Effect of High Pressure Processing and High-Temperature Short-Time Sterilization on the Quality of Sea Buckthorn Juice
2022-07-29YANGPeiqingWANGYongtaoWUXiaomengGENGHongyeLIAOXiaojunZHAOLiang
YANG Peiqing, WANG Yongtao, WU Xiaomeng, GENG Hongye, LIAO Xiaojun, ZHAO Liang,2,*
(1. National Engineering Research Center for Fruit and Vegetable Processing, Key Laboratory of Fruit and Vegetable Processing of Ministry of Agriculture and Rural Affairs, Beijing Key Laboratory for Food Non-thermal Processing, College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China; 2. Xinghua Industrial Research Center for Food Science and Human Health, China Agricultural University, Xinghua 225700, China)
Abstract: In order to select sea buckthorn (SBT) cultivars suitable for juice making, this study characterized the physicochemical properties of SBT berries from three cultivars (‘Chinese SBT’, ‘Shengguo NO.1’, ‘Shenqiuhong’) in China. It was found that ‘Chinese SBT’ showed the highest superoxide dismutase (SOD) activity ((1 029.14 ± 77.72) U/g),total phenolic content ((8.37 ± 0.20) mg gallic acid equivalent (GAE)/g), and antioxidant capacity ((11.04 ± 0.27) and(6.06 ± 0.32) mmol Trolox equivalent (TE)/100 g determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging and ferric-reducing/antioxidant power (FRAP) assay, respectively). The juice of ‘Chinese SBT’ concentrated to an over 2.5 increase in SOD activity could be developed as a functional product. The quality of SBT juice processed by high pressure processing (HPP, 500 MPa/6 min) or high-temperature short-time sterilization (HTST, 100 ℃/15 s) was comparatively evaluated. Total aerobic viable count was reduced by more than 3 (lg(CFU/mL)) by HPP and HTST. Neither yeasts nor molds were detected in the treated samples. Moreover, both treatments could increase the SOD activity whereas the HPP-treated samples exhibited higher SOD activity during storage. HPP and HTST well retained total phenols, L-ascorbic acid,and antioxidant capacity. These quality characteristics were well preserved during storage at 4 ℃. Therefore, HPP- or HTST-treated SBT juice can be developed as a functional product with high antioxidant and SOD activity.
Keywords: sea buckthorn juice; antioxidant capacity; antioxidant compounds; high pressure processing sterilization;high-temperature short-time sterilization
Sea buckthorn (L.) (SBT) is a thorny nitrogen-fixing deciduous shrub of cold arid region native to Europe and Asia. SBT berries is rich in antioxidant compounds, such as vitamins, flavonoids, carotenoids,polyunsaturated fatty acids, in which-ascorbic acid is the highest in the plant kingdom. Moreover, superoxidative dismutase (SOD, EC 1.15.1.1), which plays a vital antioxidant role in human health by scavenging superoxide anion radicals, also shows a high level in SBT berries.As SOD derived from animal blood may exhibit some biological pollution issues, plant-derived SOD appears to be a reasonable choice for food application. Thus, SBT berries have gained attention for their high nutritional and medicinal potentials. Considering that the soft and fragile berries of SBT were difficult to transport and store, the objective of this study is to develop a novel process for SBT juices with antioxidant functions.
For the processing of fruit and vegetable juices, a thermal pasteurization technology has been mostly employed for achieving microbiological safety, especially high temperature short time (HTST) involving performing heating juices in continuous flow systems at > 70 ℃ for 5–15 s. Even though HTST could effectively inactivate microorganisms,the employment of high temperature may induce the degradation of some heat-sensitive compounds, leading to the organoleptic and nutritional quality deterioration of juices.To meet the consumer demand for healthy, nutritious, and fresh-like qualities of juices, non-thermal processing has grown continuous improvements, in which high pressure processing (HPP) has been successfully industrial and shows promising application prospects. HPP implies subjecting food to the hydrostatic pressure of 100 to 600 MPa at ambient or lower temperatures to achieve microbial inactivation.As HPP only affects noncovalent bonds, most low molecular weight compounds involving color, flavor, bioactivity, are retained well. Furthermore, HPP can induce the alternation of the spatial structure of macromolecules, such as proteins and starch, which may trigger their bioactivity changes. Some previous studies found that HPP could activate the SOD activity in sliced ham cutand chestnut rose. Since SBT shows high antioxidant contents and SOD activity, employing HPP may achieve well maintenance or even enhancement of the antioxidant capacities.
The other objective of this study was to compare the antioxidant capacity and physicochemical qualities of three cultivars of SBT berries, including ‘Chinese SBT’, ‘Shengguo No. 1’ and ‘Shenqiuhong’, and select the suitable cultivar for juicing. Then, a portion of SBT juices were concentrated to obtain high SOD product, and the other portion of nonconcentrated juices were produced as not-from-concentrate(NFC) SBT juice. Then, the microbiological safety,antioxidant capacity and physicochemical qualities of SBT juices were evaluated immediately after HPP or HTST treatment, and during 31 days storage at 4 ℃.
1 Materials and Methods
1.1 Materials and chemicals
Three cultivars of SBT berries (‘Chinese SBT’,‘Shengguo No. 1’ and ‘Shenqiuhong’) were purchased from Jintudi Co., Ltd. (Inner Mongolia, China). ‘Chinese SBT’ was edible wild cultivar in China, ‘Shengguo No. 1’was bred from ‘Chinese SBT’ and ‘Mongolian SBT’, and‘Shenqiuhong’ originated from Russia. ‘Shengguo No. 1’and ‘Shenqiuhong’ were harvested in mid-August 2017,while ‘Chinese SBT’ was harvested in early October 2017,in Ordos, Inner Mongolia, China. After harvest, they were frozen immediately, transported through cold chain and stored at -20 ℃.
Pectinase (Pectinex BE XXL, 13 600 U/mL) Novozymes Biotechnology Co., Ltd., Tianjin, China; Folin-Ciocalteu reagent Beijing Solarbio Science & Technology Co., Ltd.;-ascorbic acid of high-performance liquid chromatography(HPLC)-grade, (±)-6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (Trolox), 1,1-diphenyl-2-picrylhydrazyl (DPPH)Sigma-Aldrich, Inc., St. Louis, MO, USA; Glucose and fructose of HPLC-grade Aladdin Biochemical Technology Co., Ltd.,Shanghai, China; Oxalic acid, malic acid, tartaric acid of HPLC-grade Yuanye Biotechnology Co., Ltd., Shanghai,China; Plate count agar and Rose Bengal agar Beijing Land Bridging Technology Co., Ltd.; Other chemicals were purchased from Beijing Chemicals Co., Ltd..
1.2 Instrumental and equipment
CQC30L-600 HPP machine Beijing Suyuan Zhongtian Scientific Ltd.; FT74 HTST/UHT processing unit Armfield Ltd., UK; LC-20A HPLC, UV-1800 spectrophotometer Shimadzu Corporation, Japan; WAY-2S Digital Abbe refractometer Shanghai Precision &Scientific Instrument Co., Ltd.; Color Quest XE colorimeter Hunter Laboratory, USA; PB-10 pH meter Sartorius Co.,Ltd., USA; JYZ-E19 slow juicer Jiuyang Co., Ltd., China;RE-5210A vacuum-rotary evaporator Shanghai Yangrong Biochemistry Instrument Factory.
1.3 Methods
1.3.1 Process of SBT juice production
1.3.1.1 Preparation of SBT juices
Frozen SBT berries were thawed in the shade for 24 h at 4 ℃, and cleaned with tap water. Then, the SBT berries were pulped with a slow juicer at 20 000 r/min for 1 min and depectinized by pectinase at 50 ℃ and 250 mg/L for 2 h. After filtration with double layers of gauze, a portion of the juice was concentrated to 2.2 times by vacuumrotary evaporation at 50 ℃ and –0.1 MPa. Finally, the nonconcentrated and concentrated juices were divided into two portions: one portion was filled into 60 mL polyethylene terephthalate (PET) bottles, sealed and kept at 4 ℃ until treated by HPP within 3 h; the other portion was kept at 4 ℃until treated by HTST within 3 h.
1.3.1.2 HPP and HTST treatments of SBT juices
HPP treatment: The juices were processed at 500 MPa for 6 min and then stored at 4 ℃ for 31 days. The highest temperature in the vessel was estimated to be approximately 40 ℃ and dropped quickly to ambient temperature after pressurization. The pressurization rate was approximately 180 MPa/min and the depressurization time was less than 5 s.Distilled water was used as the pressure-transmitting fluid.
HTST treatment: The juices were processed at 100 ℃for 15 s, cooled to 20 ℃ immediately, transferred aseptically into PET bottle and stored at 4 ℃ for 31 days.
These processing parameters could ensure the counts of total aerobic bacteria (TAB) in SBT juices less than 2 (lg(CFU/mL)) and ensure yeasts and moulds (Y&M) not detected, according to our previous study.
1.3.2 Microbiological analysis
The plate count method was used to count TAB and Y&M in SBT juice. Plate count agar and Rose Bengal agar were used for counting the TAB and Y&M colonies.The colonies were counted after incubation. The control groups indicated the SBT samples not treated by HPP or HTST, which was only measured at day 0 (the same as other indicators).
1.3.3 Determination of SOD activity
SOD activity was measured according to previous study. Superoxide anion can be generated when riboflavin is exposed to light, which can reduce nitro-blue tetrazolium(NBT) to formazan dye. SOD can inhibit the formation of formazan by scavenging superoxide anion and SOD activity was measured through colorimetric method. NBT solution contained 1.933 mg of methionine (Met), 45.8 mg of NBT, 0.47 mg of riboflavin and 29.0 mg of ethylene diamine tetraacetic acid (EDTA) dissolved in 1 000 mL of 0.05 mol/L phosphate buffered at pH 7.8. Samples were mixed with phosphate buffer, homogenized for 1 min, incubated for 20 min and centrifuged at 10 000 ×for 10 min. The supernatant was diluted to appropriate multiples and mixed with NBT solution,incubated at photochemical reaction chamber (illumination intensity was 4 800 lx) at 28 ℃ for 20 min, and absorbance at 560 nm was measured. All procedures were carried out in the dark except the photochemical reaction. One unit of SOD activity (U) was defined as the amount of SOD required to inhibit the initial rate of the photochemical reduction to 50%.SOD activity was calculated using the equation (1).

Where OD and ODis the absorbance after the photochemical reaction in the presence and absence of SBT samples, respectively;is the dilution ratio.
1.3.4 Determination of antioxidant compounds and antioxidant capacity
1.3.4.1 Determination of total phenols content
Extraction of total phenols and its content were measured using Folin-Ciocalteu reagent according to the procedure described by Liu Fengxia et al. The contents were expressed as gallic acid equivalent (GAE).
1.3.4.2 Determination of-ascorbic acid content
Sample extraction and the HPLC analysis were performed according to the procedure described by Liu Fengxia et al.
1.3.4.3 Determination of antioxidant capacity
The antioxidant activity was determined by evaluating the DPPH radical scavenging capacity and the ferricreducing/antioxidant power (FRAP) according to Liu Fengxia et al. The results were expressed as Trolox equivalent (TE).
1.3.5 Determination of sugars and organic acids content
Sample extraction and the HPLC analysis were performed according to the procedure described by Liu Fengxia et al.
1.3.6 Determination of other physicochemical characteristics
Color assessment was conducted at ambient temperature using a Hunter Lab Color Quest XE colorimeterin reflectance mode. Color was expressed as the follow parameters:*(lightness),* (redness),* (yellowness), Δ(total color difference), Δwas calculated using the equation (2).

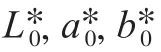
The pH value was measured using a PB-10 pH meter.The total soluble solids (TSS) content were determined as°Brix using a WAY-2S digital Abbe Refraction meter.
1.4 Statistical analysis
All experiments were performed in triplicate. The data were exhibited as the means ± standard deviation and were analysed using the Microcal Origin 2018 software. Analysis of variance and Duncan’s test were carried out and the significance was established at< 0.05.
2 Results and Analysis
2.1 Physicochemical and antioxidant properties of three SBT cultivars
2.1.1 Antioxidant compounds and antioxidant capacity
SOD activities of three cultivars showed significant differences, ranging from 245.27 to 1 029.14 U/g (fresh mass,) (Table 1). ‘Chinese SBT’ showed the highest SOD activity, and ‘Shengguo No. 1’ and ‘Shenqiuhong’ only exhibited approximately 50% and 25% of ‘Chinese SBT’, respectively.Consistently, Hou Zhiqiang et al.reported the SOD activity of SBT juices showed 1 427.5 U/mL using NBT method.

Table 1 Antioxidant compounds and antioxidant capacity in three SBT cultivars
Total phenols content in three cultivars exhibited significant difference, ranging from 2.28 to 8.37 mg GAE/g(Table 1). ‘Chinese SBT’ showed the highest total phenols content, followed by ‘Shengguo No. 1’ and ‘Shenqiuhong’.As reported in the previous studies, total phenols content were 3.44 mg GAE/gin SBT berries from Romania, and ranged from 2.13 to 2.62 mg GAE/gin seven genotypes of SBT berries from Turkey, which were consistent with the results in this study. However, total phenols content varied from 8.62 to 14.17 mg GAE/gin six cultivars of SBT berries from Russia, Germany and the Czech Republic, and Guo Ruixue et al.also observed that total phenols showed 16.00 mg GAE/gin SBT berries from China, which is higher than the results in this study.
-ascorbic acid content in three cultivars also showed significant difference, ranging from 22.05 to 232.53 mg/100 g(Table 1). ‘Shengguo No. 1’ exhibited the highest-ascorbic acid content, followed by ‘Chinese SBT’ and ‘Shenqiuhong’. Consistently,-ascorbic acid ranged from 28 to 85 mg/100 gin seven genotypes of SBT berries from Turkey, and varied from 52.86 to 130.97 mg/100 gin eight cultivars of SBT berries from Poland. However, six cultivars of SBT berries from Russia,Germany and the Czech Republic showed higher-ascorbic acid content, ranging from 394 to 573 mg/100 g. An Xiongtaofound that the-ascorbic acid in ‘Chinese SBT’is much higher than that in other cultivars, which is consistent with this study, whereas the content (859.6 mg/100 g) is higher than that in this study due to different determination method. Zheng Jie et al.also found that the ascorbic acid in wild SBT from China ranged from 250 to 1 660 mg/100 g,which increased with the altitude of the growth place increased and as the latitude decreased.
All the differences of these above-mentioned antioxidant compounds were possibly ascribed to different cultivars and climates, agricultural practices, ripeness, countries,years, and other regional conditions, as well as the different determination methods. The different content of these compounds contributed to the differences of antioxidant capacity. The DPPH radical-scavenging capacity of SBT berries ranged from 1.10 to 11.04 mmol TE/100 g(Table 1), which showed significant difference, in which‘Chinese SBT’ showed the highest, followed by ‘Shengguo No. 1’and ‘Shenqiuhong’. Similar results were obtained using FRAP method, ranging from 1.16 to 6.06 mmol TE/100 g.
2.1.2 Contents of sugars, acids, TSS and pH
As shown in Table 2, sugars were mainly identified as glucose (17.41-96.82 mg/g) and fructose (1.30-27.11 mg/g)in three cultivars, and sucrose was not detected in this study.‘Chinese SBT’ showed the highest glucose and fructose,followed by ‘Shengguo No. 1’ and ‘Shenqiuhong’. Glucose content was much higher than fructose content, indicating that glucose was the major reducing sugar in SBT berries in this study. TSS content in ‘Chinese SBT’ (18.27 °Brix)and ‘Shengguo No. 1’ (17.83 °Brix) exhibited no significant difference, and significantly higher than that in ‘Shenqiuhong’(15.47 °Brix), indicating that no direct relation between TSS content and sugar contents and other certain constituents may affect the TSS content. Consistently, Ma Xueying et al.also found that glucose and fructose in six cultivars of SBT berries from Finland and Estonia ranged from 2 to 48 mg/g and from 1 to 24 mg/g, respectively and TSS content ranged from 6.9 to 9.6 °Brix.
Organic acids content in SBT berries were mainly tartaric acid, malic acid, oxalic acid (Table 2) and-ascorbic acid (Table 1), which may contribute more to TSS content than sugars. Tartaric acid content, ranging from 21.21 to 36.80 mg/g, was the highest among organic acids,regardless of the cultivars, followed by malic acid, oxalic acid and-ascorbic acid. The pH value of ‘Chinese SBT’ was significantly lower than other two cultivars due to its highest content of organic acids. However, Ma Xueying et al.observed that malic acid (16-40 mg/g) and quinic acid(7-22 mg/g) were two major organic acids in six cultivars of SBT berries from Finland and Estonia.
As ‘Chinese SBT’ exhibit the highest antioxidant capacity and highest content of antioxidant compounds, it was selected for the subsequent juicing.

Table 2 Sugar contents, organic acid contents, TSS content and pH in three SBT cultivars
2.2 Quality comparison of SBT juices after HPP and HTST and during storage
2.2.1 Microbiological analysis
As shown in Table 3, TAB counts in non-concentrated and concentrated juices were reduced significantly by 3.54 and 3.47 (lg(CFU/mL)) after HPP, and by more than 4 (lg(CFU/mL)) after HTST, respectively. After 31 days storage at 4 ℃, TAB counts increased to 2.16 (lg(CFU/mL)) in HPP-treated non-concentrated juices, and to 2.61 (lg(CFU/mL))in HPP-treated concentrated juices. TAB counts maintained less than 1.00 (lg(CFU/mL)) in the juices treated by HTST.As Y&M counts are sensitive to HPP and HTST, Y&M maintained below the detection level (1.00 (lg(CFU/mL)))after HPP and HTST and during storage in both non-concentrated and concentrated SBT juices.

Table 3 Microbial counts in HPP- or HTST-treated SBT juice during 31-day storage at 4 ℃
HPP and HTST may achieve microbial inactivation through different mechanisms. HPP mainly acts on the disruption of the cytoplasmic membrane. The denaturation of protein or enzymes could be triggered by both treatments. Nucleic acids may be not sensitive to HPP but degrade under high temperatures. The function loss of these biomacromolecules may trigger the metabolism homeostasis perturbation and finally microbial cell death. Besides, the acidic environment in the SBT juice provided an additional hurdle for the microorganisms, enhancing the microbial inactivation and inhibiting their outgrowth.
2.2.2 Antioxidant compounds and antioxidant capacity
As shown in Fig. 1A, SOD activities were increased by 10.74% and 4.94% in non-concentrated juice, 14.37%and 11.78% in concentrated juices after HPP and HTST,respectively. After concentration, SOD activity was increased to more than 2.5 times. Consistently, Hou Zhiqiang et al.observed that SOD activity in SBT juice was significantly increased by 17.13% after HPP (500 MPa/6 min). Clariana et al.also reported that HPP (400 MPa/6 min) could increase the SOD activity of sliced ham cut by 16.1%. The increased SOD activity induced by HPP was attributed to the higher extraction from crushed cells. Moreover, Hou Zhiqiang et al.reported that the activity of SOD purified from chestnut rose was increased by 22.23%–38.02% after HPP(100–500 MPa/0–20 min), and they found that HPP increased the surface hydrophobicity of SOD which shows positive collations with the increased activity. In contrast, Hou Zhiqiang et al.reported that SOD activity in SBT juices decreased significantly by 48.9% after HTST (100 ℃/15 s),indicating HTST inactivated SOD, which is inconsistent with the results of this study. Different from the NBT methods adopted in this study, Hou Zhiqiang et al.used WST methods. WST may be oxidized to formazan, a blue dye, by superoxide radicals generated by xanthine oxidase and hypoxanthine, and SOD could inhibit the formation of formazan, then the SOD activity could be determined by the colorimetric method. The different mechanisms between these two methods may attribute to different results of the effect of HTST on SOD activity. Moreover, the SBT cultivars used in this study show lower pH which may stabilize SOD.During 31 days storage at 4 ℃, SOD activity gradually decreased by around 20%, which may be attributed to protein denaturation and degradation. SOD activity is higher in SBT juices treated by HPP than that treated by HTST during storage, which may be attributed to the better activation effect of HPP.
As shown in Fig. 1B, total phenols content in SBT juices showed no obvious changes after HPP or HTST. HPP retained phenols well, which was consistent with previous studies such as SBT juices, mango nectarsand carrot juices.Moreover, some studies also observed that total phenols contents were increased obviously after HPP in aronia berry purée, and onion. This increase of total phenols contents could be attributed to the higher extraction due to the membrane permeabilization of plant cells induced by instantaneous pressure. For HTST, Zhang Yan et al.reported that total phenols content in carrot juices decreased obviously after HTST (110 ℃/8.6 s), while Huang Wenshu et al.reported it increased by approximately 2 times in apricot nectars after HTST (110 ℃/8.6 s). There may be a balance between the higher extraction and thermal degradation induced by HTST, leading to these differences of total phenol content. Total phenols content decreased by 16.30%and 14.78% in HPP- and HTST-treated non-concentrated juices, respectively, 15.65% and 16.62% in HPP- and HTST-treated concentrated juices during storage, respectively,which may be attributed to the oxidation degradation and the polymerization with proteins.
-ascorbic acid content also showed no obvious changes in SBT juices after HPP or HTST (Fig. 1C). Consistently,Liu Fengxia et al.found that-ascorbic acid content in mango nectars showed no obvious changes after HPP(600 MPa/1 min) and HTST (110 ℃/8.6 s). Patras et al.also reported that HPP (400–600 MPa/15 min) retained the-ascorbic acid well in strawberry and blackberry purées.However, previous studies reported HTST may lead to obvious decrease of-ascorbic acid content in SBT juicesand Korla pear juices. Dhakal et al.also reported that HPP (300–600 MPa/15 min) did not induce loss of-ascorbic acid in pineapple juices, regardless of the applied pressure and holding time, while thermal treatment (75–95 ℃/60 min)induced much degradation.-ascorbic acid is vulnerable to be oxidized to dehydroascorbic acid, a compound without bioactivity, under certain circumstances such as heat, water activity, oxygen, metal ions, endogenous enzyme and alkaline pH.-ascorbic acid content decreased by 37.04% and 36.26% in HPP- and HTST-treated non-concentrated juices,31.80% and 28.43% in HPP- and HTST-treated concentrated juices during storage. The degradation of-ascorbic acid during storage was affected by storage condition, processing method and packaging. In the processing and initial storage time, the degradation of-ascorbic acid was mainly aerobic,and when the oxygen in the headspace of the package was depleted, anaerobic degradation occurred. The rate constants of aerobic degradation were 100–1 000 times higher than that of anaerobic degradation, so the degradation of-ascorbic acid may appear fast-to-slow tendency during long-time storage. The higher retention of-ascorbic acid in the concentrated juices was probably due to the interactions among various antioxidant compounds at higher contents.
HPP and HTST induced no obvious change in antioxidant capacity of SBT juices using the DPPH and FRAP methods (Fig. 1D–E). The antioxidant capacity decreased by 35.54% and 34.91% using DPPH method,25.64% and 16.92% using FRAP method in HPP- and HTST-treated non-concentrated juices, respectively, and by 39.66% and 42.79% using DPPH method, 27.89% and 18.20% using FRAP method in HPP- and HTST-treated concentrated juices during storage, respectively. The decrease of the contents of antioxidant compounds may contribute to the decrease of antioxidative capacities.

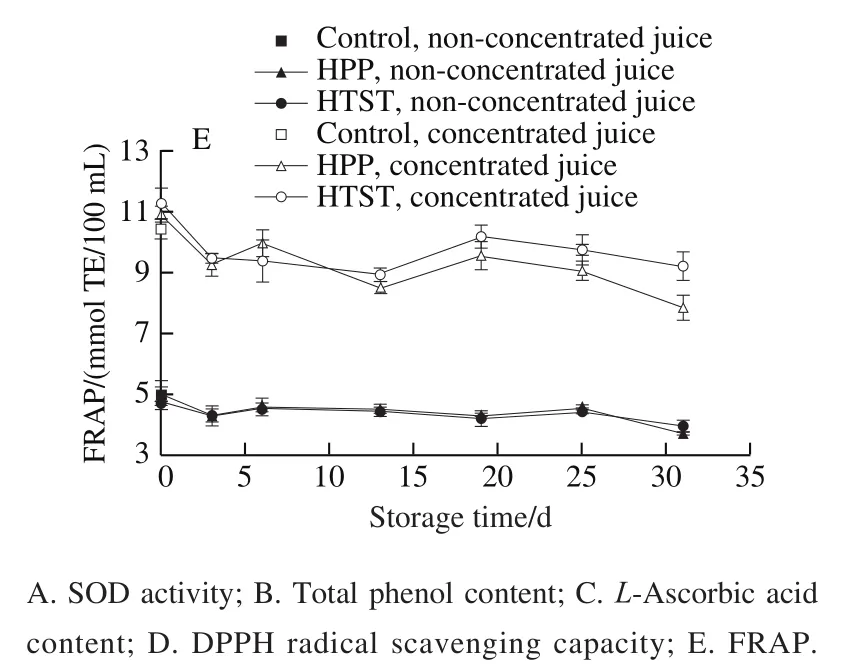
Fig. 1 Changes in antioxidant compounds and antioxidant capacity in HPP- or HTST-treated SBT juice during 31 days of storage at 4 ℃
2.2.3 Main sugars and main organic acids content
For the non-concentrated juices, glucose and fructose contents after HPP and glucose content after HTST did not change, while fructose content decreased obviously after HTST (Fig. 2A, B). For the concentrated juice, glucose content after HPP did not change and fructose content decreased obviously, glucose and fructose contents after HTST decreased obviously. The decrease of sugars content may be related to Maillard reaction induced by HTST and HPP. Fructose content exists to a greater extent in the openchain form than does glucose, so the initial stages of the Maillard reaction occur more rapidly than with glucose.After concentration, sugars in high concentration were more susceptible to Maillard reaction. Glucose and fructose content remained stable with fluctuations during storage. Maillard reaction in this study was inhibited by low temperature of 4 ℃ during storage.
Tartaric acid and malic acid content showed no obvious difference after HPP or HTST (Fig. 2C, E), indicating that HPP and HTST exhibited no effect on the two acids.Oxalic acid content in the non-concentrated juices increased obviously after HPP and HTST, while no obvious change was observed in the concentrated juices (Fig. 2D). During storage, tartaric acid content exhibited a decreasing trend only in concentrated juices, malic acid and oxalic acid in nonconcentrated juice and concentrated juice fluctuated to some extent or increased.
TSS content and pH showed no obvious change after the HPP and HTST, and during storage (data not shown).
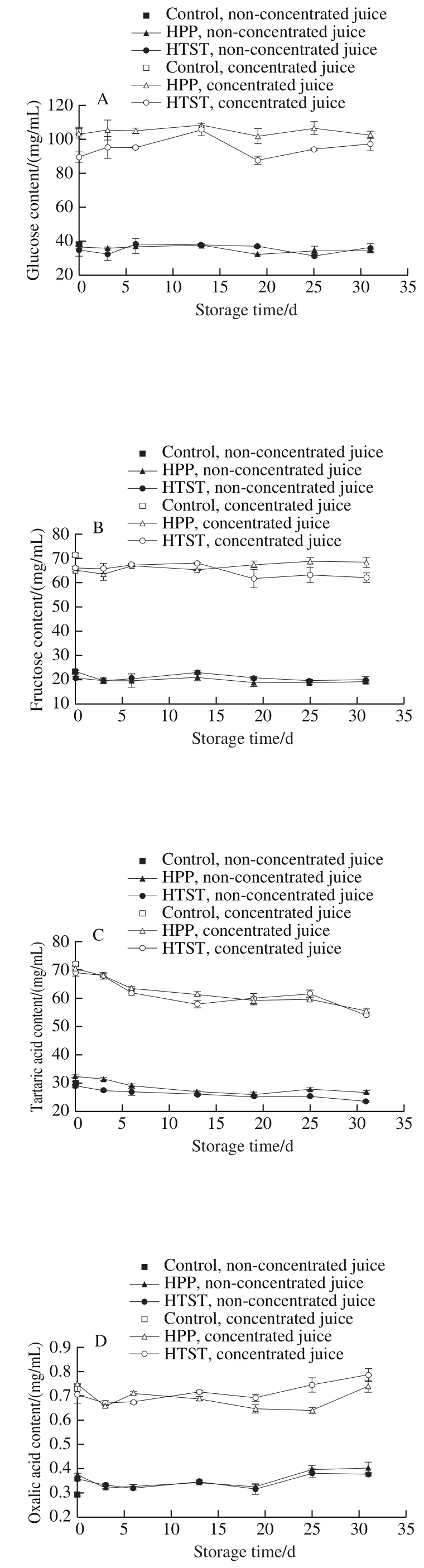

Fig. 2 Changes in major sugars and organic acids in HPP- or HTST-treated SBT juice during 31 days of storage at 4 ℃
2.2.4 Color parameters
No obvious changes of*,*, andvalues of SBT juices were observed after HPP or HTST (Fig. 3). Δvalues were less than 2.00, indicating that HPP and HTST did not lead to visual color changes.*,* and* value exhibited slight fluctuations but remained constant during storage.Δvalues during storage were less than 2.5, indicating that the color of SBT juices was well preserved. Acidic environment and high level of antioxidant compounds in SBT juices may effectively inhibit pigment degradation and color deterioration.
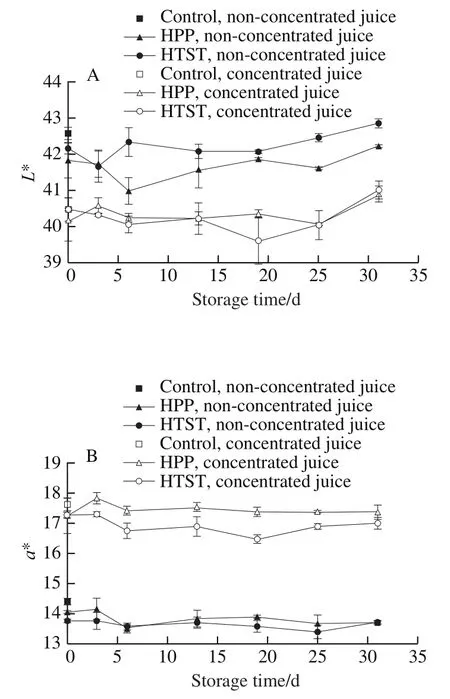

Fig. 3 Changes in color parameters in HPP- or HTST-treated SBT juice during 31 days of storage at 4 ℃
3 Conclusion
‘Chinese SBT’ was selected for juicing due to its high antioxidant capacity and contents of antioxidant compounds.HPP and HTST achieved significant microbial inactivation and ensured the microbiological safety of SBT juices during 31 days storage at 4 ℃. HPP and HTST could both increase the SOD activity whereas HPP-treated samples exhibited higher SOD activity during storage. HPP and HTST could retain contents of total phenols,-ascorbic acid, glucose,tartaric acid, malic acid, antioxidant capacity, and color and quality characteristics were well preserved during storage.As a result, the HPP and HTST-treated SBT juices performed well as antioxidant NFC juices. The high SOD activity in SBT juices, together with enzyme activation effect by HPP and enrichment effect of concentration process, has the potential to be utilised to generate functional products with high SOD activity.
