-1.With the presence of these complexes,the activation energies were changed to 112.90,156.72,and 187.09 kJ·mol-1for AP+LH1,AP+[Mn(atzc)2(H2O)2]·2H2O, and AP+Zn(atzc)2(H2O),respectively.Among the three complexes,complex LH1 exhibited the best catalytic activity for the decomposition of AP.
From the above,LH1 not only had high detonation velocity,detonation pressure,the energy of detonation,and less sensitivity to the external stimulus,but also had a good catalytic effect on AP.The crucial point in the synthesis of energetic complexes with high energy and low sensitivity is choosing a suitable ligand.The ligand should meet the following requirements.Firstly,the triazole ring of the energetic ligand introduces amino and carboxyl groups,which realizes the feasibility of a single component instead of a multicomponent function.Secondly,the energetic ligand could enhance explosive heat and oxygen balance,and decrease sensitivity,which is beneficial for the thermal decomposition of AP.What′s more,the presence of coordinated water molecules is beneficial to construct a hydrogen-bonded supramolecular network,which can reduce the sensitivity of the energetic compound LH1.
3 Conclusions
In summary,a new energetic complex[Cd(Hatzc)2(H2O)](LH1)was synthesized and fully characterized.Compound LH1 possessed promising energetic properties,including low mechanical sensitivities,high detonation pressure,high detonation velocity,and high energy of detonation.DSC experiments reveal that AP was decomposed completely in a shorter time with a large of heat released in the presence of LH1,which indicates that complex LH1 has a good catalytic effect on the thermal decomposition of AP.The present study indicates the probable application of the complex in the solid propellant field.Further investigation is currently underway.
