Effects of white noise on procedural pain-related cortical response and pain score in neonates:A randomized controlled trial
2022-07-31XuynRenLiLiSiyLinChunxiZhongBinWng
Xuyn Ren ,Li Li ,Siy Lin ,Chunxi Zhong ,Bin Wng
a Clinical Nursing Education &Research Section,Zhujiang Hospital,Southern Medical University,Guangzhou,China
b School of Nursing,Southern Medical University,Guangzhou,China
cDepartment of Pediatrics,Zhujiang Hospital,Southern Medical University,Guangzhou,China
Keywords:Facial expression Intensive care units Neonates Procedural pain Premature infant pain profile-revised Radial artery Regional cerebral oxygen saturation White noise
ABSTRACT Objectives:To evaluate the effects of white noise on pain-related cortical response,pain score,and behavioral and physiological parameters in neonates with procedural pain.Methods:A double-blind,randomized controlled trial was conducted.Sixty-six neonates from the Neonatal Intensive Care Unit in a university-affiliated general hospital were randomly assigned to listen to white noise at 50 dB (experimental group) or 0 dB (control group) 2 min before radial artery blood sampling and continued until 5 min after needle withdrawal.Pain-related cortical response was measured by regional cerebral oxygen saturation(rScO2)monitored with near-infrared spectroscopy,and facial expressions and physiological parameters were recorded by two video cameras.Two assessors scored the Premature Infant Pain Profile-Revised (PIPP-R) independently when viewing the videos.Primary outcomes were pain score and rScO2 during arterial puncture and 5 min after needle withdrawal.Secondary outcomes were pulse oximetric oxygen saturation (SpO2) and heart rate (HR) during arterial puncture,and duration of painful expressions.The study was registered at the Chinese Clinical Trial Registry (ChiCTR2200055571).Results:Sixty neonates (experimental group,n=29;control group,n=31) were included in the final analysis.The maximum PIPP-R score in the experimental and control groups was 12.00 (9.50,13.00),12.50 (10.50,13.75),respectively (median difference -0.5,95% CI -2.0 to 0.5),and minimum rScO2 was(61.22 ±3.07)%,(61.32 ± 2.79)%,respectively(mean difference -0.325,95%CI -1.382 to 0.732),without significant differences.During arterial puncture,the mean rScO2,HR,and SpO2 did not differ between groups.After needle withdrawal,the trends for rScO2,PIPP-R score,and facial expression returning to baseline were different between the two groups without statistical significance.Conclusion:The white noise intervention did not show beneficial effects on pain-related cortical response as well as pain score,behavioral and physiological parameters in neonates with procedural pain.
What is known?
● It is recommended to evaluate the effectiveness of interventions for pain relief in neonates with neurophysiological measures as well as physiological and behavioral parameters.
● Prior research findings show that white noise could reduce pain scores and improve pain-related behaviors in neonates with procedural pain,but the researcher and/or assessor were not blinded in most of the studies.
● Effects of white noise intervention on pain-related neurophysiological response need further study to explore.
What is new?
● Listening to white noise did not show beneficial effects on procedural pain in neonates in this double-blind randomized controlled study.
● Use of two assessors blinded to group allocation viewing the mute recorded videos and scoring pain independently improved the reliability of the outcome measures in neonates.
● Pain-related cortical response combined with pain scales and/or behavioral parameters may lead to a better understanding of the effects of non-pharmacological strategies on pain in neonates.
1.Introduction
Neonates admitted to the hospital experience numerous and frequent painful medical procedures for the sake of diagnosis or treatment.Procedures,such as drawing blood from the heel or the artery and/or the vein,intravenous and/or intramuscular injection,are a routine part of their medical care [1].These repeated untreated painful and stressful stimuli have been demonstrated to disrupt infant neural synaptogenesis,brain maturation,and development [2,3].Mitigating the likelihood of long-term neurodevelopmental injury in this fragile population is a growing focus for improving pediatric critical care[4].
Compared with analgesic drugs,non-pharmacological intervention is safer with fewer adverse effects.Non-pharmacological interventions for acute procedural pain in neonates have been highly recommended by international guidelines [5].A range of studies have examined various non-pharmacological pain-alleviating strategies during some painful procedures in neonates,such as oral sucrose,skin-to-skin contact,and white noise[6-8].White noise is a kind of continuous,monotonous sound in the form of resonance that suppresses disturbing sounds coming from the outside environment and that has a soothing quality [9].Given these characteristics,white noise is similar to the sounds in the mother's womb.While still in the womb,the infant is influenced by the mother’s heartbeat,and exposure to these familiar sounds and rhythms after birth has a soothing effect on the infant[10].Studies support the positive effects of white noise intervention for neonates with improvement in sleep and weight gain [9,11].Recently,several studies have evaluated the analgesic effect of white noise in neonates with procedural pain,but the results are inconsistent[8,10,12-14].
Because neonates are nonverbal,it is difficult to evaluate the effectiveness of interventions for pain relief in neonates.Physiological indicators (e.g.,heart rate [HR],respiration rate,oxygen saturation),behavioral indicators (e.g.,crying,facial expression),and pain scores by observers are commonly used to evaluate nonpharmacological interventions for pain relief in neonates [15,16].Physiological indicators can be assessed objectively,but they are likely nonspecific indicators of distress rather than being painspecific [17,18].Behavioral indicators are regarded as sensitive and specific for pain [19].However,studies suggest that neonates who do not display a change in facial expression after painful procedures may still display significant cortical responses [6,20].Pain scores are largely based on the observer’s subjective judgment of the behavioral and/or physiological parameters in neonates[21],and may not be an appropriate outcome measure for neonatal analgesia studies [22].Moreover,there is an increasing awareness of the need to not only reduce acute behavioral responses to pain in neonates,but also to protect the developing nervous system from persistent sensitization of pain pathways and potential damaging effects of altered neural activity on central nervous system development [4].Thus,the effects of interventions for pain relief in neonates,may not be precisely assessed when only using pain scales and/or behavioral parameters [4] and should include neurophysiological measures[20,23].The regional cerebral oxygen saturation(rScO2),monitored with near-infrared spectroscopy (NIRS),could be used to examine cortical activity in response to acute noxious stimuli and pharmacologic/non-pharmacologic intervention for pain relief [24,25].It has been suggested to incorporate NIRS with behavioral and physiological indicators of pain to better understand the neonate’s response to a painful procedure and the effect of pain relief measures [23].
Several studies have evaluated the analgesic effects of white noise with pain scales and physiological (HR,pulse oximetric oxygen saturation [SpO2]) and/or behavioral parameters during selected painful procedures,including heel puncture [8,13,26] and retinopathy screening[12].These studies showed that white noise intervention could reduce pain scores,and improve physiological and behavioral parameters in neonates experiencing procedural pain,however,the pain assessors were reported blinded only in one study [12].In another assessor-blinded study,Taplak et al.evaluated the effects of the breast milk smell,white noise,and facilitated tucking with pain scores,HR,and SpO2in neonates during endotracheal suctioning [14].The results showed that no statistically significant differences were found between the groups in pain score,HR,and SpO2during the endotracheal suctioning procedure[14].Further research is needed to validate the effects of white noise to reduce pain scores and improve physiological parameters in neonates with procedural pain.Moreover,the effect of white noise intervention on the pain-related neurophysiological response of neonates is unknown,and it is important to understand whether this intervention could potentially prevent harm to the neurodevelopment of neonates caused by pain.
The present study hypothesizes that administration of white noise before and during an arterial blood sampling puncture would reduce the evoked cortical responses and pain scores in neonates.This study measured the changes in pain-related cortical response(rScO2),pain score,HR,SpO2,and duration of painful expressions evoked by arterial blood sampling puncture in term infants,to evaluate the effects of white noise intervention in neonates with procedural pain.
2.Methods
2.1.Study design and setting
A double-blind randomized controlled trial was conducted using a parallel design with two groups:1)experimental group(with white noise at 50 dB);and 2) control group (with white noise at 0 dB).The study was conducted from March 2021 to August 2021 at the Neonatal Intensive Care Unit(NICU),Department of Pediatrics,in a university-affiliated Class A tertiary general hospital in Guangzhou,China.
2.2.Participants
The convenience sampling method was applied in this study.Inclusion criteria were neonates with gestational age between 37 and 42 weeks;scheduled for arterial blood sampling via puncture;having had at least one painful procedure after birth,and with signed informed consent forms from parents or legal guardians.Exclusion criteria were neonates who had a condition that might influence their physiological response to pain,such as congenital anomalies,surgery,severe illness requiring treatment with sedatives,muscle relaxants,or antiepileptic drugs,or had any other condition preventing them from listening to the white noise,or with oxygen therapy,or with axillary temperature ≥37.3°C.
2.3.Sample size calculation
Effect size was interpreted using Cohen’s d (small effect:0.2;medium effect:0.5;large effect:0.8)[27].Variables with a medium or large effect were regarded as being clinically meaningful.Based on a previous study,in which a white noise intervention reduced pain scores in neonates experiencing painful procedures with a large effect [8],the effect size d was determined to be 0.80 in the present study.G*Power (version 3.1.9.7) was used to estimate the sample size [27].We chose “Tests→Means→two independent groups” and used the following settings “Two tails,Effect size d=0.8,α err prob=0.05,Power=0.8,Allocation ratio N2/N1=1”resulting in a required sample size of 52 with 26 subjects per group.Because of the possibility that technical failure could occur in any one of the physiological recordings (i.e.,video,NIRS,or pulse oximetry) and potential missing data in the study,the final sample size was 66 neonates(33 per group)anticipating a 20%incomplete data.
2.4.Randomization and blinding procedure
Blocked randomization(block size=six)was performed using a computer-generated randomization list with an equal allocation ratio by the investigator.Blocked randomization was used to reduce accidental selection bias and achieve balance in the allocation of neonates to treatment arms.This approach increases the probability that each arm will contain an equal number of individuals by sequencing participant assignments by blocks.Enrolled neonates were assigned sequential screening numbers corresponding to the computer-generated random numbers by the investigator.According to the screening number order,66 neonates were separated into 11 blocks of six.One graduate student who was blinded to the study hypotheses served as the research assistant.Neonates in each block were assigned in a 1∶1 ratio to the experimental and control groups according to the random number order,and the group allocation was written on cards and placed in sealed,opaque envelopes by the research assistant.Without wearing earplugs,the research assistant was required to behave neutrally and similarly across conditions and to prepare the music player to play white noise at 50 dB for intervention or 0 dB for sham intervention.All prepared music players were identical in appearance.The data collector and the two nurses who performed the arterial puncture were required to wear earplugs.Two trained assessors viewed the mute recorded videos and scored pain independently.Throughout the study,the investigator,clinicians,participants,and parents were blinded to group allocation.
2.5.Study intervention
The procedural pain stimulus was a radial artery puncture to collect a clinically necessary blood sample in the morning shift.Before the intervention,all neonates were awake and lay in nesting position in their incubators with some environmental noise attenuation.The nesting position was a standard of care in this unit to make neonates comfortable.The background sound and operational sound in the NICU were under 50 dB meeting the recommended standards for the newborn ICU [28].To ensure both groups experienced the radial artery puncture similarly,the arterial punctures were performed by two skilled nurses with a disposable venous infusion needle(0.55 mm×20.00 mm)through the access port door of the incubator.Only data from neonates for the first arterial puncture attempt was studied.
The research assistant placed the prepared music player about 20 cm away from the head of neonates in their incubators.Based on previous studies with white noise at 45-65 dB playing 5 s to 15 min before the procedure and continuing after its completion[13,14,29],the music player was turned on 2 min before the procedure and continued until 5 min after needle withdrawal in this study.Neonates in the experimental group were exposed to 50 dB white noise from the album “Colic Baby:White Noise for Babies” using the episode “Calm Rain for Colic Baby Sleep” delivered on a continuous loop (available at https://music.163.com/#/song?id=565877614).Neonates in the control group were exposed to 0 dB white noise for the sham intervention.To maintain resemblance to a real-life scenario,ambient noise was also not modulated.Both groups were in the same clinical setting with the only difference being the white noise intervention.The research assistant was present during the study to collect and record any adverse events,but was not involved in data collection,analysis,and any care delivered to the neonates.
2.6.Outcome measures
Primary outcomes were pain score and rScO2during arterial puncture and 5 min after needle withdrawal.Secondary outcomes were mean SpO2and HR during arterial puncture and duration of painful expressions.
Pain score was assessed using the Premature Infant Pain Profile-Revised(PIPP-R)which is a seven-item multidimensional measure of pain revised from the Premature Infant Pain Profile(PIPP).It has been widely evaluated and validated for acute pain assessment in preterm and term neonates [31,32].The indicators include three behavioral (facial actions:brow bulge,eye squeeze,nasolabial furrow),two physiological (changes in HR and oxygen saturation),and two contextual(gestational age[GA]and behavioral state[BS])variables.Physiological and behavioral items are numerically scored based on a 4-point scale (0,1,2,and 3).Conversely,the contextual items are numerically reverse,scored (3,2,1,and 0).Items of the GA and BS were only scored if there were changes in any of the physiological and/or behavioral variables in response to the stimulus.Both the PIPP and PIPP-R have a 0-18 range of total scores for full-term neonates.A total PIPP score of 6 or less indicates minimal to no pain,while scores greater than 12 indicate moderate to severe pain [33].There is no report on the delineation of mild,moderate,or severe pain based on PIPP-R scores,so the level of pain in this study was categorized similarly to the PIPP score.The facial actions in PIPP-R were considered as kind of painful expressions,including brow bulge,eye squeeze,and nasolabial furrow.The duration of painful expressions was defined as the time between the appearance of any facial actions and the disappearance of all three facial actions.
The pulse oximeter and electrocardiographic monitor (EDAN Instruments,Shenzhen,China)was used to measure SpO2and HR.A double-channel NIRS (EnginMed,Suzhou,China) was used to monitor data for the rScO2.
2.7.Data collection procedure
From 5 min before the arterial puncture until 5 min after its completion,the rScO2,HR and SpO2were collected by the investigator.The timeline of the data collection procedure is presented in Fig.1.The emitter optodes of NIRS were placed about 2 cm below and slightly posterior to the C3/C4 position according to the international electroencephalography 10-20 system [30].The two pairs of optodes were fastened bilaterally with a fabric cap over the somatosensory cortex symmetrically on each side of the neonatal head.The pulse oximeter probe was placed on one of the neonatal feet.From 2 min before the arterial puncture to 5 min after its completion,neonatal facial expressions and the electrocardiographic monitor that displayed HR and SpO2were recorded on the mute mode by two video cameras respectively,for calculation of the pain score,duration of painful expressions and physiological measures.
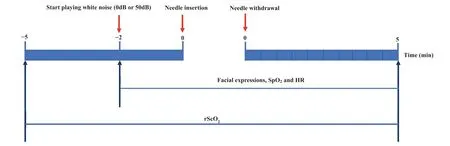
Fig.1.The timeline of the procedure.
At the end of each trial,all data were imported into the computer and marked with three time points (quiet/awake state,needle insertion and needle withdrawal) by the investigator.The videos were scored independently using the PIPP-R by two trained observers,who were blinded to the intervention.One of the observers calculated the duration of painful expressions.The stable value displayed before the procedure represented the baseline HR and SpO2.The mean rScO2of the two optodes was calculated 30 s before the procedure in a quiet state and defined as the baseline rScO2.Based on previous studies,the minimum rScO2was defined as the lowest value of rScO2in 20 s after the needle penetrating the skin[22,30].During arterial puncture,the mean rScO2,HR and SpO2were calculated,and the maximum pain score was assessed.During 5 min after the needle withdrawal,rScO2and pain score were assessed for every 30-s interval,and the 10 assessment time points were labeled as T1 to T10,respectively.Demographic and clinical data of each neonate were retrospectively collected from the electronic medical record.
2.8.Data analysis
Data were analyzed using SPSS version 26(IBM Co.,Armonk,NY,USA),and graphs were generated using OriginPro version 2021(OriginLab Corporation,Northampton,MA,USA).Exploratory data analysis and Shapiro-Wilk tests were performed to determine the normality of the data distribution.Baseline characteristics were reported,using frequencies and percentages for categorical variables,and mean±SD or median(P25,P75)for continuous variables.The paired t-test or Wilcoxon signed-rank test were used for data comparison before and during arterial puncture in the same group.The primary analysis was conducted according to a modified intent-to-treat (mITT) analysis set.The mITT analysis set included all the randomized patients who received the intervention,measured baseline rScO2,and at least one postbaseline rScO2,excluding participants who failed to pass the hearing screening during hospitalization.
For the primary outcome analyses,the inter-rater consistency among the two trained observers evaluating pain intensity was measured by the intra-class correlation coefficients(ICCs),using the mean of the two assessors’pain scores for comparison between the two groups.The Mann-Whitney U test with the Hodges-Lehmann estimator was used to calculate median differences in maximum PIPP-R score between the two groups.Analysis of covariance adjusted for baseline rScO2was used to assess the difference between two groups in the minimum and mean rScO2,with mean differences expressed with their two-sided 95% CIs.A generalized linear mixed-effect model was used(group,time point,the group ×time interaction,and baseline were considered as fixed effects,subject as random effects)to test statistical significance between two groups.A first-order autoregressive correlation structure was used to model correlations among the repeated measurements.Point estimates and corresponding 95%CIs for the least-squares means at each time point were derived.Secondary outcomes were evaluated as follows:analysis of covariance adjusted for baseline HR was used to assess the mean difference in the mean HR between the two groups;the Mann-Whitney U test was used to assess the difference in SpO2during arterial puncture,and the duration of painful expressions.All data were double entered and checked,and any discrepancies were resolved by referring to the original data.The level of significance was set at 0.05.
2.9.Ethical approval
The procedures followed in this study complied with the ethical standards established by the Ethics Committee of the hospital and received approval from the committee (2020-KY-096-02).The study conformed to the standards set by the Declaration of Helsinki and Good Clinical Practice guidelines and was registered at the Chinese Clinical Trial Registry (ChiCTR2200055571).Informed written consents were obtained from the parents or legal guardians of the recruited neonates.
3.Results
3.1.Characteristics of participants
A total of 66 neonates scheduled for arterial blood sampling were included in the initial sample.Six neonates were excluded due to failure to pass hearing screening and/or technical problems.Thus,60 neonates were included in the final analysis(experimental group:n=29;control group:n=31).The flowchart in Fig.2 shows patient enrollment,allocation,and analysis numbers.Gestational age was 37-41 weeks,postnatal age at the study time was 1-24 days,and 38(63%)were male.There were no significant differences in baseline characteristics between the two groups.Table 1 shows the characteristics of the final sample of neonates.No adverse events occurred that were related to the intervention.
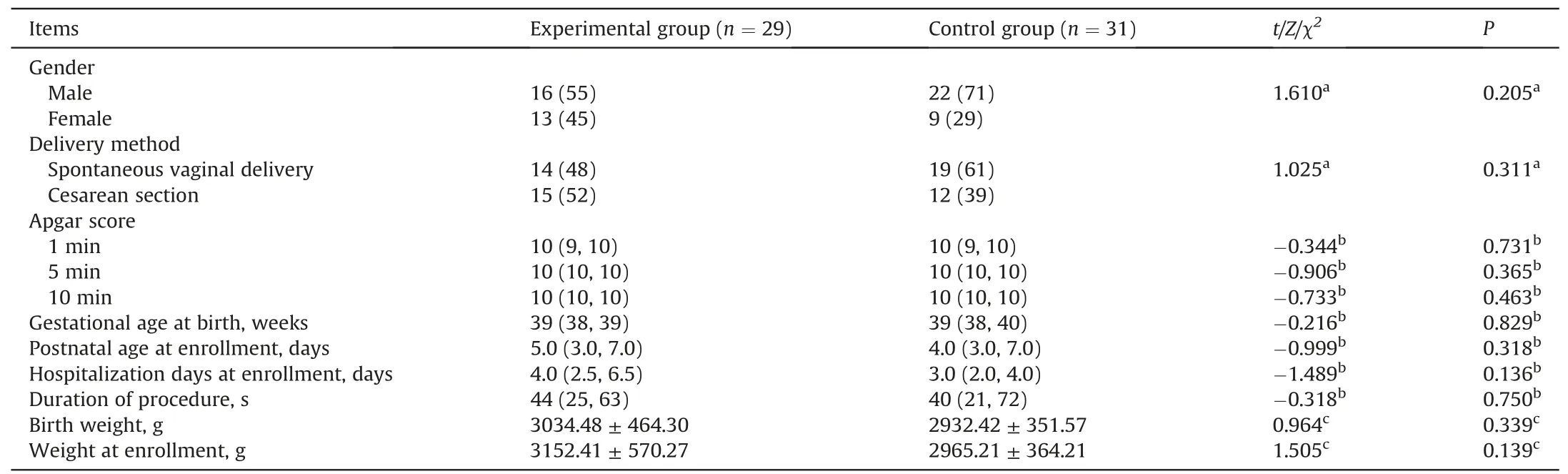
Table 1 Characteristics and clinical data of neonates.
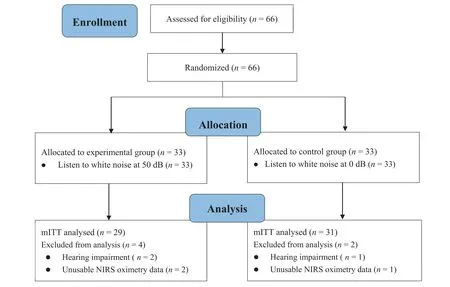
Fig.2.Flow diagram.
3.2.Pain score
The ICCs of the PIPP-R score between two assessors during arterial puncture was 0.891,and during 5 min after the needle withdrawal ranged from 0.801 to 0.953(Appendix A).As shown in Table 2,the maximum PIPP-R score in the experimental group was lower than the control group,but the difference between the two groups was not statistically significant (median difference -0.5,95%CI-2.0 to 0.5,Z=-0.885,P=0.376).There was a statistically significant difference noting the time effect,but no significant difference in group effect and interaction effect in PIPP-R score during the 5 min after needle withdrawal(Table 3).The graphical trend for the estimates of the PIPP-R score derived from the generalized linear mixed-effect model was presented in Fig.3.The PIPP-R score gradually decreased towards baseline over time in both groups by 5 min after the needle withdrawal.Notably,although the differences were not statistically significant,the trend of PIPP-R score in the experimental group decreased to almost 6 points faster than in the control group.
3.3.rScO2
There was no significant difference between the two groups in the baseline rScO2(t=0.406,P=0.666).As shown in Table 2,both groups demonstrated significant decreases in rScO2after the needle penetrated the skin (P <0.01).However,the minimum rScO2did not differ significantly between two groups (mean difference -0.325,95% CI -1.382 to 0.732;P=0.540).There was still no significant difference in the mean rScO2during arterial puncture between two groups (mean difference -0.138,95%CI -1.249 to 0.972;P=0.804).There was a statistically significant difference noting the time effect,but no significant difference in group effect and interaction effect in rScO2during 5 min after the needle withdrawal(Table 4).The graphical trend for the estimates of the rScO2derived from the generalized linear mixed-effect model was presented in Fig.4.The values of rScO2gradually recovered towards baseline over time in both groups during the 5 min after the needle withdrawal.Notably,although the differences were not statistically significant,the trend of rScO2in the experimental group returned to baseline levels more rapidly and steadily than that of the control group.
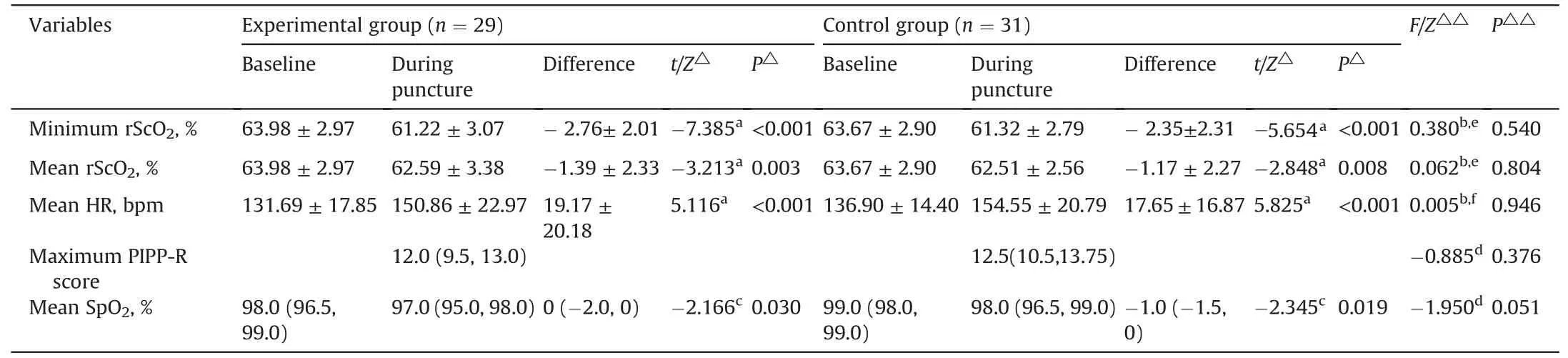
Table 2 The rScO2, PIPP-R score,HR,and SpO2 before and during arterial puncture in neonates.
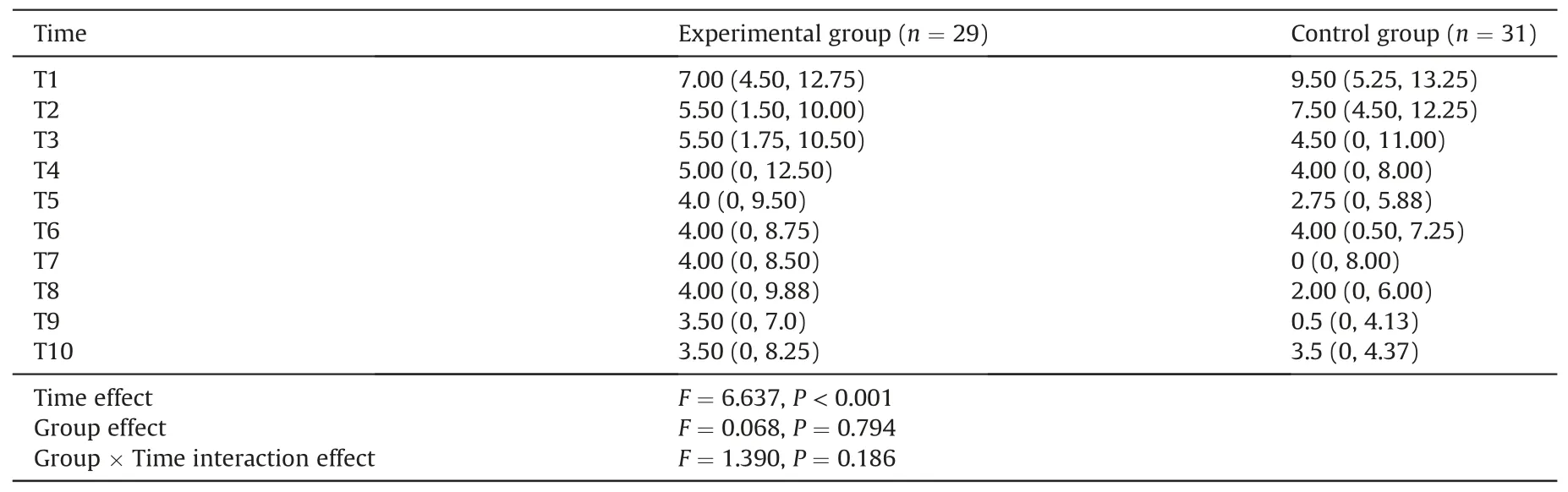
Table 3 Comparison for PIPP-R scores during the 5 min after needle withdrawal.
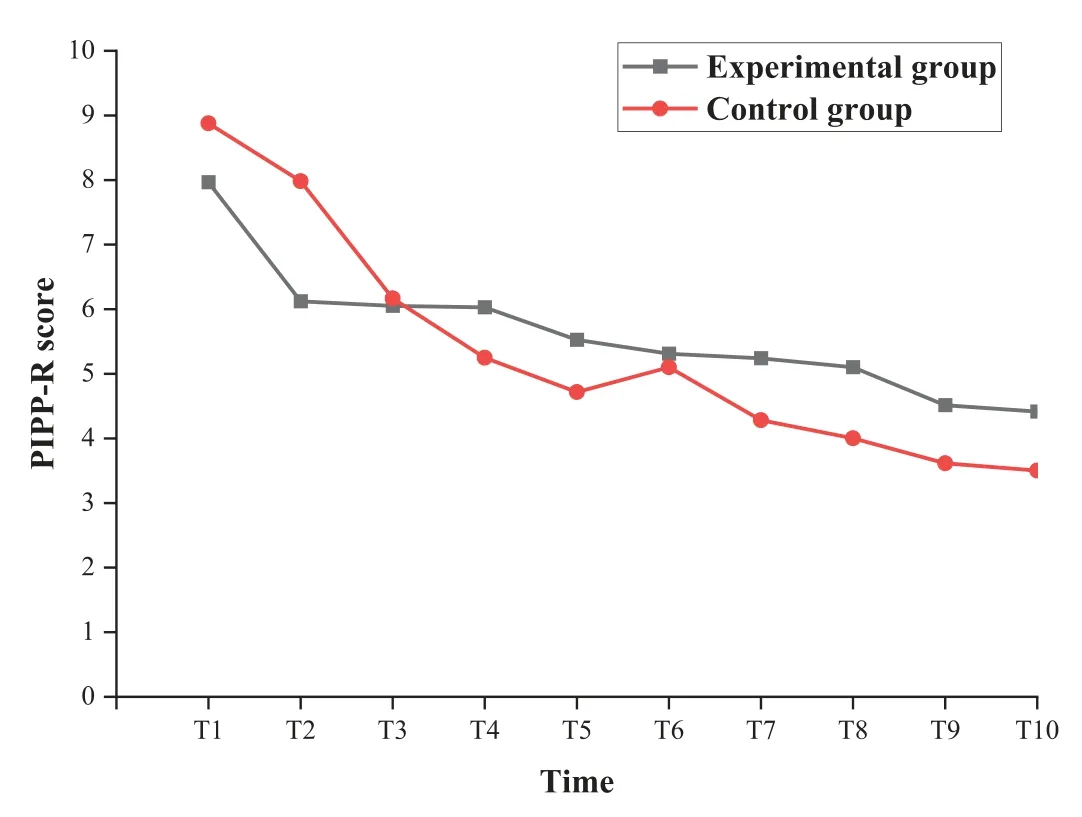
Fig.3.Graphical trend for the estimates of the PIPP-R score derived from the generalized linear mixed-effect model.The PIPP-R score was assessed every 30 s during the 5 min after the needle withdrawal,and the 10 assessment time points were labeled as T1 to T10,respectively.PIPP-R=premature infant pain profile.
3.4.HR and SpO2
There were no significant differences between two groups in baseline HR (t=-1.250,P=0.216),and SpO2(Z=-7.915,P=0.056).As shown in Table 2,both groups showed a significant decrease SpO2(P <0.05),and increase in HR (P <0.001) during arterial puncture.However,the difference in the HR (mean difference 0.33,95% CI -9.32 to 9.98,P=0.946) and SpO2(median difference -1,95% CI -2 to 0,P=0.051) during arterial puncture was not significant between two groups.
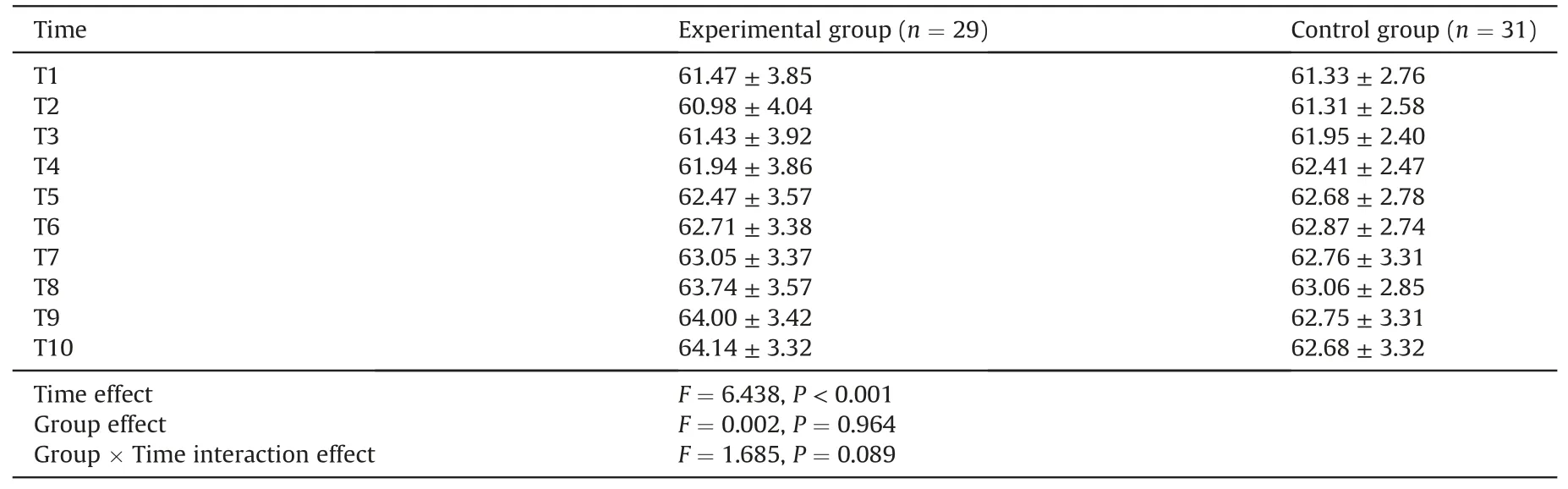
Table 4 Comparison for rScO2 during the 5 min after needle withdrawal.
3.5.Duration of painful expressions
Although the duration of painful expressions in the experimental group (median [IQR],58.00 [33.50,135.00] s) was shorter than the control group(median[P25,P75],64.00[43.00,156.00]s),it was not statistically significant (median difference-9,95%CI -37 to 18,Z=-0.474,P=0.635).
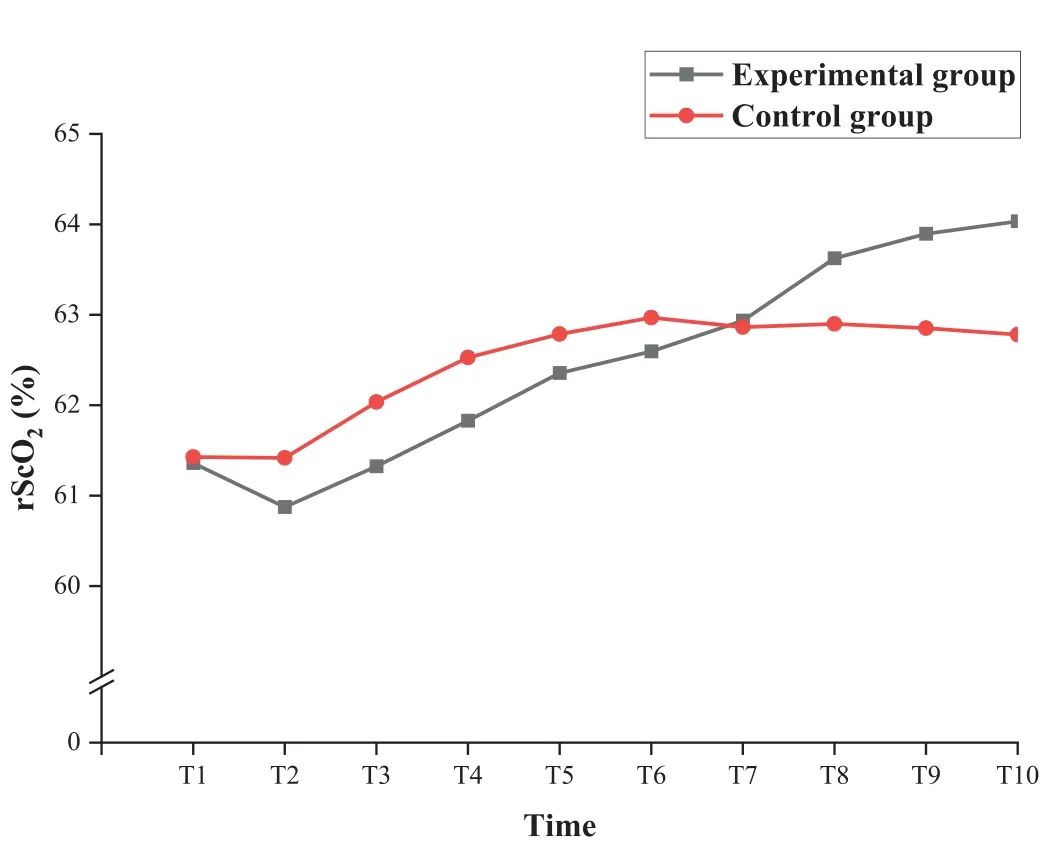
Fig.4.Graphical trend for the estimates of the rScO2 derived from the generalized linear mixed-effect model.
The rScO2was assessed every 30 s during the 5 min after the needle withdrawal,and the 10 assessment time points were labeled as T1 to T10,respectively.rScO2=regional cerebral oxygen saturation.
4.Discussion
This double-blind randomized controlled study measured the effect of listening to white noise 2 min before,during,and within 5 min after the painful procedure in neonates.There were no significant differences in cortical response (rScO2) as well as PIPP-R score,physiological parameters (HR,SpO2),and behavioral parameters (duration of painful expressions).
Pain scales based on the observations of behavioral and/or physiological indicators (HR,SpO2) are commonly used measurements to evaluate the effects of non-pharmacological intervention for neonatal pain [34].A clinically significant reduction in PIPP/PIPP-R scores was defined as two points in previous studies[35,36].In this study,both the nurses who performed the arterial puncture and the assessors were blinded to the group allocation.The results showed that neonates in both experimental and control groups may experience moderate to severe intensity of pain during the arterial blood sampling(median PIPP-R score was 12 and 12.5,respectively).The reduction of 0.5 points in the PIPP-R score during arterial puncture in the experimental group has no clinical significance,but the PIPP-R score in the experimental group decreased close to mild pain faster than in the control group after the needle withdrawal.These results were similar to a single-blind study in which the assessor was blinded to the group allocation during endotracheal suctioning [14].Taplak &Bayat et al.divided 86 premature infants randomly into four groups and provided breast milk smell,white noise,facilitated tucking,and usual care [14].Results showed that the PIPP-R score(median,11.5,10,8,12,respectively),HR,and SpO2were not different during the procedure.
The results of this study were inconsistent with the following unblinded studies in terms of pain score[8,10,13,26,29].Kucukoglu et al.performed an unblinded randomized controlled study to evaluate the effect of white noise intervention for relieving procedural pain caused by vaccination in premature infants,finding that the mean PIPP score in the experimental group was 8.14 points lower than 14.35 points in the control group,statistically significant[10].In the other unblinded trials,white noise intervention was applied during heel lance,the consistent conclusion was drawn that white noise intervention was effective in reducing the pain score [8,13,26].However,the pain scales used were based on the assessor's subjective judgment of the behavioral and/or physiological parameters.None of these studies mentioned the blinding of the procedure performers and/or pain assessors.The unblinded study design and pain score as the primary outcome may lead to an overestimation of the effect.The findings of the above studies suggest that white noise may be a potential soothing intervention to help neonates recover from the painful procedure,but it did not show significant effects in reducing pain scores in this more rigorous double-blind study.
It is of great importance to evaluate the effect of interventions on pain-related neurobiology-based parameters in neonates[4].In the present study,there were significant changes in both groups in rScO2after the needle penetrating the skin,which was similar to a previous study [37].Painful stimuli in neonates activated nociceptive pathways from the periphery to the cortex,as a result,the cerebral oxygen consumption increased and the rScO2decreased.The findings show that both maximum and mean reductions from baseline in rScO2were not different between the two groups during the procedure,indicating that listening to white noise from 2 min before the painful procedure does not reduce the cortical response during the procedure.However,the trend of rScO2in the experimental group returning to baseline more rapidly and steadily after the needle withdrawal is noteworthy.White noise intervention may relieve pain by regulating cognition and/or promoting the release of endorphins from the brain [38,39].In previous studies,white noise was provided from 5 s to 15 min before painful procedures and continued during and after the procedures[14,29],but those studies did not measure pain-related neurobiology-based parameters in neonates.In this study,white noise intervention was provided for neonates from 2 min before the procedure and continued until 5 min after its completion.The duration of white noise in this study may be not long enough to show clinically significant effects.Further research is needed to determine the effectiveness of playing white noise on pain-related neurophysiological parameters with a longer duration before and after a painful procedure.
In our study,HR increased and SpO2decreased significantly during the procedure in both groups.These findings indicate the neonates experienced pain triggered by the arterial blood sampling,which caused excitement of the sympathetic nervous system leading to a short-term increase in HR and oxygen consumption.However,there was no statistical difference between the two groups,which was consistent with the results of Taplak &Bayat et al.[14],but inconsistent with the results of Kahraman et al.[13].In the study conducted by Kahraman et al.,sixty-four neonates were randomly assigned to four groups:white noise,recorded mother's voice,MiniMuffs,and control.Results showed that the pain score and HR of the premature neonates in the white noise group were significantly lower than in the control group,while mean SpO2in the white noise group was higher than in the control group[13].The following speculations may explain these apparent conflicting results:HR and SpO2are not sensitive and specific indicators to evaluate the effects of interventions in neonatal pain relief;and the effect of white noise is not strong enough to reduce the impact of painful stimuli on HR and SpO2during the procedure.
The average duration of painful expression in the experimental group was shorter than in the control group.Although the difference is not statistically significant,it also suggests that the white noise intervention may contribute to neonatal behaviors recovering from a painful procedure.Future studies are needed with large sample size and longer white noise intervention to determine its effect on the duration of painful expression caused by procedural pain.
5.Limitations
There are limitations in this study.First,the relatively small sample size estimated with the expected effect size was not powered enough to observe subtle effects that white noise might have on procedural pain in neonates,additional double-blind randomized controlled studies with larger samples are needed.Second,this study evaluated the effect of white noise in neonates only from 2 min before and during the procedure,and within 5 min after the needle withdrawal.Further studies are needed to investigate the effect of white noise on pain-related parameters starting earlier before the painful stimulation and continuing longer after they return to the baseline.Third,due to the small head size of the included neonates,the two-optode NIRS with an optimum placement was used in this study to evaluate the effect of white noise on neonatal regional cortical response during and after the arterial blood sampling.A multi-channel NIRS with smaller optodes would be more appropriate for larger areas of cortical response in future studies.
6.Conclusion
Listening to white noise before and during a painful procedure did not show beneficial effects on pain-related cortical response,pain score,and behavioral and physiologic parameters in neonates.Though not statistically significant,the level of rScO2,pain score,and duration of painful expression in the experimental group showed a trend to return to baseline faster than in the control group,implying the potential soothing effect of white noise in neonates with procedural pain.Additional double-blind randomized controlled studies are needed to determinate the effects of white noise on procedural pain in this vulnerable population.
Data availability statement
The datasets in the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Xuyan Ren:Conceptualization,Methodology,Formal analysis,Investigation,Writing-original draft,Writing-review&editing,Funding acquisition.Li Li:Conceptualization,Methodology,Writing -original draft,Writing -review &editing,Funding acquisition.Siya Lin:Investigation,Writing -review &editing.Chunxia Zhong:Resources,Writing-review&editing.Bin Wang:Resources,Writing -review &editing.
Declaration of competing interest
The authors have declared no conflict of interest.
Funding
This work was supported by grants from Guangdong Nurse Association [gdshsxh2021a058] and Department of Science and Technology of Guangdong Province [2014A020212396].
Acknowledgments
The authors would like to thank all the medical and nursing staff in the Neonatal Intensive Care Unit at Zhujiang Hospital,Southern Medical University for their kind assistance with data collection.Special thanks to Ms.Cuilan Zhang and Ms.Liping Diao for their support to the study.The authors are grateful to Dr.Keela Herr from The University of Iowa for her helpful comments and revision to the manuscript,and Dr.Ying Wu from Department of Biostatistics,Southern Medical University for her support in statistic analysis.To those neonates and their family who participated in this study,the authors extend special thanks.
Appendices.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2022.06.007.
杂志排行
International Journal of Nursing Sciences的其它文章
- Preventive strategies for feeding intolerance among patients withsevere traumatic brain injury:A cross-sectional survey
- Effects of multidisciplinary exercise management on patients after percutaneous coronary intervention:A randomized controlled study
- Implementation strategies to improve evidence-based practice for post-stroke dysphagia identification and management:A before-andafter study
- Development and validation of a rapid psychosocial well-being screening tool in patients with metastatic breast cancer
- The relationship between acceptance of illness and quality of life among men who have sex with men living with human immunodeficiency virus:A cross-sectional study
- Implementation and evaluation of the peer-training program for village health volunteers to improve chronic disease management among older adults in rural Thailand
