Free-radical evolution and decay in cross-linked polytetrafluoroethylene irradiated by gamma-rays
2022-07-26FeiXiangShaGuoJunChengZiYueXuanYangLiuWeiHuaLiuXiaoDongZhangZhongFengTang
Fei-Xiang Sha · Guo-Jun Cheng · Zi-Yue Xuan · Yang Liu · Wei-Hua Liu ·Xiao-Dong Zhang · Zhong-Feng Tang
Abstract Electron spin resonance techniques were employed to investigate the effects of the absorbed dose and post-irradiation conditions on the evolution and decay of free-radicals in cross-linked polytetrafluoroethylene(XPTFE), induced by γ-ray radiation. Chain-end free-radicals, chain alkyl free-radicals, and tertiary alkyl free-radicals were detected when XPTFE was irradiated under Ar atmosphere. The corresponding peroxy free-radicals were formed upon exposure of irradiated XPTFE to air;the freeradicals concentration first increased linearly with increasing absorbed dose and then gradually saturated.The free-radicals yield under air atmosphere was greater than that under Ar, and the peroxy free-radicals were preserved for a relatively long time when irradiated XPTFE was stored under air atmosphere. The chain alkyl free-radicals may be converted to chain end free-radicals by β-scission,while chain end free-radicals are more sensitive to oxygen than chain alkyl free-radicals. When the annealing temperature was raised above the α-transition temperature of XPTFE,the decay of the free-radicals was greatly affected and accelerated by the motion of the molecules over the long range.
Keywords Polytetrafluoroethylene · Cross-linked polytetrafluoroethylene·Electron spin resonance·Decay·Radiation · γ-ray
1 Introduction
Polytetrafluoroethylene (PTFE) is a type of unbranched and linear polymer that has been widely used in the chemical,electronic,and machining industries owing to its excellent chemical resistance, good thermal stability,superior dielectric properties, and self-lubrication ability[1–3].However,a small dose of radiation can significantly compromise the mechanical properties of PTFE; thus, its wider application in strong radiation fields is restricted.However, it has been found that cross-linked PTFE(XPTFE) can be prepared from PTFE by irradiation under inert atmosphere above the melting point of PTFE [4–6].XPTFE exhibits superior wear and irradiation resistance compared to PTFE, while retaining some of the excellent properties of PTFE. Therefore, XPTFE may have critical applications in high-precision aerospace,nuclear,and other strong radiation fields. Nevertheless, when XPTFE is irradiated by certain high-energy rays,such as gamma-rays(γ-ray), free-radicals are generated on the polymer chains.This process has high potential to degrade the performance of XPTFE via certain chemical reactions[7–9]and directly affects the reliability and lifespan of XPTFE in the course of use [10, 11]. Therefore, investigating free-radicals evolution and decay in XPTFE under different radiation environments is of great significance.

In this study, the effects of the absorbed dose and postirradiation conditions (e.g., oxygen, storage time, and annealing temperature) on the evolution of free-radicals in XPTFE, induced by γ-ray radiation, are investigated using the ESR technique.
2 Experimental
2.1 Materials
Virgin PTFE sheets (Daikin Fluorochemicals (China)Co., Ltd.) with a thickness of approximately 2.0 mm and an average molecular weight of approximately 1.6 × 106were used in this experiment. The XPTFE samples were prepared by electron beam irradiation of molten PTFE in a specially designed irradiation vessel at a temperature of 335 ± 5 °C under N2[18]. The doses absorbed by the samples (hereinafter adsorbed doses) were 90, 500, and 2000 kGy, and the obtained samples are denoted as XPTFE90, XPTFE500, and XPTFE2000, respectively.After cooling the mixture to room temperature,no residual free-radicals were detected.
2.2 Irradiation and annealing treatment
Before irradiation,the samples were cut into small strips of 2.0 mm × 2.0 mm × 10.0 mm and placed in standard nuclear magnetic resonance (NMR) tubes under air atmosphere or sealed in NMR tubes in a glove-box filled with Ar, respectively. The samples and NMR tubes were irradiated by γ-rays generated by a60Co source at room temperature. Each sample was irradiated for 17.0 h, where the total absorbed dose for each sample was 0,20,50,100,200, and 500 kGy. The samples were annealed at 30–280 °C in 50 °C increments under air for 15.0 min using a muffle furnace.
2.3 DSC measurements
The samples were weighed and placed in an aluminum crucible with a pierced lid.The crucible was then placed in a differential scanning calorimetry (DSC) device (DSC 3 + , METTLER TOLEDO). Nitrogen at a flow rate of 50 mL/min was used as the protective gas.The temperature was raised and lowered at a rate of 10 °C/min.The heating and cooling processes were repeated to eliminate the effect of thermal stress on the specimen.The crystallinity(χc)of the sample was calculated using the software accompanying the analytical instrument by applying the following formula [28]:

where ΔHc is the enthalpy of fusion and ΔHf is the enthalpy of the crystal phase transition of PTFE (82 J/g)[28].
2.4 ESR measurements
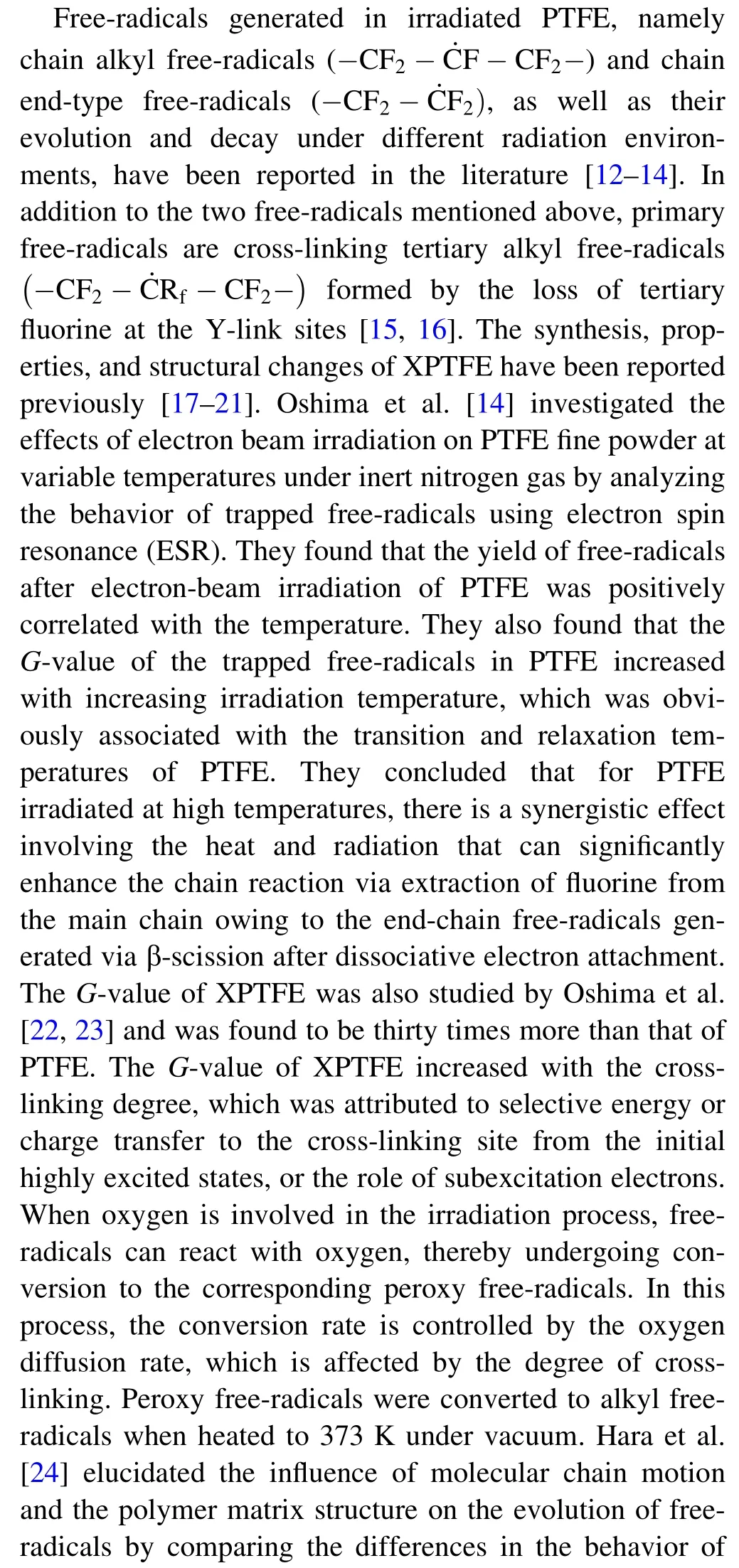
Before testing, the bottom portion of the inverted NMR tube was annealed to eliminate the free-radicals formed in the NMR tubes. ESR measurements were performed at room temperature and at different time intervals after irradiation to investigate the decay of the free-radicals.ESR spectra were acquired at room temperature with a microwave power of 0.998 mW and 100 kHz field modulation using an X-band spectrometer (JES-FA200). The free-radicals concentration of the samples was obtained from the ratio of the sample absorption peak area to that of the manganese standard sample and was calculated as follows:
IsoSimu/FA (version 2.4.3, JEOL RESONANCE Incorporated) software was used to simulate the experimental spectra.
3 Results and discussion
3.1 DSC studies
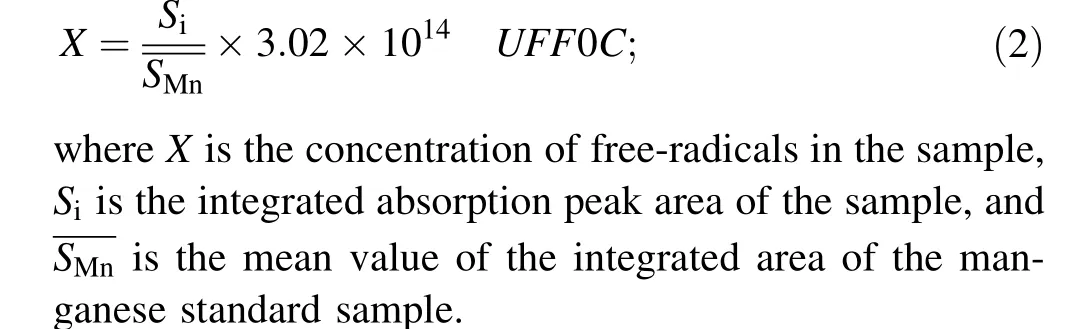
DSC is an effective method for studying the melting and crystallization behavior of samples. The difference between the first and second cooling curves of the samples may be due to the thermal history (processing conditions,cooling rate,etc.)of the samples.To eliminate the effect of the thermal history of each sample and maintain consistency in the experiments, the second heating and cooling curves were chosen for inter-sample comparison.As shown in Fig. 1a, as the absorbed dose increased, the melting point and enthalpy of XPTFE gradually decreased, and the melting range became broader. The melting point of a polymer is usually affected by its crystallinity, chain structure,molecular weight,and other factors.When PTFE is irradiated by electron beam at high temperature, the generated free-radicals can easily react to form a crosslinked network structure owing to the high mobility of the chain segments. The cross-linked network structure may disrupt the original crystalline structure of PTFE,leading to an imperfect crystal structure. The degree of imperfection increased with increasing absorbed dose,and the imperfect crystals tended to melt at lower temperatures, which lowered the melting point. The broadening of the melting range was also due to the imperfect crystals. When the radiation dose absorbed by XPTFE was low, the extent of cross-linking was low,where some of the molecular chains broken by irradiation may form additional crystalline structures, leading to an increase in the melting enthalpy and crystallinity.As the cross-linking degree increased,the cross-linked structure prevented crystallization, leading to a decrease in the melting enthalpy and crystallinity.
These results indicate that different XPTFE samples have different crystalline regions. The melting point,melting enthalpy, and crystallinity of the samples are summarized in Table 1.
3.2 Types and concentration of free-radicals
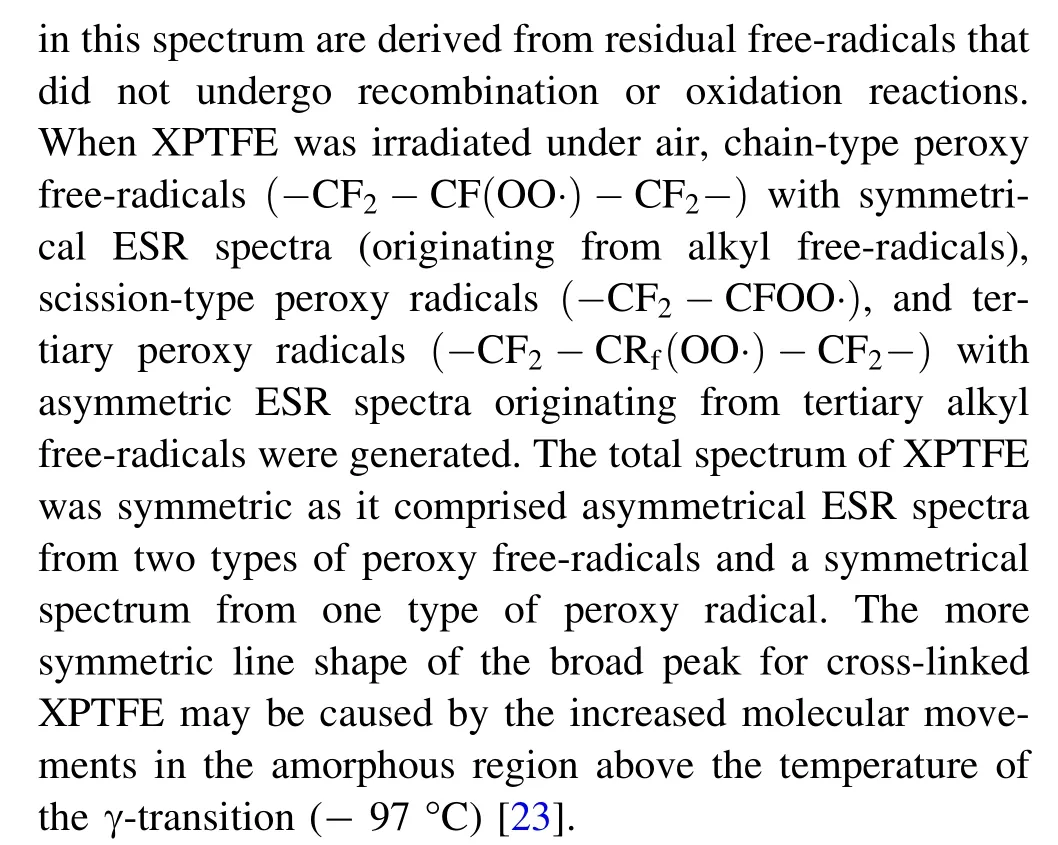
The ESR spectra of PTFE and XPTFE at an absorbed dose of 100 kGy when irradiated under Ar and air are shown in Fig. 2. Because the g-factor and hyperfine splitting obtained from Fig. 2 are the same, it is deduced that the paramagnetic center does not change significantly with the degree of cross-linking [22, 29]. When the samples were irradiated under Ar, as shown in Fig. 2a, some peaks overlapped due to the poor resolution of the ESR technique, which may be due to the distribution of hyperfine splitting caused by the steric angular dependency of the Fβatoms on the p-orbitals of the free-radicals [23]. The spectra of PTFE and XPTFE were observed to be composed of a relatively low-strength double quintet (2 × 5 lines) and a triple triplet (3 × 3 lines), as previously reported [30, 31]. The strength of both sets of peaks increased as the cross-linking degree of the samples increased. Besides, the intensity of the broad single peak(marked by the diamond symbol with a solid line) in the ESR spectrum of XPTFE at approximately 323 mT also increased with increasing cross-linking degree of the samples [22, 32]. For the samples irradiated under air atmosphere (Fig. 2b), except for some small peaks where the intensity increased with the degree of cross-linking,the broad peak at approximately 323 mT in the ESR spectrum of XPTFE was relatively symmetrical compared to that of PTFE, and the degree of symmetry also increased with the degree of cross-linking. In short, there are differences in the free-radical species formed in PTFE and XPTFE, as indicated by their different ESR spectra,but the same types of free-radicals are formed in XPTFE irradiated under different atmospheres,as indicated by the similar shapes of the ESR spectra. The variations in the type and concentration of free-radicals generated in XPTFE by irradiation under air and Ar at room temperature are discussed hereinafter.
Fig. 1 (Color online) DSC thermograms of initial samples. a Heating curves; b cooling curves

Table 1 Melting point, melting enthalpy, and crystallinity of PTFE and XPTFE
Because the same types of free-radicals were present in XPTFE irradiated under different conditions and due to the moderate cross-linking of XPTFE500, XPTFE500 was chosen as a representative for further study. The ESR spectrogram of XPTFE500 with an absorbed dose of 100 kGy after irradiation under Ar and air is shown in Fig. 3a. The stick diagrams of the ESR spectra for the chain alkyl free-radicals and chain end-type free-radicals are shown in Fig. 3b. The characteristic peaks are marked as P1and P2. Considering the ESR spectra of XPTFE500 irradiated under Ar and the ESR stick plots, the double quintet is attributed to chain alkyl free-radicals, and the triple–triplet with relatively low strength (approximately one eleventh of the former) is assigned to chain end-type free-radicals, based on analysis of hyperfine splitting (hfs)caused by the interaction between the unpaired electron and fluorine nucleus [24]. Moreover, these two types of free-radicals are widely recognized to be trapped in the crystalline regions [33]. The parameters used to simulate chain alkyl free-radicals were one F(α) atom(ΔHα≈9.0 mT) and four equivalent F(β) atoms(ΔHβ≈3.3 mT). The parameters used to simulate chain end-type free-radicals were two F(α) atoms(ΔH′α≈10.0mT) and two F(β) atoms (ΔH′β≈1.3mT). In the simulation,the intensity of the chain alkyl free-radicals was eleven times stronger than that of the chain end-type free-radicals. The experimental and simulated spectra are shown in Fig. 3c.The simulated results are consistent with the experimental results.In addition,the broad single peak was thought to be associated with cross-linked tertiary alkyl free-radicals that were perceived to be trapped in cross-linked amorphous regions[33].Cross-linking greatly changes the molecular conformation near the cross-linking sites and reduces the ionization or excitation potential.Thus,free-radicals can be selectively generated in this area.This broad single peak is believed to be caused by hyperfine splitting due to the F atoms on the Cβand Cβatoms depending on the disordered configuration of these atoms in the cross-linked regions,as well as the poor resolution of the ESR spectrogram.


Fig. 2 (Color online) ESR spectra of PTFE and XPTFE with an absorbed dose of 100 kGy (a); under Ar (b) and under air (c)
From comparison of the peak intensity in Fig. 3, it is clear that the intensity of the peaks for the chain end-type free-radicals and chain alkyl free-radicals trapped in the crystalline regions was basically the same for the samples irradiated under Ar and air; however, the intensity was slightly lower for the sample irradiated under air. This observation indicates that the free-radicals trapped in the crystalline regions were relatively stable during irradiation.When the sample was irradiated under air, the signals of the tertiary alkyl free-radicals generated by irradiation under Ar were masked by the broad peak of the peroxy free-radicals. At room temperature, the reactions between free-radicals and migration of the free-radicals are hindered by the low mobility of the XPTFE molecular chains [24].The decay behavior of free-radicals within a certain time at room temperature for the samples irradiated under air is mainly due to reactions with oxygen. These reactions depend mainly on the rate of oxygen diffusion in the polymer matrix. Thus, the free-radicals trapped in the crystalline region,where oxygen could not easily penetrate,were relatively stable. Only an exceedingly small portion of these free-radicals moved to the crystal surface and subsequently reacted with oxygen. In contrast, tertiary alkyl free-radicals trapped in the cross-linked disordered region were relatively prone to transformation into tertiary peroxyl free-radicals due to the diffusion of oxygen.

Fig. 3 (Color online) a ESR spectra of XPTFE500 with absorbed dose of 100 kGy under Ar and air, respectively. The characteristic peaks are marked by arrows (P1: chain end-type free-radicals and chain alkyl free-radicals; P2: chain alkyl free-radicals). b ESR stick plots for chain alkyl free-radicals and chain end-type free-radicals.The heights of the sticks are positively related to the relative strength of the lines[24].c Experimental and simulated spectra of PTFE with absorbed dose of 100 kGy
The concentrations of free-radicals in XPTFE90,XPTFE500, and XPTFE2000 after irradiation with 100 kGy under Ar and air are illustrated in Fig. 4. For the same absorbed dose, more free-radicals were generated in XPTFE with a greater degree of cross-linking. Oshima et al. [22] attributed the higher concentration of free-radicals in XPTFE to selective energy or charge transfer to the cross-linking site from the initial highly excited states, or the role of sub-excited electrons.At room temperature,the PTFE chain segments are rigid due to the tight packing of its molecules,and the rotational freedom of the–CF2–unit along the molecular chain axis is limited even in the amorphous regions due to the larger size of the F atom(van der Waals’radius 1.35 A˚)compared to that of H atom(van der Waals’radius 1.1 A˚).Cross-linking further reduced the mobility of the molecular chains. Thus, the possible reactions between the free-radicals were reduced because of the lower crystallinity and restricted migration caused by cross-linking, which may be the reason for the higher concentration of free-radicals. Cross-linking of XPTFE under irradiation in the molten state formed a mesh structure, which led to poor mobility of the molecular chains.The crystals formed during the cooling and crystallization processes were not perfect. When the cross-linking degree increased, the crystallinity of XPTFE decreased. Owing to the weak intermolecular forces in XPTFE, the probability of reaction between free-radicals in the non-crystalline regions is much lower than that in the crystalline regions.Therefore, XPTFE with a higher degree of cross-linking had a higher concentration of free-radicals.As illustrated in Fig. 4, the concentration of free-radicals produced by irradiation under air was greater than that generated under Ar. The evolution of free-radicals in XPTFE under different atmospheres is discussed in detail hereinafter.
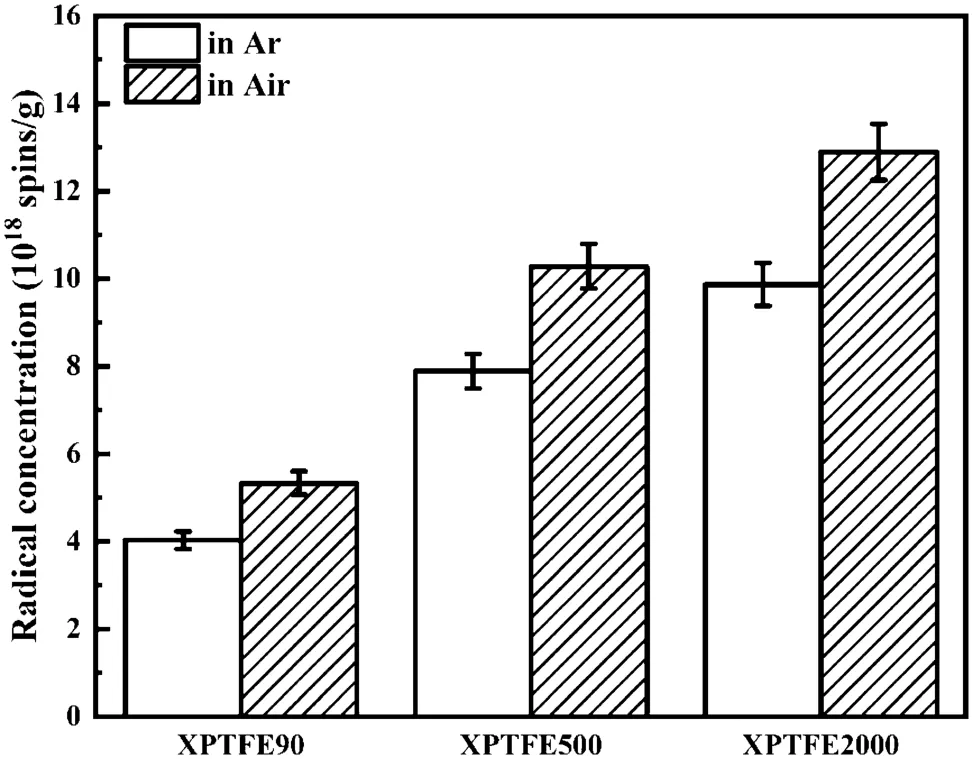
Fig. 4 Concentration of radicals in XPTFE after irradiation with 100 kGy under Ar and air, respectively
The ESR spectra of XPTFE500 subjected to different irradiation doses under Ar and air are shown in Fig. 5. No residual free-radicals were detected when the absorbed irradiation dose was 0 kGy because the EB irradiation was carried out at a high temperature of approximately 335 °C. After EB irradiation, the sample was slowly cooled and the free-radicals gradually decayed. Under Ar atmosphere (Fig. 5a), the intensity of the ESR peak for the chain end-type free-radicals (marked by the arrow with a dotted line), chain alkyl free-radicals (marked by the arrow with solid line), and cross-linking tertiary alkyl free-radicals (marked by the diamond symbol with solid line) increased with increasing radiation dose within a certain range. However, under air (Fig. 5b), the intensity of the ESR signal of free-radicals trapped in the crystalline regions increased with increasing radiation dose,whereas the intensity of the broad single peak associated with peroxy free-radicals first increased and then decreased with increasing absorbed dose. When the sample was irradiated under air, more free-radicals were generated as the absorbed dose increased. However, only a small number of free-radicals trapped in the crystal regions was converted to the corresponding peroxy freeradicals, whereas the tertiary alkyl free-radicals trapped in the cross-linked disordered region reacted with oxygen to form tertiary alkyl peroxyl free-radicals. Therefore, the intensity of the broad single peak increased, and the spectrum became less symmetrical within a certain dose range. At an absorbed dose of 500 kGy, the broad single peak became less intense, which may be due to almost complete destruction of the cross-linked structure of XPTFE500 at such high absorbed doses. In this case, air can easily diffuse into the polymer matrix, causing the free-radicals located in the amorphous region to decay.

Fig. 5 (Color online) ESR spectrum of XPTFE500 irradiated under Ar (a) and air (b) with different doses

Fig. 6 (Color online) Variation of free-radical concentration in XPTFE exposed to different irradiation doses under Ar (a) and air (b)
The variation in the radical concentration of XPTFE irradiated under Ar and air at different doses is shown in Fig. 6. Figure 6a and b shows that the concentration of free-radicals in XPTFE first increased linearly as the absorbed dose increased and then gradually saturated. The saturation concentration of radicals in XPTFE90,XPTFE500,and XPTFE2000 after irradiation under Ar was calculated to be around 5.6 × 1018, 9.7 × 1018, and 17.6 × 1018spins/g, respectively. Similarly, the saturation concentration of radicals in XPTFE90, XPTFE500, and XPTFE2000 after irradiation under air was calculated to be approximately 4.9 × 1018, 10.2 × 1018, and 21.2 × 1018spins/g, respectively. Because 100 kGy was the end point of the linear increase in the free-radical concentration, the radiation chemical yield (G (˙R )) of the samples was calculated for radiation doses below 100 kGy. G (˙R )is defined as the number of free-radicals formed per 100 eV of energy absorbed and was calculated according to the following formula:
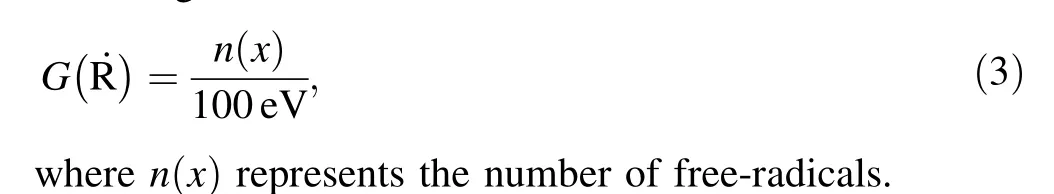
The G R˙values of XPTFE90, XPTFE500, and XPTFE2000 after irradiation under Ar were calculated to be approximately 0.81, 1.12, and 1.91, respectively. The G (˙R )values for XPTFE90, XPTFE500, and XPTFE2000 after irradiation under air were calculated as approximately 0.94, 1.74, and 2.26, respectively. XPTFE with a higher degree of cross-linking had a larger G (˙R ). Moreover, the G (˙R )value of XPTFE after irradiation under air was greater than that of the sample irradiated under Ar. As discussed in the previous section, the movement of the XPTFE chain segments was hindered by cross-linking and the possible reactions between free-radicals were reduced.The crystallinity of XPTFE decreased as the degree of cross-linking increased,resulting in smaller crystal regions.The probability of free-radical reactions in the amorphous and disordered regions is much lower than that in the crystalline regions, owing to the low intermolecular forces of XPTFE. Therefore, XPTFE with a higher degree of cross-linking had a higher concentration of free-radicals.
3.3 Evolution and decay of free-radicals at room temperature


Fig.7 (Color online)Variation of ESR spectrum for XPTFE500 irradiated at100 kGy dose under Ar and kept under Ar(a);irradiated under Ar and subsequently exposed to air (b); irradiated under air and kept under air (c); plot of radical concentration versus storage time (d)
where,H1is the height of the P1peak in the ESR spectrum and H2is the height of the P2peak in the ESR spectrum.
The variation in the ESR spectra of XPTFE500 irradiated under Ar with an absorbed dose of 100 kGy against the storage time at room temperature is shown in Fig. 7a.For XPTFE500 irradiated and kept under Ar(Fig. 7a),the intensity of the peak in the ESR spectra representing chain end-type free-radicals and chain alkyl free-radicals gradually decreased with time,whilethoseassociatedwithtertiaryalkylfree-radicalsremained relatively stable. Tertiary alkyl free-radicals trapped in the cross-linked disordered regions of XPTFE remained relatively stable,which is not only due to the weak intermolecular forces in XPTFE, but also because there was little translational diffusion of the segments in the para-crystalline region below the melting point for the rigid chain segments, in addition to the restricted rotational freedom of the –CF2– unit along the molecular chain axis,and cross-linking.Combined with Fig. 8,it can be seen that the PHR decreased over time,indicating that under Ar,the chain-alkyl free-radicals decayed faster than the chain-end free-radicals. Several possible reactions have been reported.Forsythe and Hill et al.showed that in the radiation chemistryoffluoropolymers,fluorinetransferishighlyunlikely[34].The β-scission reaction of the main-chain free-radicals
at a specified temperature were reported by Hara et al.[24].Therefore,the β-scission reaction of main-chain free-radicals may reasonably explain the decrease in the PHR under Ar at room temperature. As shown in Fig. 7d, only approximately 50%of the free-radicals remained after five days.

Fig. 8 (Color online) PHR of XPTFE500 irradiated and kept under different atmospheres versus storage time
To investigate the influence of oxygen engagement on free-radical evolution, XPTFE500 was irradiated under Ar and subsequently exposed to air atmosphere, as shown in Fig. 7b. The free-radical profile rapidly changed. Over time,the peaks corresponding to tertiary alkyl free-radicals gradually became covered by those of peroxy free-radicals.The peak intensities of the two types of free-radicals trapped in the crystalline regions gradually decreased until the peaks disappeared completely. Finally, only an asymmetric broad peak associated with the peroxy free-radicals was observed after 3240 min. The evolution of free-radicals was significantly affected by the participation of oxygen. The free-radicals were converted to the corresponding peroxy free-radicals by the diffusion of oxygen in the XPTFE matrix. In this case, the free-radicals also undergo oxidation reactions with oxygen, in addition to reactions between the free-radicals; the former dominates and is related to the rate of oxygen diffusion in the polymer matrix. In contrast, as shown in Fig. 8, the PHR increased with time when the sample was exposed to air, where the behavior differed significantly from that of the sample kept under Ar,indicating that chain end free-radicals were more sensitive to oxygen than chain alkyl free-radicals. Eventually, the scission-type peroxy free-radicals were transformed into the corresponding alkyl radicals by eliminating the COF2group. Chain-type peroxy free-radicals are converted to carboxyl and terminal carboxyl groups[35].This eventually led to shortening of the PTFE chain ends,which compromised the mechanical properties. As shown in Fig. 7d, compared to the sample kept under Ar, the total radical concentration decreased more slowly in this case owing to the participation of oxygen.
To mitigate the influence of the oxygen diffusion rate on the evolution of free-radicals, XPTFE500 was irradiated and kept under air.Owing to the relatively long irradiation time, it was assumed that oxygen had completely diffused into the polymer matrix, except for in the crystalline regions where it is difficult for oxygen to diffuse during the irradiation process. As shown in Fig. 7c, the peaks of the two types of free-radicals trapped in the crystalline regions gradually became less intense until they completely disappeared, and the intensity of the broad single peak first increased and then decreased over time.Tertiary alkyl freeradicals trapped in the cross-linked disordered regions are oxidized to the corresponding peroxy free-radicals. The free-radicals trapped in the crystalline region slowly move to the surface of the crystalline phase, where they easily react with the oxygen present in the amorphous region.Finally, over time, these peroxy free-radicals gradually disappear.As expected,as illustrated in Figs. 7d and 8,the total radical concentration is the parameter that decayed the slowest with time,whereas the PHR increased rapidly with time. This further verifies the conclusion that chain-end free-radicals are more sensitive to oxygen than chain alkyl free-radicals.
In general, there are significant differences in the stability of chain alkyl free-radicals and chain end-type freeradicals in different post-irradiation atmospheres. Combined with previous conclusions, chain end-type free-radicals are more sensitive to oxygen than chain alkyl freeradicals. The possible reactions of free-radicals in XPTFE irradiated at room temperature by γ-rays are as follows:
Irradiation stage:
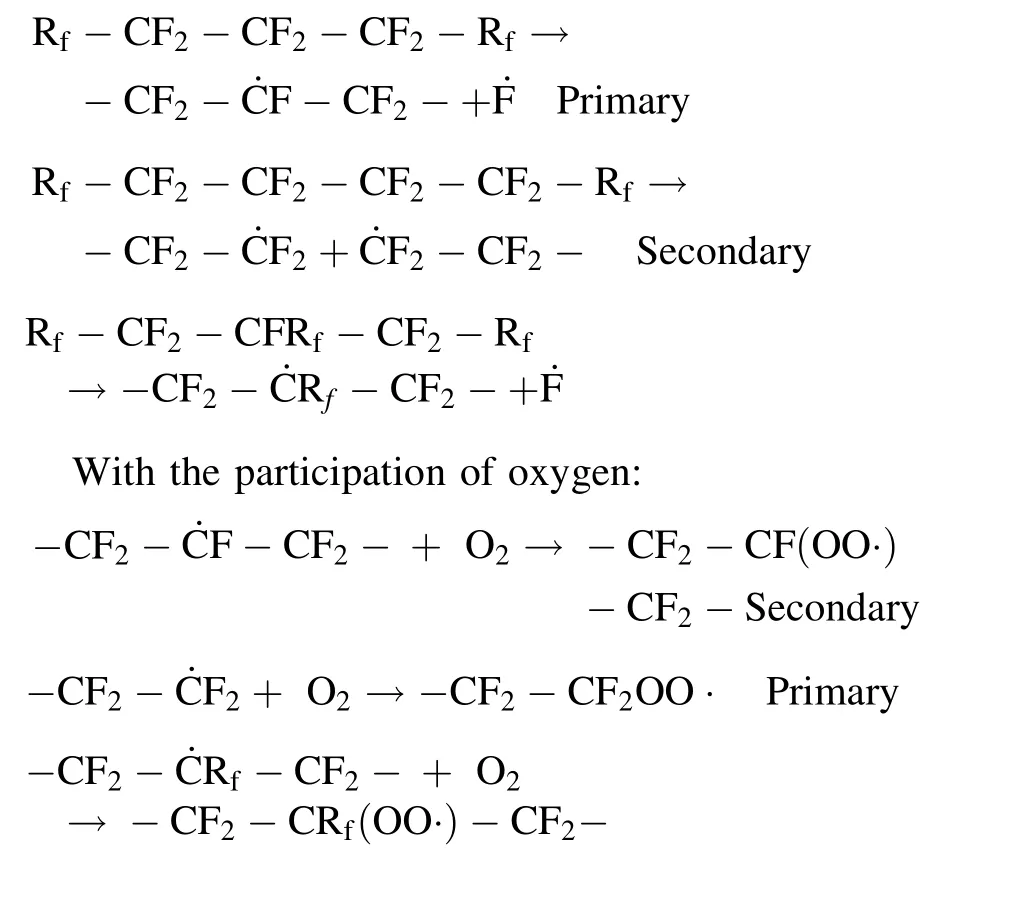

3.4 Evolution and decay of free-radicals at elevated temperatures
The free-radicals in XPTFE500 are relatively stable at room temperature under air atmosphere, which may result in significant variations in their physical properties during long-term storage and use [8]. Therefore, it is essential to investigate the decay behavior of free-radicals in XPTFE500 at elevated temperatures under air, which is generally related to the motion of the molecular chain segment. Thus, XPTFE500 was thermally treated (from room temperature to 280 °C)under air atmosphere to track the decay of free-radicals. Figure 9a shows the ESR spectrum of XPTFE500 exposed to an irradiation dose of 100 kGy under air atmosphere, with heat treatment. The ESR peaks gradually became less intense with increasing temperature and finally disappeared when the temperature reached 280 °C. No noticeable variations in the shape of the ESR spectra were detected below the temperature of 230 °C. No generation of new free-radicals was observed with increasing temperature because the temperature was relatively low and was not sufficient to break the molecular chains or induce new reactions in XPTFE500.In this case,the free-radicals decayed mainly by reacting with oxygen,and migration of the free-radicals was accelerated at elevated temperatures.Oshima et al.[36]concluded that α,β,and γ relaxation processes occurred in PTFE at approximately 130,19,and - 97°C,respectively.By introducing cross-linking, the α relaxation temperature became lower,while γ relaxation occurred at higher temperatures, and no β relaxation was observed. As shown in Fig. 8b, the freeradicals trapped in XPTFE decayed fastest within the temperature interval of 80–130 °C, indicative of the α relaxation process (corresponding to long-range molecular motion). Migration and recombination of the free-radicals,as well as the diffusion of oxygen,were accelerated above the α relaxation temperature. Therefore, the decay rate of samples in this temperature interval increased significantly.In conclusion, it is feasible to achieve rapid decay of trapped free-radicals by increasing the temperature to accelerate the migration rate of the molecular chain segments.
4 Conclusion

Fig. 9 (Color online) Temperature-dependent changes in ESR spectra of XPTFE500 with 100 kGy irradiation dose under air (a); variation in free-radical concentration with temperature (b)
Three categories of radicals (chain alkyl free-radicals,chain end free-radicals, and tertiary alkyl free-radicals)were formed in XPTFE by γ-ray radiation at room temperature under Ar atmosphere. These free-radicals are easily oxidized to the corresponding peroxy free-radicals in air. The free-radical concentration first increased linearly as the dose of absorbed radiation increased, and then gradually saturated.The radical saturation concentration of XPTFE90, XPTFE500, and XPTFE2000 irradiated under Ar was calculated to be around 5.6 × 1018, 9.7 × 1018,and 17.6 × 1018spins/g, respectively. Similarly, that for XPTFE irradiated under air atmosphere was calculated to be approximately 4.9 × 1018, 10.2 × 1018, and 21.2 × 1018spins/g, respectively. Under the same irradiation conditions, the concentration of free-radicals in XPTFE increased as the degree of cross-linking increased.The total radical concentration in XPTFE was higher in air because of the high stability of peroxyl radicals formed in the presence of oxygen. By comparing the changes in the PHR of the chain alkyl free-radicals with that of the chain end free-radicals under different atmospheres,it is deduced that chain alkyl free-radicals may be converted to chain end free-radicals by β-scission, whereas chain end freeradicals are more sensitive to oxygen than chain alkyl freeradicals. At temperatures above the α relaxation temperature of XPTFE,the free-radicals decay much faster because of accelerated migration and recombination of the freeradicals, as well as faster diffusion of oxygen.
Author contribution All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Fei-Xiang Sha, Zi-Yue Xuan, and Yang Liu. The first draft of the manuscript was written by Fei-Xiang Sha, reviewed and edited by Guo-Jun Cheng, Wei-Hua Liu, Xiao-Dong Zhang, and Zhong-Feng Tang. All authors read and approved the final manuscript.
杂志排行
Nuclear Science and Techniques的其它文章
- Spatial resolution and image processing for pinhole camera-based X-ray fluorescence imaging: a simulation study
- Picosecond time-resolved X-ray ferromagnetic resonance measurements at Shanghai synchrotron radiation facility
- Nonrecursive residual Monte Carlo method for SN transport discretization error estimation
- Density fluctuations in intermediate-energy heavy-ion collisions
- Isospin effects on intermediate mass fragments at intermediate energy-heavy ion collisions
- Identification of anomalous fast bulk events in a p-type pointcontact germanium detector
