Conjugated Protonated Double Bonds and Triple Bonds
2022-08-15WUXinyaoLISidian
WU Xinyao,LI Sidian
(Institute of Molecular Science,Shanxi University,Taiyuan 030006,China)
Abstract:Boron as a prototypical electron-deficient element exhibits unique structures and bonding,as demonstrated in the protonated double bonds in D2hB2H6(1)and protonated triple bonds in D3hB2H5+(2).Based on extensive first-principles theory calculations and detailed bonding analysis,we present herein the possible existence of conjugated protonated double bonds in C2hB4C10H14(3)and conjugated protonated triple bonds in D3dB4H82+(4),extending the delocalized B−H−B 3c-2e bonds to boron-containing conjugated systems.Substituting B with Be in 2 and 4 generates the monoanion D3hBe2H5-(5)and neutral D3dBe2B2H8(6)which possess similar protonated triple bonds and conjugated protonated triple bonds,respectively.The IR,Raman,and photoelectron(PE)spectra of the concerned species are computationally simulated to facilitate their experimental characterizations.
Key words:first-principles theory calculations;borenes;borynes;conjugated protonated double bonds;conjugated protonated triple bonds
As a prototypical electron-deficient element in the periodical table,boron exhibits strong propensity to form multi-center-two-electron(mc-2e)bonds in both polyhedral molecules and bulk solids[1-3],as demonstrated in the protonated double bonds(PDBs)inD2hB2H6( 11)which contains two equivalent 3c-2e B−H−B σ bridges(two delocalized σ bonds as shown in Fig.S1)and protonated 3c-2e B-H-B triple bonds(PTBs)inD3hB2H5+( 22)which possesses three equivalent 3c-2e B−H−B σ bridges(three delocalized σ bonds as shown in Fig.S2)[4-6].B2H6( 11)with two PDBs and B2H5+( 22)with three PTBs can thus be viewed as the boron analogs of ethyleneD2hC2H4 with one C=C double bond(1σ+1π)and acetyleneD∞hC2H2with one C≡C triple bond(1σ+2π),respectively.They should therefore belong to borenes and borynes,respectively.It is well known that ethylene C2H4can be further extended to form 1,3-butadieneC2hC4H6( 77)which encompasses two conjugated double bonds,while acetylene C2H2can be used as precursors to generate 1,3-diacetyleneD∞hC4H2( 88)with two conjugated triple bonds.However,there have been no conjugated protonated double bonds(CPDBs)or conjugated protonated triple bonds(CPTBs)reported to date in chemistry in either theory or experiments.In this work,based on extensive first-principles theory calculations and detailed adaptive natural density partitioning(AdNDP)[7-8]and electron localization function(ELF)[9]bonding analyses[10-12],we predict in this work the conjugated boron-containing speciesC2hB4C10H14( 33)andD3dB4H82+( 44)which possess CPDBs and CPTBs,respectively.CPTBs are also found to in the Be-substitutedD3dBe2B2H8( 66).Such boron-containing conjugated species are expected to form a novel series of unsaturated compounds with important applications in both chemistry and materials science.
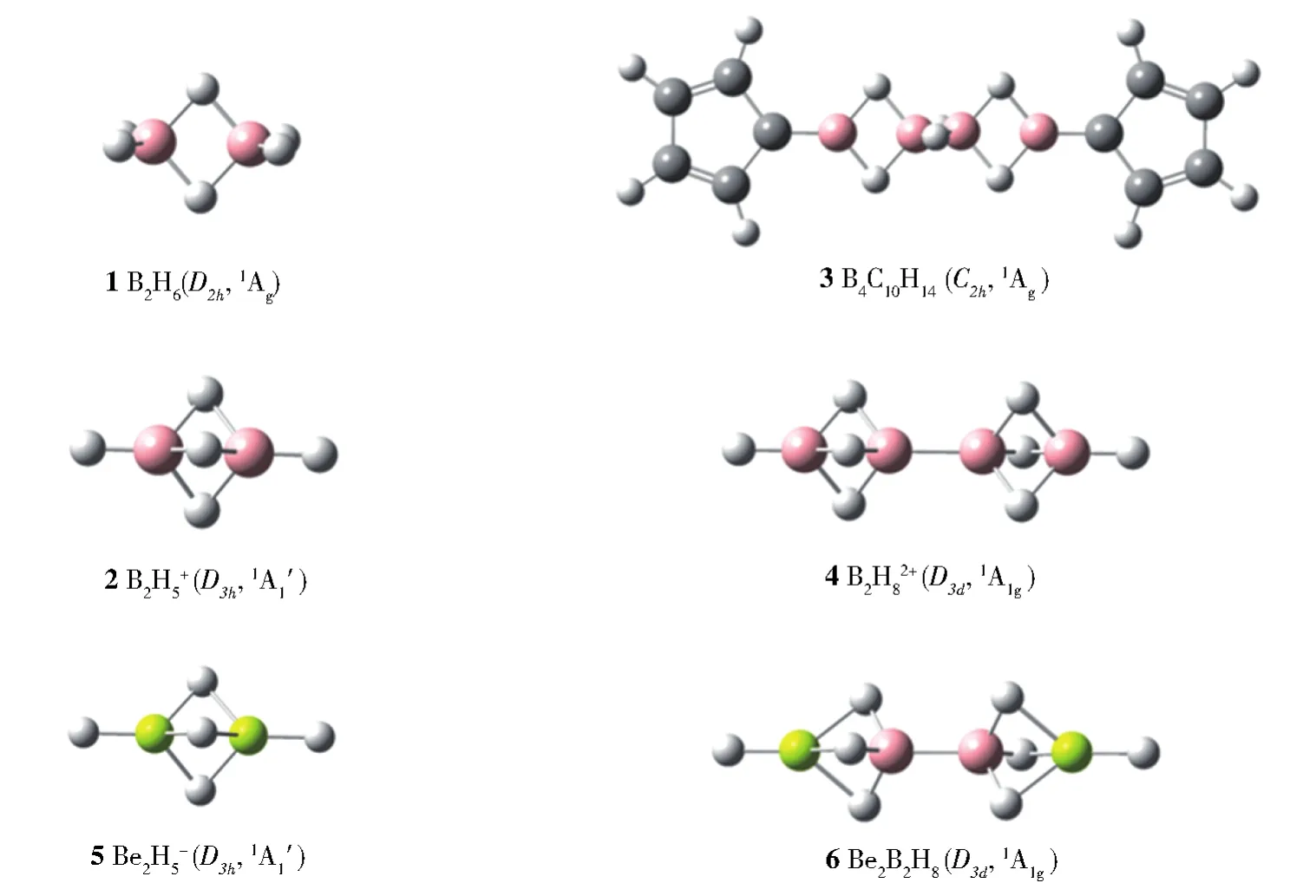
Fig.1 Optimized structures of D2hB2H6(1),D3hB2H5+(2),C2hB4C10H14(3),D3dB4H82+(4),D3hBe2H5-(5),and D3dBe2B2H8(6)at PBE0/6-311+G(d,p)level
The theoretically optimizedC2hB4C10H14( 33)at PBE0/6-311+G(d,p)level[13]turns out to be one of the three lowest-lying isomers of the system containing cyclopentadienyl(Cp)rings within 0.20 eV(Fig.S3).It has the HOMO-LUMO energy gap of∆Egap=3.75 eV and lowest vibrational frequency of 10 cm-1.Detailed ELF and AdNDP bonding analyses presented in Fig.2(a)and Fig.S4 indicate thatC2hB4C10H14( 33)possesses a bonding pattern similar to that of the well-known conjugated 1,3-butadieneC2hC4H6( 77).Natural bonding orbital(NBO)analysis indicates that the two equivalent B atoms connected to the cyclopentadienyl(Cp)ring in B4C10H14( 33)carry the positive natural charge ofqB=+0.40|e|,while their C neighbors in the Cp ring possess the negative natural charge of qC=−0.5|e|,indicating that there exist obvious charge transfers from B atoms to the Cp rings.As briefed in Figure 2(a)and detailed in Fig.S4,B4C10H14( 33)possesses 2 2c-2e B−C σ bonds to bridge the B4chain and two Cp units with the occupation numbers of|ON|=1.99|e|,1 2c-2e B−B σ bond between the two B2H3units on the left and right with ON=1.95|e|,10 2c-2e CC σ bonds on the Cp rings with ON=1.96-1.98|e|,2 2c-2e B−H σ bonds with ON=1.96|e|,8 2c-2e C-H σ bonds with ON=1.99|e|,4 2c-2e C−C π bonds on the Cp rings with ON=1.88|e|,and 2 2c-2e B−C π bonds connecting the B4chain and two Cp rings with ON=1.72|e|,in an overall symmetry ofC2h.More interestingly and importantly,as highlighted in the box in Figure 2(a),B4C10H14( 33)contains 2 equivalent 3c-2e B-H-B σ bonds over the B2unit on the left and 2 equivalent 3c-2e B-H-B σ bonds over the B2unit on the right,forming the first conjugated protonated double bonds(CPDBs)reported in chemistry.As highlighted in Figure 2a,such two CPDBs inC2hB4C10H14( 33)are obviously analogous to the two conjugated double bonds in 1,3-butadieneC2hC4H6( 77)which features 2 equivalent 2c-2e C−C σ bonds with ON=1.99|e|and 2 equivalent 2c-2e C−C π bonds with ON=1.93|e|(a 2σ+2π system).The calculated ELF pictures in Figure 2a well support the existence of two conjugated protonated double bounds unveiled by AdNDP analysis in bothC2hB4C10H14( 33)andC2hC4H6( 77).
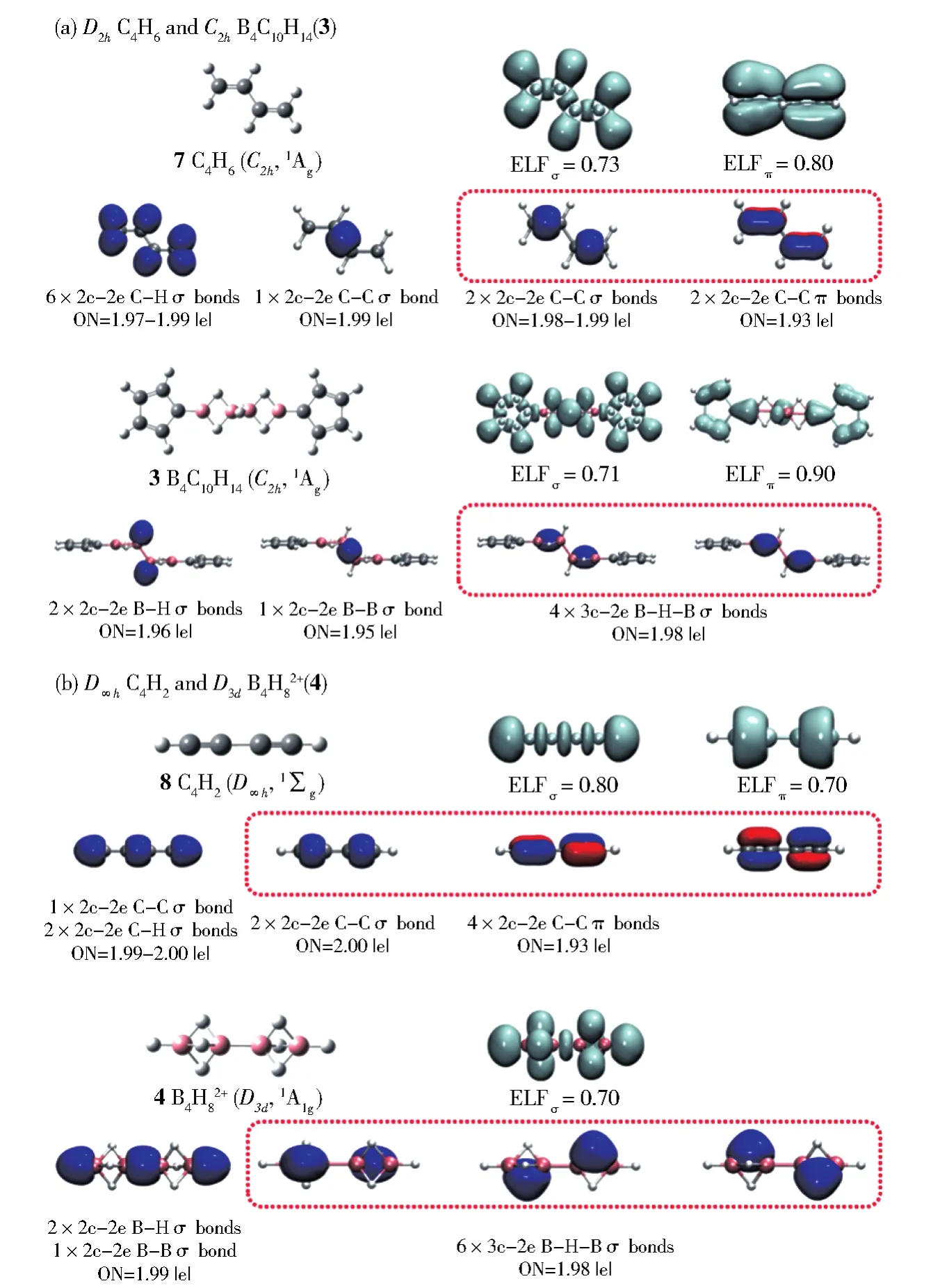
Fig.2 Comparison of the ELF and AdNDP bonding patterns of(a)C2hC4H6(7)and C2hB4C10H14(3)and(b)D∞hC2H2(8)and D3dB4H82+(4),with the conjugated double bonds and triple bonds highlighted in red boxes
Extensive TGMin2 GM searches[14-16]show thatD3dB4H82+( 44)is the well-defined global minimum of the dication with the HOMO-LUMO energy gap of ΔEgap=9.94 eV at PBE0/6-311+G(d,p)level(Fig.S5).It contains two conjugated protonated triple bonds(CPTBs)analogous to the two conjugated C≡C triple bonds in linear 1,3-diacetyleneD∞hC4H2( 88),as demonstrated in their AdNDP bonding patterns in Figure 2(b).B4H82+( 44)contains 9 pairs of valence electrons,including 2 2c-2e B-H σ bonds at the two ends with ON=1.99|e|,1 2c-2e B-B σ bond at the center with ON=1.99|e|,3 3c-2e B-H-B σ bonds ON=1.98|e|on the B2unit on the left,and 3 3c-2e B−H−B σ bonds ON=1.98|e|on the B2unit on the right,in an overall symmetry ofD3d.The two CPTBs in B4H82+( 44)appear to be similar to the two conjugated triple bonds in 1,3-diacetyleneD∞hC4H2( 88)which possesses 2 2c-2e C−C σ bond with ON=2.00|e|and 4 2c-2e C−C π bonds with ON=1.93|e|(a 2σ+4π system).The ELF pictures depicted in Figure 2(b)further evidence the existence of two conjugated triple bonds in bothD3dB4H82+( 44)andD∞hC4H2( 88).Fig.S6 summarizes the obtained conjugated protonated double and triple bondsin B4C10H14( 33)and B4H82+( 44),compared with the protonated double and triple bonds in B2H6( 11)and B2H5+( 22).
Substituting two B atoms inD3hB2H5+( 22)andD3dB4H82+( 44)with two Be atoms generates the monocationD3hBe2H5-( 55)and neutralD3dBe2B2H8( 66),respectively.Detailed AdNDP analysis show that,similar toD3hB2H5+( 22),there exist 3 equivalent 3c-2e Be−H−Be σ bonds with ON=1.97|e|inD3hBe2H5-( 55)(Fig.S7a)and,analogous to B4H82+( 44),there have two sets of conjugated 3c-2e Be-H-B σ bonds with ON=1.97|e|inD3dBe2B2H8( 66)(Fig.S7b),indicating that two Be atoms are involved in the formation of the conjugated system.Interestingly,Be2H5-( 55)possesses a huge HOMULUMO gap of ΔEgap=6.35 eV at PBE0 level,suggesting its high stability in thermodynamics and viable possibility to be observed in gas-phase experiments.The calculated negative nucleus independent chemical shifts values of NICS=−37.4,−37.8,−20.3,and−26.7(NICS)[17-18]at the centers of three equivalent protonated triple bonds in B2H5+( 22),B4H82+( 44),Be2H5-( 55),and Be2B2H8( 66),respectively,strongly suggest that three protonated B−H−B,Be−H−Be,or Be−H−B triple bonds evenly distributed aroundC3the molecular axes generate local aromaticities to help stabilize the concerned systems.
Figure 3(a)and(b)depict the simulated IR and Raman spectra ofD3dB4H82+( 44).The high-symmetry B4H82+( 44)exhibits two strong IR peaks at 1 241(a2u)and 1 396(a2u)and two strong Raman peaks at 2 180(eg)and 2 815(a1g)cm-1,respectively.The strongest Raman peak 2 180(eg)is a typical radial breathing vibration mainly involving the concerted vibrations of the two equivalent CPTBs.The lowsymmetryC2hB4C10H14( 33)has one main IR peak at 1 660(bu)(Fig.3(c))and three strong Raman absorptions at 1 126(ag),1 577(ag)and 1 700(ag)cm-1(Fig.3(d)),respectively.The IR,Raman,and photoelectron(PE)spectra of Be2H5-and IR,Raman,and UV-vis spectra of Be2B2H8were simulated in Fig.S8,with Be2H5−possessing an exceptionally large first detachment energy of VDE=5.04 eV.The simulated PE spectroscopy of Be2H5-and UV-vis spectroscopy of Be2B2H8were calculated using the TD-DFT-PBE0 method[19].
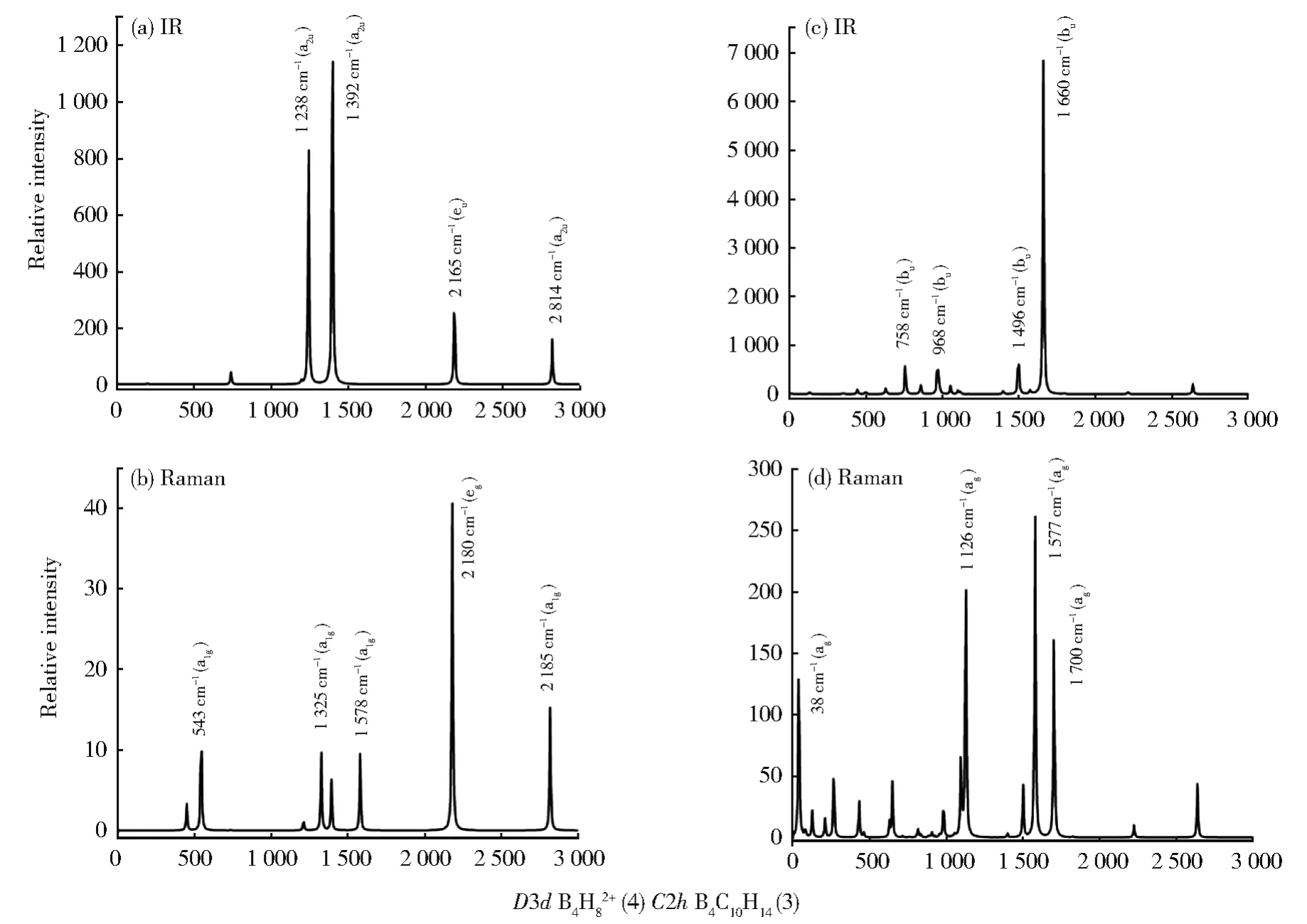
Fig.3 Computationally simulated(a)IR and(b)Raman spectra of D3dB4H82+(4)and(c)IR and(d)Raman spectra of C2hB4C10H14(3)at PBE0/6-311+G(d,p)level
In summary,based on extensive first-principles theory calculations and bonding analysis,we have presented in this work the existence of conjugated protonated double bonds and conjugated protonated triple bonds in chemistry.The two B-H2-B CPDBs in B4C10H14( 33)turn out to be similar to the two conjugated C=C double bonds in 1,3-butadiene C4H6( 77),while the two B-H3-B CPTBs in B4H82+( 44)are analogous to the two conjugated C≡C triple bonds in 1,3-diacetylene C4H2( 88).Similar Be-H3-B CPTBs exist in neutral Be2B2H8( 66).CPDBs and CPTBs are also possible to exist in two-dimensional borophenes and borophynes,respectively.Experimental syntheses are invited to characterize such species to open a new area of boron-containing conjugated nanomaterials.
