黄连内生真菌Albifimbria viridis的化学成分研究
2022-09-02艾洪莲马旭军位盼盼曹锐格黄蓉李正辉
艾洪莲,马旭军,位盼盼,曹锐格,黄蓉,李正辉
(中南民族大学 药学院,武汉430074)
黄连(Coptis chinensisFranch)属于毛茛科多年生草本植物,是一种常见的中草药.在我国,绝大多数都分布在陕西、四川、湖南、贵州、湖北等地的山谷凉湿荫蔽密林中[1],在临床上应用很广泛、需求量大.由于掠夺性采挖,使得野生黄连数量逐年降低,少数种类面临灭绝[2].黄连的药用部位多为根和根茎,具有多种有效成分,主要含有黄酮、甾体、多糖、木脂素、香豆素、萜类、有机酸、挥发油、生物碱等多种化学成分[3],研究发现黄连具有抗菌、镇静催眠、抗病原体、抗炎、解热、降糖等药理活性[4].
植物内生菌存在于健康植物组织中,不会对其宿主呈现出明显感染症状,是植物微生态系统的重要组成部分,亦是重要的微生物资源[5].在长期共同进化过程中,植物内生真菌与宿主表现出互利共生的关系.植物内生真菌利用宿主提供的物质条件完成自身的生长繁殖,另一方面内生真菌也可以产生一些具有抗病虫害活性的次生代谢产物,从而提高宿主的生存能力[6].研究表明内生真菌几乎存在于所有的药用植物中,从药用植物内生真菌可以得到多种药用成分[7].为了进一步研究黄连内生真菌的化学成分,本文对黄连内生真菌Albifimbria viridis的次生代谢产物进行了研究.利用凝胶Sephadex-20、硅胶柱色谱层析及反相高效液相色谱等一系列分离方法,分离纯化得到8个化合物(图1).

图1 化合物1~8的结构式Fig.1 Chemical structures of compounds 1-8
1 实验部分
1.1 仪器与材料
仪器:核磁共振波谱仪(600 MHz,德国Bruker);酶标仪(TECAN);质谱仪(Premier P776,美国waters).
材料:FUJI C18 填料,粒径为20~45 μm,孔径为100 A(赛谱锐思);Sephadex-LH 20(瑞典Amersham biosciences);柱色谱硅胶(200~300 目)与薄层色谱硅胶板(青岛海洋化工);分析型HPLC 为Agilent 1260,色谱柱为Agilent ZORBAX SB-C18柱(4.6 mm×250 mm),径粒为5 μm;制备型HPLC 为Agilent 1260,色谱柱为Agilent Eclipse XDB-C18柱(9.4 mm×250 mm),径粒为5 μm;显色溶剂:香草醛溶液和硫酸乙醇溶液;观察紫外波长:254 nm 和365 nm;色谱甲醇和乙腈(湖北弗顿);96 孔板(NEST);MTT(VETEC);DMEM培养基(赛默飞世尔生物化学).
1.2 发酵培养
1.2.1 菌种来源
该内生真菌分离自黄连新鲜植株的根部,自编号为L05.通过ITS 序列测定结果对比得知,该序列与Albifimbria viridis(A.viridis)的 最 大 相 似 度 达100%.基因库登录号为MT110687.1,故将其鉴定为Albifimbria viridis.目前,该菌株保藏于中南民族大学药学院微生物菌种库.
1.2.2 培养基
大米培养基:大米100 g,蒸馏水100 mL,置于650 mL组培瓶中.在121 ℃条件下高压蒸汽灭菌20 min.
1.3 提取分离
1.3.1A.viridis真菌的分离纯化
取黄连的新鲜块根,自来水冲洗干净后切成小段,75%乙醇润洗60 s,无菌水漂洗3 次.0.1%升汞浸泡消毒40 s,无菌水漂洗3 次.过滤纸(经过高压灭菌)吸干水分,剪去黄连根部两端的切口后从中划开.无菌条件下将这些小块接至PDA 培养基(含青链霉素混合液)中,置于25 ℃的恒温培养箱培养.待真菌长出后,挑取菌丝进行纯化培养得单一菌株.
1.3.2A.viridis菌株的扩大发酵
A.viridis菌株接种于液体培养基中,置于25 ℃恒温培养,得菌悬液.称取100 mL蒸馏水与100 g大米于650 mL组培瓶中,高压蒸汽灭菌(121 ℃,20 min)得大米培养基.将已培养好的A.viridis菌悬液在无菌条件下取1.0 mL 接种在大米培养基(共180 瓶)中,于25 ℃的恒温室中培养30 d.
1.3.3A.viridis菌株次级代谢产物的提取分离
无水甲醇浸泡大米发酵物4 次,每次20 L,得提取液.减压浓缩后,得浸膏4800 g.将加热至60 ℃的热水与浸膏按体积比1∶1混悬.用石油醚、乙酸乙酯和正丁醇萃取混悬液,每种溶剂萃取4次,每次10 L,得萃取液.减压浓缩乙酸乙酯萃取液,得乙酸乙酯部位110.0 g.
称取1700 g 硅胶(300~400 目)和过量石油醚混悬均匀后,湿法装柱.将110.0 g 硅胶(200~300 目)和乙酸乙酯部位进行拌样,旋干至细沙状,研磨,干法上样.经石油醚∶乙酸乙酯(15∶1→0∶1)和乙酸乙酯∶甲醇(15∶1→0∶1)洗脱,得75 个流分.根据色谱行为相似性进行TLC 分析,合并流分得12 个组段(1~12).半制备HPLC 分离纯化(乙腈∶水= 45∶55,3 mL/min)组段1,得化合物1(32.9 mg,21.5 min),更换色谱条件(乙腈∶水=40∶60,3 mL/min),得化合物2(5.0 mg,7.0 min).组段10(2.0 g)使用Sephadex LH-20(100%甲醇)分离将样品分为5 个组段(10-1~10-5).半制备HPLC分离纯化(乙腈∶水=55∶45,3 mL/min)组段10-4,得化合物3(6.0 mg,15.2 min).组段11(7.3 g)C18中压反向制备柱分离(洗脱系统为甲醇-水,10∶90→1∶0 梯度洗脱)分为7个组段(11-1~11-7).组段11-4 半制备HPLC 分离纯化(乙腈∶水=50∶50,3 mL/min),得化合物4(2.3 mg,44.5 min)、化合物5(3.8 mg,34.4 min)和化合物6(2.8 mg,15.5 min).半制备HPLC 分离纯化(乙腈∶水= 40∶60,3 mL/min)组段11-6,得化合物7(1.2 mg,8.3 min).组段12(5.7 g)中压反向制备柱分离(洗脱系统为甲醇-水,10∶90→1∶0梯度洗脱)将样品分为8 个组段(12-1~12-8).组段12-3 半制备HPLC分离纯化(乙腈∶水= 39∶61,3 mL/min),得化合物8(3.7 mg,20.0 min).
2 结构鉴定
化 合 物1:C29H36O9,白 色 粉 末;ESI-MS m/z:551.22693([M+Na]+);1H NMR(600 MHz,Chloroform-d)δ 7.62(1H,dd,J= 15.0,11.6,2.1,1.0 Hz,H-8"),6.53(1H,t,J= 11.3 Hz,H-9"),5.92(1H,d,J=2.6 Hz,H-7"),5.90-5.85(2H,m,H-4,10"),5.78(1H,d,J= 1.0 Hz,H-10"),5.76(1H,d,J= 1.0 Hz,H-2"),5.42(1H,dq,J= 5.1,1.6 Hz,H-10),5.23(1H ,d ,J= 6.9 Hz,H-5"),4.38(1H,d,J=12.6 Hz,Ha-15),4.06(1H,dt,J=8.8,2.4 Hz,H-6"),3.99(1H,d,J= 12.5 Hz,Hb-15),3.81(2H,t,J= 5.9 Hz,H-2,4"),3.62(1H,ddd,J= 12.0,6.0,2.6 Hz,H-11,13"),3.46(1H,s,H-13"),3.11(1H,d,J= 4.1 Hz,Ha-13),2.80(1H,d,J= 4.1 Hz,Hb-13),2.45(1H,dd,J= 15.3,8.4 Hz,Ha-13),2.25(3H,d,J=1.4 Hz,H-12"),2.17(1H,t,J= 4.8Hz,Hb-3),1.88-1.83(4H,m,H-7,8),1.69(3H,s,H-16),1.35(3H,d,J= 6.0 Hz,H-14"),0.83(3H,s,H-14).13C NMR(150MHz,Chloroform-d)δ 166.29(C-11"),166.00(C-1"),155.54(C-3"),143.22(C-9"),140.56(C-9),134.61(C-7"),126.06(C-8"),119.83(C-2"),118.81(C-10"),118.61(C-10),103.40(C-5"),82.24(C-6"),79.73(C-4"),79.19(C-2),76.46(C-13"),73.86(C-4),67.84(C-11),65.63(C-12),63.45(C-15),49.19(C-5),48.03(C-13),43.24(C-6),34.73(C-3),27.65(C-8),23.40(C-16),20.38(C-7),16.02(C-12"),13.15(C-14"),7.43(C-14).以上波谱数据与文献[8]基本一致,鉴定化合物1为Roridin J.
化 合 物2:C27H34O9,白 色 粉 末;ESI-MS m/z:525.21210([M+Na]+);1H NMR(600 MHz,Chloroform-d)δ 8.03(1H,ddd,J=15.8,11.6,1.1 Hz,H-8"),6.67(1H,t,J= 11.3 Hz,H-9"),6.15(1H,d,J= 11.0 Hz,H-10"),6.04(1H,d,J= 15.7 Hz,H-7"),5.80(1H,dd,J= 8.3,4.1 Hz,H-4),5.43(1H,dd,J= 5.3,1.8 Hz,H-10),4.79(1H,d,J=12.1 Hz,H-15b),4.51(1H,ddd,J=11.4,5.3,2.4 Hz,H-5b"),4.21(1H,d,J= 12.1 Hz,H-15a),4.16-4.13(1H,m,H-2"),3.98(1H,td,J= 11.9,3.3 Hz,H-5a"),3.86(1H,d,J= 5.1 Hz,H-2),3.56(1H,d,J= 5.4 Hz,H-11),3.12(1H,d,J=3.9 Hz,H-13b),2.80(1H,d,J= 4.0 Hz,H-13a),2.48(1H,dd,J= 15.5,8.2 Hz,H-3b),2.35(1H,dddd,J= 11.1,6.4,4.1,2.0 Hz,H-3a),2.26(s,2H),1.99-1.87(4H,m,H-7b,8,4b"),1.79(1H,dt,J=11.5,3.0 Hz,H-7a,4a"),1.75(3H,s,H-16),0.87(3H,s,H-14).13C NMR(150 MHz,Chloroform-d)δ 7.45(C-14),10.10(C-6"),20.10(C-7),23.45(C-16),27.62(C-8),32.29(C-4"),33.35(C-3"),35.01(C-3),44.30(C-6),47.95(C-13),51.04(C-5),61.04(C-5"),63.38(C-15),65.14(C-12),66.78(C-11),74.09(C-2"),75.42(C-2),77.16(C-4),117.79(C-10),125.71(C-5""),127.40(C-2""),138.73(C-4""),138.89(C-3""),141.14(C-9"),165.41(C-6""),166.07(C-11"),174.63(C-1"),以上波谱数据与文献[9]基本一致,鉴定化合物2为疣孢菌素A(verrucarin A).
化合物3:C29H34O10,白色粉 末;ESI-MS m/z:565.20642([M+Na]+);1H NMR(600 MHz,Chloroform-d)δ 7.99(1H,ddd,J=15.7,11.6,1.1 Hz,H-8"),6.71-6.52(1H,m,H-9"),6.08(1H,dt,J=11.1,0.9 Hz,H-7"),6.00(1H,dd,J= 15.7,0.8 Hz,H-10"),5.92(1H,dd,J= 8.3,4.3 Hz,H-4),5.77-5.72(1H,m,H-10),5.72-5.64(1H,m,H-2"),5.19(1H,dd,J= 4.7,2.0 Hz,H-8),4.54(1H,dd,J= 12.5,1.3 Hz,H-15),4.48(1H,d,J= 11.4 Hz,H-5"b),4.20(1H,d,J= 12.4 Hz,H-15a),4.12(1H,d,J= 3.1Hz,H-5"a),3.84(1H,d,J= 5.1 Hz,H-11),3.79(1H,d,J= 5.6 Hz,H-2),3.11(1H,d,J=3.9 Hz,H-13b),2.84(1H,d,J= 4.0 Hz,H-13a),2.50(3H,d,J= 23.9 Hz,H-3a,4"),2.27(3H,d,J= 1.3 Hz,H-3b,7),1.94(3H,s,H-18),1.76(3H,d,J=1.4 Hz,H-16),0.80(3H,s,H-14).13C NMR(150 MHz,Chloroform-d)δ 171.04(C-19),165.95(C-2)(C-11"),165.79(C-1"),165.65(C-6"),157.23(C-3"),140.11(C-9"),138.92(C-8"),136.63(C-9),127.96(C-7"),125.25(C-10"),123.93(C-10),117.81(C-2"),79.02(C-2),74.91(C-8),68.88(C-8),67.09(C-11),65.42(C-12),64.51(C-15),60.53(C-5"),49.07(C-5),48.06(C-13),42.24(C-6),40.29(C-4"),34.92(C-3),26.49(C-7),21.15(C-18),20.62(C-16),17.24(C-6"),7.14(C-7).以上波谱数据与文献[10]基本一致,鉴定化合物3为verrucarin L acetate.
化合 物4:C31H38O10,白色粉 末;ESI-MS m/z:593.23755([M+Na]+);1H NMR(600 MHz,Chloroform-d)δ 7.64(1H,dddd,J= 15.0,11.6,2.1,1.1 Hz,H- 8"),6.56(1H,t,J= 11.4 Hz,H-9"),5.95(1H,dd,J= 15.3,2.6 Hz,H-7"),5.90(1H,dd,J= 8.4,4.5 Hz,H-4),5.79(1H,dq,J= 11.1,1.0 Hz,H-10"),5.71-5.68(1H,m,H-10),5.63(1H,t,J=1.3 Hz,H-2"),5.52(1H,dd,J=8.6,3.4 Hz,H-5"),5.18(1H,d,J= 4.9 Hz,H-8),4.41-4.33(2H,m,H-3b),4.06(1H,dt,J= 8.6,2.4 Hz,H-6"),3.83(1H,d,J= 5.1 Hz,H-2),3.75(1H,d,J= 5.5 Hz,H-11),3.66(1H,dq,J= 8.6,6.0 Hz,H-3a),3.10(1H,d,J= 4.0 Hz,H-13),2.83(1H,d,J= 4.1 Hz,H-13),2.65(1H,dd,J= 12.4,3.4 Hz,H-4"a),2.47(1H,dd,J= 15.4,8.3 Hz,H-3a),2.27(3H,d,J=1.3 Hz,H-12"),2.23-2.14(2H,m,H-7),1.92(3H,s,H8-Ac),1.76-1.74(1H,m,H-4"b),1.34(3H,d,J= 6.1 Hz,H-14"),0.91-0.64(3H,m,H-14).13C NMR(150 MHz,Chloroform-d)δ 171.11,166.37(C-1"),165.99(C-11"),143.30(C-9"),135.10(C-9),126.17(C-8"),124.12(C-10),118.76(C-2"),118.55(C-2"),100.84(C-5"),82.11(C-6"),79.16(C-2),73.57(C-4),68.90(C-8),67.34(C-11),65.51(C-12),64.62(C-15),49.19(C-5),48.01(C-13),47.88,42.25(C-6),34.80(C-3a),26.41(C-7),21.06(8-Ac),20.65(C-16),18.35(C-12"),16.57(C-14"),7.38(C-14).以上波谱数据与文献[11]基本一致,鉴定化合物4为Calcarisporin B1.
化 合 物5:C27H32O8,白 色 粉 末;ESI-MS m/z:507.20172([M+Na]+);1H NMR(600 MHz,Chloroform-d)δ 8.06(1H,ddd,J=15.7,11.6,1.1 Hz,H-8"),6.64-6.58(1H,m,H-9"),6.10(1H,dt,J=11.0,0.9 Hz,H-10"),6.02-5.97(1H ,m,H-7"),5.83(1H,q,J= 1.1 Hz,H-2"),5.45(1H,dq,J=4.6,1.5 Hz,H-10),4.48-4.40(1H,m,H-4),4.14(1H,ddd,J= 11.3,8.9,4.5 Hz,H-5"),3.96(1H,d,J=12.6 Hz,H-15),3.85(1H,d,J=5.1 Hz,H-2),3.75(1H,d,J= 5.4 Hz,H-11),3.14(1H,d,J=4.0 Hz,H-13),2.83(1H,d,J=4.0 Hz,H-13),2.55-2.48(3H,m,H-3,4"),2.27(1H,d,J=1.3 Hz,H-12"),2.16(1H,ddd,J= 15.3,5.2,4.3 Hz,H-3),2.04-1.91(3H,m,H-16),1.82(2H,pd,J=6.3,2.8 Hz,H-7,8),0.83(3H,s,H-14).13C NMR(150 MHz,Chloroform-d)δ 166.26(C-1"),165.97(C-6""),165.70(C-1""),156.77(C-3"),140.62(C-9),139.64(C-4""),139.27(C-3""),127.54(C-2""),125.66(C-5""),118.77(C-10),118.27(C-2"),79.19(C-2),75.47(C-4),67.42(C-11),65.61(C-12),63.45(C-15),60.58(C-5"),48.98(C-5),48.21(C-13),43.18(C-6),40.34(C-4"),35.25(C-3),27.79(C-8),23.42(C-16),20.89(C-7),17.34(C-6"),7.11(C-14).以上波谱数据与文献[12]基本一致,鉴定化合物5为verrucarin J.
化 合 物6:C29H40O9,白 色 粉 末;ESI-MS m/z:555.25836([M+Na]+);1H NMR(600 MHz,Methanol-d4)δ 7.59(1H,m,H-8"),6.70(1H,t,J=11.4 Hz,H-9"),6.17(1H,dt,J= 15.4,6.6 Hz,H-7"),5.88(1H,dd,J= 7.8,3.5 Hz,H-4),5.75(1H,t,J= 1.4 Hz,H-2"),5.65(1H,d,J= 11.4 Hz,H-9"),5.41(1H,dd,J= 5.5,1.7 Hz,H-10),4.27(1H,d,J= 12.4 Hz,H-15),4.03(1H,d,J=12.4 Hz,H-15),3.84(d,J= 5.6 Hz,H-11),3.75(1H,d,J= 5.2 Hz,H-2),3.70(1H,t,J= 6.5 Hz,H-5"),3.10(1H,d,J= 4.0 Hz,H-13),2.89(1H,J= 4.0 Hz,H-13),2.54(1H,dd,J= 15.5,7.8 Hz,H-3),2.38(1H,t,J= 6.5 Hz,H-4"),2.18(1H,d,J= 1.3 Hz,H-12"),1.98(2H,m,H-7),1.80(1H,dt,J= 8.4,2.5 Hz,H-8),1.70(1H,s,H-16),1.13(3H,d,J= 6.4 Hz,H-14"),0.79(3H,d,J=1.7 Hz,H-14).13C NMR(150 MHz,Methanol-d4)δ 167.41(C-1"),167.19(C-11"),159.39(C-3"),145.89(C-9"),141.75(C-9),128.85(C-8"),119.41(C-10),117.94(C-10"),117.50(C-2"),80.23(C-2),76.24(C-4),68.12(C-11),66.39(C-12),63.74(C-15),60.43(C-5"),50.02(C-5),49.17(C-13),44.40(C-6),37.29(C-3),28.76(C-8),23.08(C-16),22.05(C-7),7.30(C-14).以上波谱数据与文献[13]基本一致,鉴定化合物6为Isotrichoverrin A.
化 合 物7:C29H40O9,白 色 粉 末;ESI-MS m/z:555.25879([M+Na]+);1H NMR(600 MHz,Methanol-d4)δ 7.62(1H,ddt,J= 15.5,11.5,1.2 Hz,H-8"),6.71(1H,td,J= 11.4,0.9 Hz,H-9"),6.13(1H,m,H-7"),5.89(1H,dd,J= 7.9,3.5 Hz,H-4),5.77(1H,q,J= 1.2 Hz,H-2"),5.67(1H,d,J= 11.4 Hz,H-10"),4.28(1H,d,J= 12.4 Hz,H-15),4.02(1H,m,H-15),3.85(1H,d,J= 5.6 Hz,H-11),3.75(1H,d,J=5.1 Hz,H-2),3.72-3.68(2H,m,H-5"),3.12(1H,d,J= 4.0 Hz,H-13),2.91(1H,d,J= 4.0 Hz,H-13),2.56(1H,dd,J= 15.4,7.8 Hz,H-3),2.39(2H,td,J= 6.6,1.1 Hz,H-4"),2.19(3H,d,J= 1.3 Hz,H-12"),1.98(1H,m,H-3),1.72(3H,d,J= 1.4 Hz,H-16),1.12(3H,d,J= 6.4 Hz,H-14"),0.81(3H,s,H-14).13C NMR (150 MHz,Methanol-d4) δ 167.46(C-1"),167.22(C-11"),159.45(C-3"),145.79(C-9"),141.82(C-9),129.00(C-8"),119.35(C-10),118.08(C-10"),80.21(C-2),76.29(C-4),68.11(C-11),66.41(C-12),63.74(C-15),60.43(C-12),50.03(C-5),44.38(C-6),37.29(C-3),28.75(C-8),23.07(C-16),22.04(C-7),18.66(d,J=13.9 Hz),7.31(C-14).以上波谱数据与文献[13]基本一致,鉴定化合物7为Isotrichoverrin B.
化合物8:C29H40O10,白色粉末;ESI-MS m/z:571.25323([M+Na]+);1H NMR(600 MHz,Methanol-d4)δ 7.62(1H,ddt,J= 15.4,11.4,1.5 Hz,H-8"),6.76(1H,t,J= 11.4 Hz,H-9"),6.13(1H,dd,J= 15.5,3.2 Hz,H-7"),5.91 - 5.73(1H,m,H-4),5.66(1H,dd,J= 4.4,2.7 Hz,H-10),4.05(1H,s,H-16),4.04(1H,s,H-2"),3.89 - 3.66(2H,m,H-2),3.05(1H,d,J= 4.0 Hz,H-13),2.49(1H,dd,J= 15.3,8.3 Hz,H-3),1.09(3H,dd,J= 16.4,6.6 Hz,H-12"),0.82(3H,s,H-14).13C NMR(150 MHz,Methanol-d4)δ175.55(C-1"),168.07(C-11"),145.35(C-9"),144.73,(C-9),141.35(C-7"),127.46(C-8"),118.98(C-10),117.86(C-10"),84.41(C-6"),80.48(C-2),76.66(C-2"),75.98(C-4),70.79(C-13"),70.50(C-5"),67.99(C-11),66.43(C-12),65.94(C-16),64.76(C-15),50.61(C-5),49.57(C-13),45.49(C-6),37.50(C-3"),35.68(C-3),34.78(C-4"),24.09(C-8),21.00(C-7),18.26(C-14"),15.06(C-12"),8.01(C-14).以上波谱数据与文献[14]基本一致,鉴定化合物8 为16-hydroxyroridind A.
3 细胞活性
3.1 实验方法
取处于对数生长期,状态良好的MCF-7和A549细胞调整细胞密度后接入96 孔板,每孔100 mL 细胞悬液,同时设置空白组.37 ℃培养7 h(在细胞孔周围加入100 mL 无菌PBS).待细胞贴壁后,分别在给药组细胞中加入不同浓度的化合物(40、30、20、10、5、1 μmol/L),每组3个复孔,37 ℃继续培养24 h,培养完成后,每孔加入100 mL MTT(MTT:10%5 mg/mL MTT+90 % 完全培养基),37 ℃孵育4 h,吸出培养基后,每孔加入100 mL DMSO 震荡10 min.;用酶标仪测定各孔吸光值OD570,并计算IC50.
3.2 实验结果
利用MTT 法检测化合物1~8 对MCF-7 和A549细胞的体外细胞毒活性,结果显示化合物4、6、8 对MCF-7(人乳腺细胞)具有显著的抑制活性,详见表1.
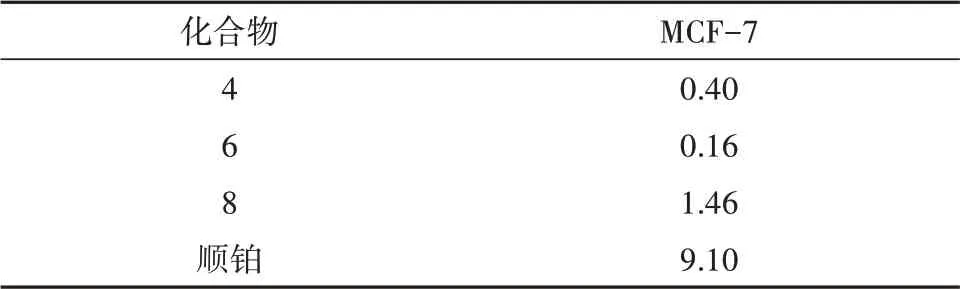
表1 化合物4、6、8的细胞毒活性Tab.1 The cytotoxic activity of compounds 4,6 and 8(IC50,μmol/L)
4 结语
内生真菌与其宿主植物在长期进化过程中,形成良好共生关系的同时,又可以产生结构新颖、活性显著的次生代谢产物[15-16].从黄连内生真菌A.viridis发酵产物的乙酸乙酯部位首次分离得到8 个大环内酯类化合物,利用MTT 法检测了化合物1~8对MCF-7和A549细胞的体外细胞毒活性,结果显示化合物4、6、8 对MCF-7(人乳腺细胞)具有显著的抑制活性.本论文丰富了黄连内生真菌的次生代谢产物结构类型,为进一步产业开发奠定了物质基础.
