Ginger-indirect moxibustion plus acupuncture versus acupuncture alone for chronic fatigue syndrome:a randomized controlled trial
2022-07-28MATingtingWUJieYANGLijieFENGFenYANGHuilinZHANGJinhuaZHONGYanjinNINGQingHUANGLirongLINYoubingYANJueCHENGuiquanHOUTianshuWANGLiRENYuanfangTANJing
MA Tingting,WU Jie,YANG Lijie,FENG Fen,YANG Huilin,ZHANG Jinhua,ZHONG Yanjin,NING Qing,HUANG Lirong,LIN Youbing,YAN Jue,CHEN Guiquan,HOU Tianshu,WANG Li,REN Yuanfang,TAN Jing
MA Tingting,WU Jie,YANG Lijie,FENG Fen,Center of Preventive Medicine,Hospital of Chengdu University of Traditional Chinese Medicine,Chengdu 610075,China
YANG Huilin,ZHANG Jinhua,ZHONG Yanjin,NING Qing,HUANG Lirong,LIN Youbing,REN Yuanfang,College of Acupuncture and Tuina,Chengdu University of Traditional Chinese Medicine,Chengdu 610075,China
YAN Jue,CHEN Guiquan,Department of acupuncture,Center of Preventive Medicine,Affiliated TCM Hospital of Southwest Medical University,Luzhou 646600,China
HOU Tianshu,WANG Li,Center of Preventive Medicine,Chengdu Integrated TCM and Western Medicine Hospital,Chengdu 610021,China
TAN Jing,Center of Chinese Evidence-Based Medicine,Sichuan University West China Hospital,Chengdu 610041,China
Abstract OBJECTIVE:To assess the efficacy and safety of gingerindirect moxibustion for chronic fatigue syndrome (CFS).METHODS:In this central randomized,controlled trial,290 CFS participants were recruited and randomly allocated to group A (ginger-indirect moxibustion plus acupuncture) or group B (acupuncture alone).The study consisted of a treatment period of 8 weeks with a total of 24 treatments (3 sessions per week,every other day),and a follow-up period of 12 weeks.The outcome was measured by Fatigue Severity Scale (FSS),Psychological Health Report (SPHERE),the Self-rating depression scale (SDS) and the Hamilton anxiety scale(HAMA) at baseline,2,4,6,8,12 and 20 weeks.RESULTS:With the treatment undergoing,the changes of FSS,SPHERE,SDS and HAMA scores in both groups increased gradually,and the effect maintained at the 12thweek.Between groups,significantly higher score changes were seen in group A in FSS after 4 weeks treatment (11.94 vs 9.12,95%CI:0.94,4.7) and in SPHERE after 2 weeks treatment (3.7 vs 2.27,95%CI:0.56,2.31).But for SDS and HAMA,the improvement did not differ significantly between groups.No severe adverse events were reported.CONCLUSION:Ginger-indirect moxibustion is a safe and effective intervention to relieve fatigue and accompanying physical symptoms of CFS.
Keywords:ginger-indirect moxibustion;fatigue syndrome,chronic;treatment outcome;safety;randomized controlled trial
1.INTRODUCTION
Chronic Fatigue Syndrome (CFS) is the presence of debilitating fatigue which lasts for a minimum of six months and is not relieved by rest,accompanied by nonspecific symptoms,1such as sleep disturbance and pain.The prevalence of CFS is estimated 40% globally,while most of suffers are young and women.2CFS affects patients’ working and body condition,and hence places burden on society.Due to the unclear pathophysiological mechanisms of CFS,3no specific pharmacological treatment is recommended and patients may benefit from alternative therapies such as acupuncture,4yoga,5-7cognitive behavior therapy or graded exercise therapy.Moxibustion treatment is a method of direct or indirect warm stimulation on acupoints by burning mugwort.According to the theory of Traditional Chinese Medicine(TCM),Yang Qideficiency is the main reason for fatigue,and moxibustion is widely used for fatigue relief due to its warm effect.8,9However,systemic reviews based on RCTs didn’t draw firm conclusion on the effectiveness of moxibustion in the treatment of CFS,due to the limited and poor quality of researches.
For CFS patients,ginger-indirect moxibustion on Governor Vessel (GV,Dumai) is regarded as one of the best approaches in TCM,with the advantage of strongYang-warming function.Therefore,we designed a randomized controlled trial to investigate the effect and safety of ginger-indirect moxibustion by comparing ginger-indirect moxibustion plus acupuncture with acupuncture alone.For the clinical operability,moxa box moxibusion over ginger slices was applied from Dazhui(GV14) to Yaoshu (GV2) as well as the same level of 1st bladder meridian on the back to reduce burning and enhance therapeutic effect.
2.METHODS
2.1.Design
This randomized,controlled trial was conducted at the Center of Preventive Medicine of Hospital of Chengdu University of TCM,the Center of Preventive Medicine of Chengdu Integrated TCM and Western Medicine Hospital,the Center of Preventive Medicine and Department of acupuncture of Affiliated TCM Hospital of Southwest Medical University,and the Center of Preventive Medicine of Deyang People’s Hospital,between February 2016 and December 2017.This trial followed the rules of the Declaration of Helsinki and the Good Clinical Practice Guidelines,with unique registration number (ChiCTR-INR-16007810) at http://www.chictr.org/cn/.The protocol was approved by the Ethics Committee of Teaching Hospital of Chengdu University of TCM in 2015 (No.2014KL-018).Written informed consent will be obtained from every patient before participation.Patients were informed that a single acupuncture therapy or a combined therapy of acupuncture and moxibustion would be compared,and no matter which therapy they would receive,there would be positive effective.
The study consisted of a 7-day run-in period prior to randomization,a treatment period of 8 weeks with a total of 24 treatments (3 sessions per week,every other day),and a follow-up period of 12 weeks.After characteristic laboratory abnormalities exclusion in the run-in period,CFS patients were randomly allocated to group of ginger-indirect moxibustion plus acupuncture (treatment group,group A),or acupuncture (control group,group B)in a 1∶1 ratio by central randomization.
2.2.Participates
Adult patients aged 18 to 55 meeting the CFS diagnostic criteria were recruited.The criteria was (a) chronic fatigue should be lasting and can’t be explained by illness,(b) debilitating fatigue predated and was accompanied by at least 4 of 8 designated symptoms which persisted or recurred during 6 or more consecutive months of illness:post-exertional malaise lasting more than 24 h;unrefreshing sleep;impaired short-term memory or concentration which is severe enough to cause substantial reduction in previous levels of occupational,educational,social,or personal activities;headaches of a new type,pattern,or severity;muscle pain;multi-joint pain without swelling or redness;sore throat;and tender on cervical/axillary lymph nodes.Exclusion criteria were (a) fatigue associated with the following diseases:organ failure;chronic infections (e.g.,acquired immune deficiency syndrome,hepatitis B or C);rheumatic and chronic inflammatory diseases;major neurologic diseases (e.g.,multiple sclerosis,neuromuscular diseases,epilepsy or other diseases requiring ongoing medication that could cause fatigue;stroke,head injury with residual neurologic deficits);diseases requiring systemic treatment (e.g.,organ or bone marrow transplantation,systemic chemotherapy,radiation of brain,thorax,abdomen,or pelvis);major endocrine diseases (e.g.,hypopituitarism,adrenal insufficiency);sleep apnea and narcolepsy,(b) the presence of pregnancy or lactation,(c) the physical contraindications to acupuncture and moxibustion treatment.
Parts of participants were recruited from the Medical Examination Center of responding hospitals.The other ways of participant recruitment were posters displayed in hospitals and communities,advertisements on local newspapers.Before trial entry,a face-to-face medical assessment including history,physical and mental state examinations were held by the clinical trial coordinator to ensure participants meet the criteria of CFS.1Then laboratory tests recommended by the International Chronic Fatigue Syndrome Study Group were taken to exclude alternative diagnoses.
2.3.Randomization and blinding
Pre-programmed central randomization process was carried out in this trial to obtain the adequate allocation.Only appointed investigators could ask for random number and group assignment through any of the two ways:pre-approved mobile phone text or web,by providing information about eligible patients’ name,birthday,and gender.Once the unique random number and group assignment were generated,a feedback,containing a random number,the group assignment code,and the patient’s basic information would be automatically sent back through the Center computer system.
The outcome assessors and statistical analysts were blinded to the intervention assignments throughout the trial.
2.4.Intervention
Practitioners:certified acupuncturists who had a TCM license and at least 2-year clinical experiences were trained to participate in this trial.The SOP training included the method of central randomization,criteria of inclusion and exclusion,location of acupoints and the correct manipulation of ginger-indirect moxibustion.In each clinic,one acupuncturist was responsible for diagnosis and giving patient informed consent.Another acupuncturist was in charge of treatment in line with SOP.Ginger-indirect moxibustion plus acupuncture group(group A):acupuncture:Baihui (GV20),Qihai (CV6),Zusanli (ST36),Taixi (KI3) or Ganshu (BL18),Pishu(BL20),Shenshu (BL23) were selected alternatively based on the meridian and acupoint theories,10,11and a review of the ancient and modern literature.Sishen-cong(EX-HN1) was added if sleep problem and poor memory were reported.Neiguan (PC6) and Sanyinjiao (SP6) were added if patients suffered extra problems of sleep or digestion.Except for Baihui (GV20),Qihai (CV6) and Sishen-cong (EX-HN1),all the other acupoints were punctured bilaterally by sterile disposable stainless needles (40 or 25 mm in length and 0.25 mm in diameter;Hwato,Suzhou,China,PRC).The depth of puncture was as follow:
Baihui (GV20),Sishen-cong (EX-HN1):horizontal needling to a depth of 0.5 to 0.8cun;
Ganshu (BL18):oblique needling at a depth of 0.5 to 0.8cun;
Qihai (CV6),Neiguan (PC6) and Taixi (KI3):perpendicular needling to a depth of 0.5 to 0.8cun;
Sanyinjiao (SP6),Zusanli (ST36),Pishu (BL20) and Shenshu (BL23):perpendicular needling to a depth of 0.5 to 1cun.
Twirling and rotating,lifting and thrusting manipulation was applied to promoteQiarrival and then needles were retained for half an hour.
Ginger-indirect moxibustion:Ginger-indirect moxibustion on Governor Vessel and Bladder Meridian was applied after needle withdrawal.Patients were asked to expose the whole back completely.Five or six three-hole Moxa boxes (the number of boxes depended on the patients’ height;Figure 1A) were placed paralleled from Dazhui (GV14) to Yaoshu (GV2) over ginger slices for 2 mm in thickness.The distance between the center of nearby holes was 4.8 cm to ensure the temperature of moxa stick (pure-mugwort moxa stick;20 cm in length and 18 mm in diameter;Hwato,Suzhou,China,PRC;Figure 1B) covered the Governor Vessel and the 1st line of Bladder Meridian.During the 45-minute gingerindirect moxibustion treatment,the depth of moxa sticks was regulated every 5 min to maintain full combustion and obtain equal warm stimulation.
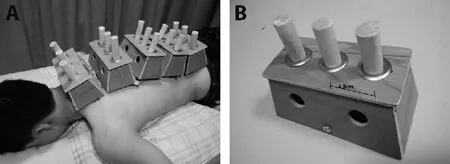
Figure 1 Ginger-indirect moxibustion group
Patients in group a received 24 sessions of acupuncture and moxibustion treatment over a period of 8 weeks (1 session per day,every two sessions with an interval of one day,3 sessions per week).
Acupuncture group (group B):in group B,patients received the same acupuncture treatment and treatment sessions as in group A.
2.5.Outcome measures
Primary outcomes:the improvement of the Fatigue Severity Scale (FSS) score was measured as the primary outcome.The FSS is a nine-statement questionnaire with a ranging score of 1-7 of each statement.The higher score indicates worse fatigue.12It was assessed at baseline,weeks 2,4,6,8 (the end of treatment),12 and 20.
Secondary outcomes:the secondary outcomes included the change of the Somatic and Psychological Health Report (SPHERE),the Self-rating depression scale(SDS),and the Hamilton anxiety scale (HAMA).The 34-item SPHERE assessed a wide range of physical and psychological symptoms identifying the key clinical features of prolonged fatigue states,with higher scores indicating greater fatigue and mental distress.13The SDS and HAMA are specific scales to assess the symptoms of anxiety/depression,with higher scores indicating greater mental distress.All the secondary outcomes were assessed at baseline,weeks 2,4,6,8,12 and 20.
Sample size:the sample size was determined based on the primary outcome.14As reported in the previous literature,there was an improvement of 8.38 on the FSS score after treatment with acupuncture.We anticipated an improvement of no less than 8.88 on the FSS score after acupuncture and moxa treatment in this trial.Given this estimate,with 90% power and a 5% level of significance,at least 145 patients in each group were required to allow for a 10% withdrawal rate.
Statistical methods:the primary statistical analyses were conducted in full analysis set (FAS),which based on the intent-to-treat (ITT) population,consisting of all randomized population who toke at least once treatment and at least one primary outcome evaluation on treatment.For missing data at endpoint (9 cases),a last observation carried forward (LOCF) method was used.The sensitivity analyses based on the per-protocol set (PPS)and complete case set by dropping those with at least once missing of outcome were also conducted as supplementary,respectively.
Quantitative data were shown by mean with standard deviation (SD) or median with percentile (QL-QU),while qualitative variables were expressed by frequency and proportion.The change of outcome assessed during treatment (weeks 2,4,6,8) and follow-up (weeks 12 and 20) comparing with baseline were shown by mean and 95% certificate interval (CI) in the line chart,by setting the absolute value at baseline as starting point.The outcome difference between group A and group B across various times were shown by mean difference (MD) and 95%CI.All the statistical analyses were performed with R software version 3.6.3 (R Development Core Team,Vienna,Austrian),and aP<0.05 was considered statistically significant.
Quality control:a strict monitoring system was established to assess the performance of the trial.Independent quality monitors supervised the manipulation on the site and the completion of case report forms monthly.After the fill-in of electro-CRF,quality monitors checked and revised the data to maintain accuracy.
3.RESULTS
3.1.Patient characteristics
Approximately 500 patients were screened and 290 were enrolled in the trial.After exclusion of patients who were repeatedly or erroneously randomized (n=14),the intention-to-treat population comprised all the remaining 276 patients (138 in group A,138 in group B).The study flow is depicted in Figure 2.The baseline characteristics of the population were similar between two groups(Table 1).
3.2.FSS improvement
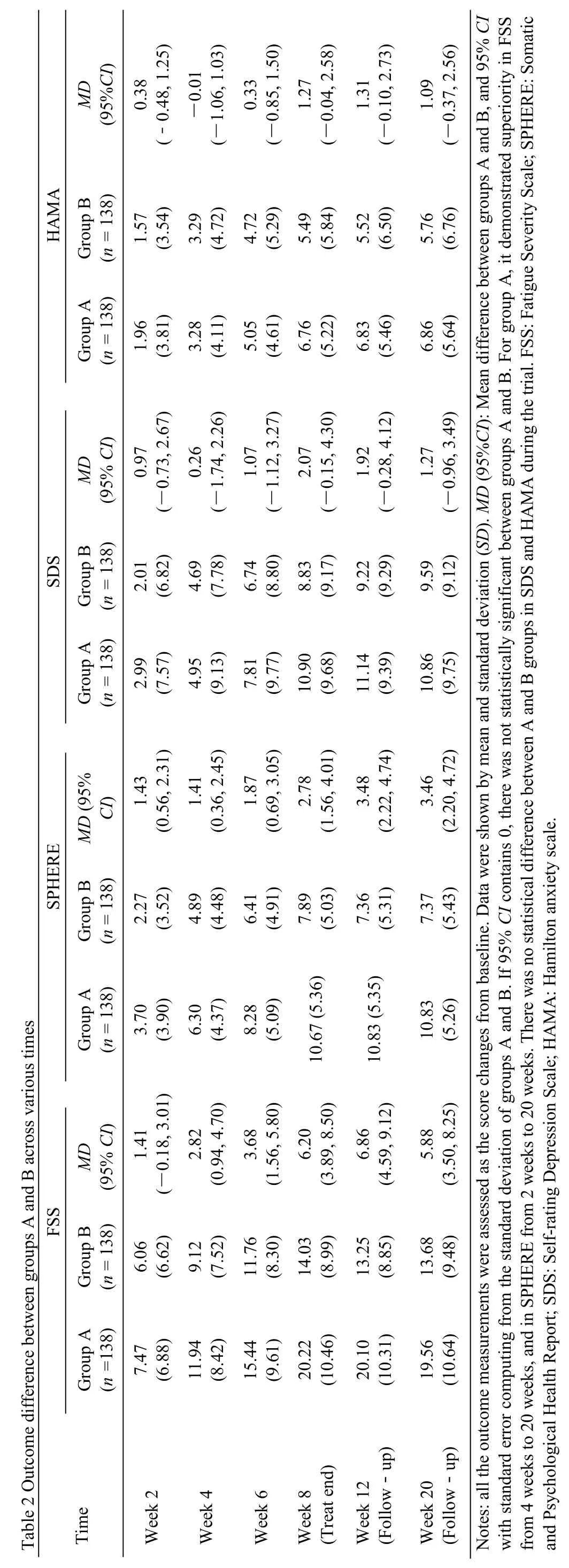
The FSS scores continually decreased from baseline in both groups after 2-week,4-week,6-week and 8-week treatment and during the follow-up period (Figure 3).Between groups,significant differences were observed,and significantly higher score changes were observed in Groups A after 4 weeks treatment (Table 2).All the symptom scores improved from baseline in both groups.The continual improvement was seen as treatment continued,and sustained during the following 12 weeks after the end of treatment (Figure 3).
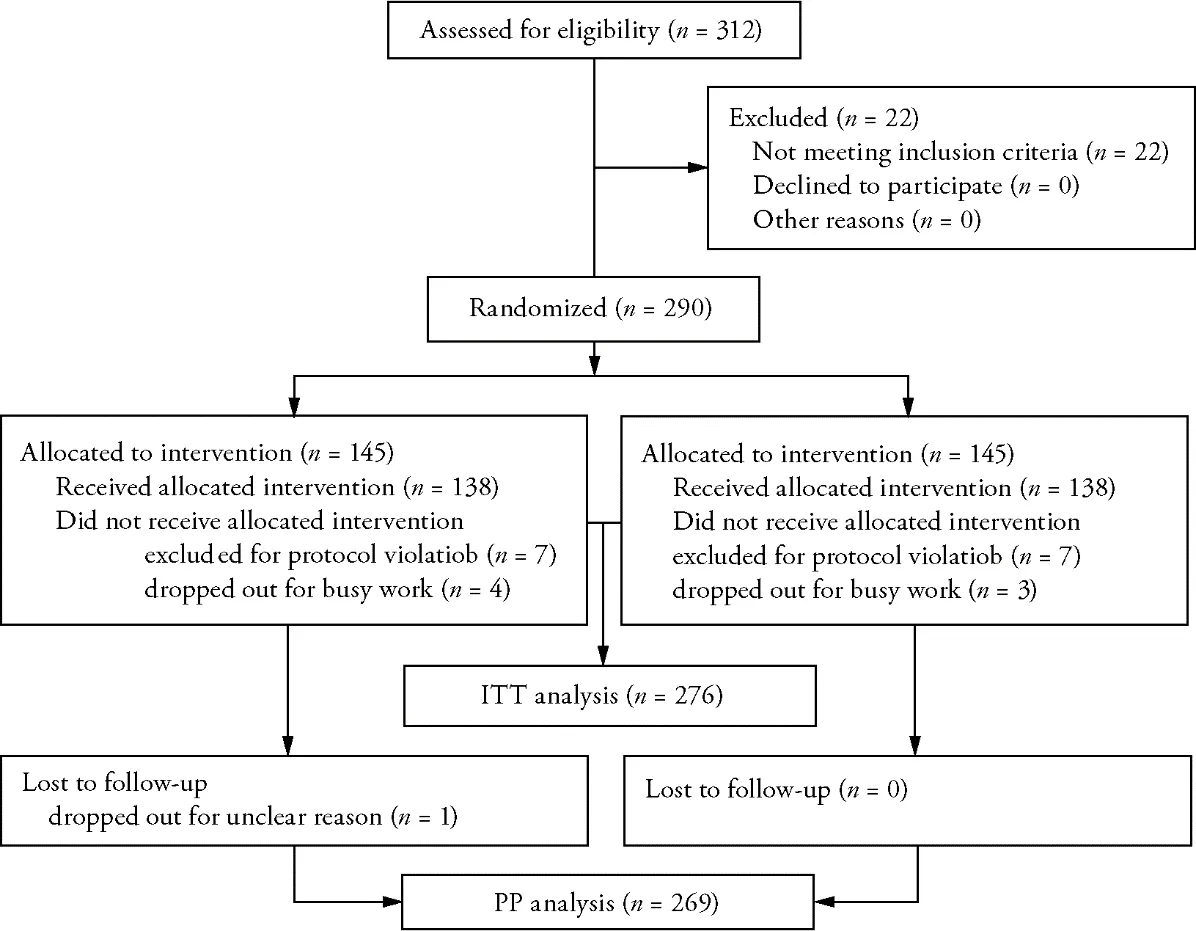
Figure 2 Study flow

Figure 3 Outcome change during treatment and follow-up
Between groups,significant differences were observed in SPHERE,in which significantly higher score changes were seen in group A after 2 weeks treatment and sustained until the end of follow-up period (Table 2).But for SDS and HAMA,the improvement in symptom scores did not differ significantly between the groups.
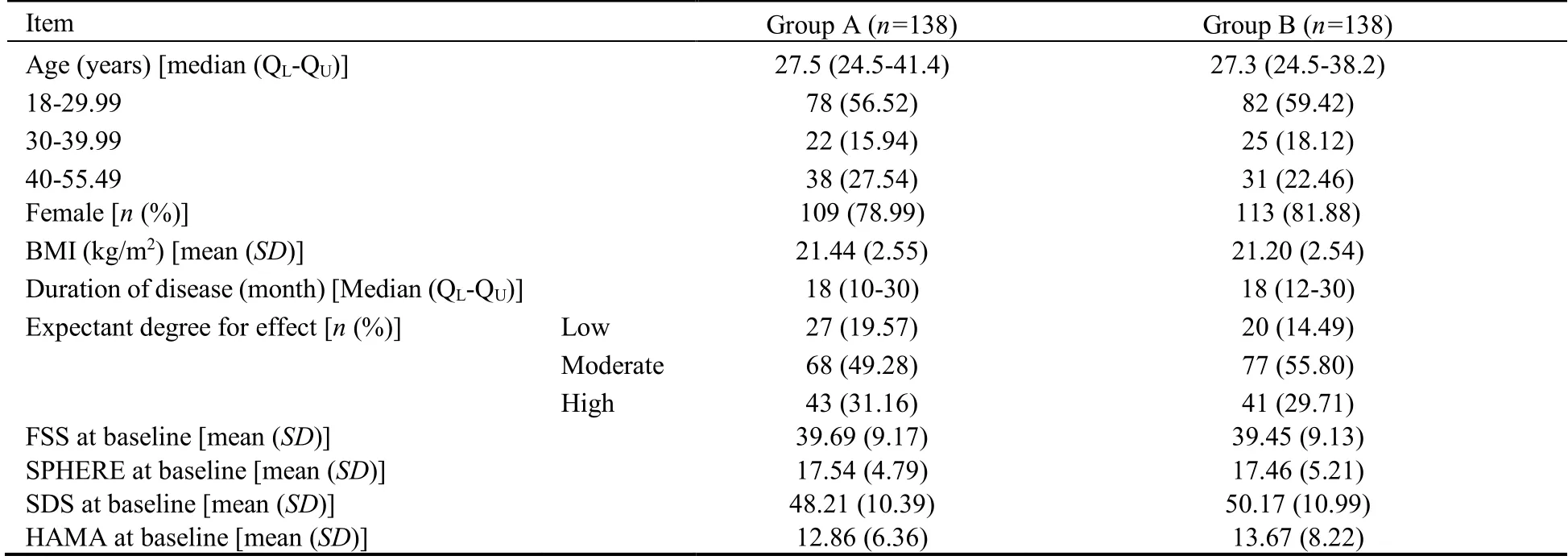
Table 1 Characteristics of patients at baseline
3.3.Safety assessments
Totally 12 adverse events (8 in group A,4 in group B)were reported,in which mild skin burns (5/6) and mild oral ulcer (1/6) were or may be related with moxibustion;mild faint during needling (1/6) and pain or hematoma at the puncture point (5/6) were related with acupuncture.No severe adverse events were reported.
4.DISCUSSION
Due to the nonorganic pathophysiology,CFS is associated with suboptimal health status and the present treatment of CFS is given based on limited evidence from clinical studies.15-17CFS is named fatigue syndrome in TCM theory andYang Qideficiency is considered as the main reason.Moxa therapy is used forYangQideficiency syndrome for thousands of years in China.As one of the special traditional Chinese moxibustion methods,the ginger-indirect moxibustion on the back(Governor Vessel and Bladder Meridian) is characterized as strong yang-warming effect through enlarging moxa therapy area from single points to theYangVessels and extending moxa therapy time from half an hour to 45 min.Although the ginger-indirect moxibustion on the back is considered to be one of the optimal treatments for fatigue symptoms relief and has been applied in clinics,its effect lacks evidence from high quality randomized controlled trials.Therefore,we designed the randomized controlled trial to evaluate the effect and safety of the gingerindirect moxibustion on Governor Vessel and 1st line of Bladder Meridian on the back in the treatment of CFS,for the purpose of providing a valid treatment approach for CFS.The results indicated that ginger-indirect moxibustion is a safe and effective intervention to relieve fatigue and accompanying physical symptoms.
So far,acupuncture is thought to be effective in relief of CFS symptoms,which fits in with the result of our study.3,11,18In our trial,an improvement of 9.12 on the FSS score after 4-week acupuncture treatment was observed,and the improvement achieved 14.03 at the end of 8-week acupuncture treatment and maintained the similar level at the 12th week,indicating a longterm effect of acupuncture.The similar improvement was also found in SPHERE.Also,acupuncture therapy is enc-ouraged in the treatment for anxiety and depression.19,20Although patients in our trial didn’t reach the diagnosis of anxiety and depression,the improvement of SDS and HAMA scores was detected in group A(acupuncture group).
In this trial,we found significant differences in FSS and SPHERE score change between groups of acupuncture alone and the combination of ginger-indirect moxibustion with acupuncture at most of all visit times,suggesting the positive effect of ginger-indirect moxi-bustion in CFS.With the ginger-indirect moxibustion treatment prolonged,the change of FSS and SPHERE scores increased gradually (from 7.47 to 20.22 in FSS;from 3.70 to 10.67 in SPHERE),and the effect persisted during 12-week follow-up.21,22The observation that indirect moxibustion was effective in relieving CFS symptoms has also been reported in other RCTs.21,22The therapeutic effect of moxibustion may be explained by its advantage in vagus nerve activation and antioxidant activity modification.The minimum clinically important difference (MCID) is used to interpret the clinical relevance of results.However,there is a lack of consensus about the size of MCID in fatigue.23The lasted evidence from the study of MCID for seven measures of fatigue in Swedish patients with systemic lupus erythematosus indicated the score change of 15.2 in FSS may be the clinically meaningful difference.In our study,patients were included as CFS,and to all of them,fatigue measured by FSS has been continually improved after treatment.In FSS,the improve rates were from18.82% to 50.94% in group A and 15.36% to 35.56% in group B,which was clinically different.
However,the differences in anxiety and depression improvement between groups were not statistically significant,demonstrating that acupuncture may play more important role in mental distress relief than moxa therapy.The efficacy of moxibustion in anxiety or depression was lack of evidence supporting,because research of its effect in mental disease is rare,so more studies are required to clarify the result and explore the underlying mechanism.On the other hand,in this trial,patients in group A didn’t meet the diagnostic criteria of depression (SDS ≥ 50) and in group B just met minimum diagnostic criteria;patients in both groups didn’t meet the diagnostic criteria of anxiety (HAMA ≥14),which may explain part of the result.
For the large sample,our research was one of the few studies to provide evidence for ginger-indirect moxibustion in treating CFS.The strengths of our study included a central randomization by independent company to confirm adequate allocation concealment and comparability between groups,and a strict quality control system by designated person to assure the standardization of the procedure and reliable results.
There are limitations of our study as well:(a) due to the purpose of our trial and in consideration of patient compliance,a group of ginger-indirect moxibustion alone was not designed;(b) a waiting-list control group was not designed,which may not exclude self-healing from the therapeutic effect;(c) anxiety was assessed by HAMA,but not by self-rating anxiety scale,which was a similar self-rating scale as SDS in measuring depression; (d) objective indicators,such as immunity indexes were not assessed,which will be studied in the further research to enrich results.
In conclusion,the ginger-indirect moxibustion on Governor Vessel and 1st line of Bladder Meridian on the back is safe and effective in relieving fatigue and accompanying physical symptoms,and the effects could last at least 12 weeks after an 8-week treatment.But its effect for psychological improvement in CFS was not clearly detected,which needs more research to verify.
5.ACKNOWLEDGMENTS
We would like to thank Chengdu BM Clinical Researches Ltd.,for their cooperation and support in the central randomization scheme,e-case report form management and clinical quality monitoring.Our gratitude is also extended to clinical staffs evolving in the trial performance and effect assessment in all participant Hospitals.
杂志排行
Journal of Traditional Chinese Medicine的其它文章
- Mechanisms of immune regulation for acupuncture on chronic respiratory diseases
- Mechanism of Huashi Xingyu Qingre recipe (化湿行淤清热方) in treating oral lichen planus based on network pharmacology and clinical trial verification
- Anti-inflammatory mechanism of rhein in treating asthma based on network pharmacology
- An international multicentric phase Ⅲ randomized controlled trial of time-acupoints-space acupuncture for the prevention of chemotherapy-induced fatigue in patients with early stage breast cancer:a study protocol
- Mining intrinsic information of convalescent patients after suffering coronavirus disease 2019 in Wuhan
- Menstrual cycle characteristics as an indicator of fertility outcomes:evidence from prospective birth cohort study in China
