竹灵消化学成分的研究
2022-07-22司盈盈孙彦君薛贵民赵珍珠冯卫生
司盈盈,孙彦君,薛贵民,赵珍珠,冯卫生*
(1.河南中医药大学药学院,河南 郑州 450046;2.河南省中药开发工程技术研究中心,河南 郑州 450046)
竹灵消Cynanchuminamoenum(Maxim.) Loes.隶属萝藦科鹅绒藤属,别名雪里蟠桃、老君须、婆婆针线包等。产于辽宁、河北、河南、山东、山西、安徽、浙江、湖北、湖南、陕西、甘肃、贵州、四川、西藏,生长于海拔100~3 500 m的山地疏林、灌木丛中或山顶、山坡草地上,朝鲜和日本也有分布[1]。根可药用,具有清热凉血、利胆、解毒的功效,主治阴虚发热、虚劳久咳、咯血、胁肋胀痛、呕恶、泻痢、产后虚烦、无名肿毒、蛇虫或疯狗咬伤。民间用作治妇女血厥、产后虚烦、妊娠遗尿、疥疮及淋巴炎等[2]。关于竹灵消的化学成分研究报道很少,目前仅从中分离得到四十余个化合物[3-4],且研究均集中在根部,其他部位的化学成分则未见报道。鹅绒藤属植物的主要化学成分为C21甾体及其苷类[5-7],而竹灵消作为鹅绒藤属植物中的一种,主要化学成分也是C21甾体类化合物,除此之外,还含有少量苯乙醇衍生物、生物碱、甘油酯、三萜、脂肪酸等其他类型成分[3-4]。本实验对竹灵消中的化学成分进一步研究,从中分离得到了14个化合物,化合物2、4~6、8、10、12为首次从该属植物中分离得到,化合物3、7、9、11、13~14为首次从该植物中分离得到。
1 材料
ARX-400 MHz 核磁共振仪、Bruker maxis HD型飞行时间质谱仪(德国Bruker公司);FLEXA HP50型中压制备系统(天津博纳艾杰尔公司);LC-8A型高效液相色谱仪(日本岛津公司);柱层析Sephadex LH-20(瑞士Parmacia Biotech 公司);柱层析硅胶(200~300目,青岛海洋化工厂)。所用分析纯和色谱纯试剂(北京化工厂和天津第三化学试剂厂)。
竹灵消全株采自河南省伏牛山,经沈阳药科大学中药学院贾景明教授鉴定为鹅绒藤属植物竹灵消Cynanchuminamoenum(Maxim.) Loes.。
2 提取与分离
将采集的竹灵消(湿质量6.5 kg)粉碎,用95%乙醇回流提取3次,每次1.5 h,合并提取液,减压浓缩至无醇味得到总提物。将总提物用2 L水分散,分别加入等体积的石油醚、二氯甲烷、乙酸乙酯、正丁醇依次萃取,萃取液减压浓缩后得到相应的萃取部位。取乙酸乙酯部位(37 g)过硅胶柱,以二氯甲烷-甲醇(0∶1~1∶0)梯度洗脱,得到16个流分(A-P)。流分O过凝胶柱得到5个亚流分O1~O5,O2过中压柱色谱,80%甲醇水洗脱部分经HPLC甲醇-水(68∶32)纯化得化合物1(12.3 mg);O3过中压柱色谱,40%甲醇水洗脱部分经HPLC甲醇-水(34∶66)纯化得化合物4(9.5 mg);O5过中压柱色谱,50%甲醇水洗脱部分经HPLC纯化甲醇-水(42∶58)得化合物5(8.7 mg)。流分M过凝胶柱得化合物2(2.6 mg)。流分N过中压柱色谱,40%甲醇洗脱部分过凝胶,得化合物3(16.3 mg)。流分L过凝胶得到4个亚流分L1~L4,L1经HPLC纯化得化合物7(10.3 mg)。流分J过中压柱色谱,40%甲醇洗脱部分过凝胶,得化合物9(11.8 mg)。流分B和流分C析出白色粉末,经洗涤、重结晶后分别得化合物12(24 mg)、13(37 mg)。流分A过凝胶柱,在洗脱的过程中析出无色针状结晶,经重结晶得化合物14(8.6 mg)。取正丁醇萃取部位(23 g)过硅胶柱,以二氯甲烷-甲醇(0∶1~1∶0)梯度洗脱,得到5个流分Fr.1~Fr.5。Fr.3过中压柱色谱,60%甲醇洗脱部分过凝胶得化合物6(8.2 mg);40%甲醇洗脱部分过凝胶得化合物8(4.4 mg)、10(5.6 mg)。Fr.4过中压柱色谱,60%甲醇洗脱部分过凝胶得化合物11(3.8 mg)。
3 结构鉴定
化合物1:无色粉末,ESI-MSm/z:505.2[M+H]+。1H-NMR (400 MHz,DMSO-d6)δ:6.39 (1H,brs,H-18),5.37 (1H,d,J=4.2 Hz,H-6),5.26 (1H,dd,J=16.8,7.5 Hz,H-16),5.04 (1H,d,J=5.5 Hz,4′-OH),4.59 (1H,dd,J=9.5,1.4 Hz,H-1′),4.15 (1H,dd,J=8.3,7.2 Hz,H-15α),3.62 (1H,t,J=9.1 Hz,H-15β),3.44 (1H,m,H-3),3.36 (1H,d,J=7.8 Hz,H-17),3.30 (3H,s,3′-OCH3),1.42 (3H,s,H-21),1.15 (3H,d,J=6.1 Hz,H-6′),0.85 (3H,s,H-19);13C-NMR (100 MHz,DMSO-d6)δ:174.8 (C-14),143.3 (C-18),139.9 (C-5),120.0 (C-6),117.7 (C-13),113.5 (C-20),96.9 (C-1′),80.0 (C-3′),76.5 (C-3),75.1 (C-4′),74.7 (C-16),71.6 (C-5′),66.8 (C-15),56.4 (3′-OCH3),55.3 (C-17),52.4 (C-8),39.8 (C-9),38.0 (C-10),38.0 (C-4),36.5 (C-1),35.8 (C-2′),29.3 (C-2),29.2 (C-7),27.5 (C-12),24.4 (C-21),23.3 (C-11),18.1 (C-19),17.5 (C-6′)。以上数据与文献[8]基本一致,故鉴定为白薇苷甲。
化合物2:黄色针晶,ESI-MSm/z:455.1[M+Na]+,433.1[M+H]+。1H-NMR (400 MHz,DMSO-d6)δ:8.14 (2H,d,J=8.0 Hz,H-2′,6′),6.91 (2H,d,J=8.0 Hz,H-3′,5′),6.81 (1H,s,H-8),6.40 (1H,s,H-6),5.54 (1H,s,H-1″),3.84~3.31 (4H,m,H-2″-5″),1.12 (3H,d,J=6.1 Hz,H-6″);13C-NMR (100 MHz,DMSO-d6)δ:176.1 (C-4),161.4 (C-7),160.4 (C-5),159.4 (C-4′),155.7 (C-9),147.5 (C-2),132.1 (C-3),129.6 (C-2′,6′),121.6 (C-1′),115.5 (C-3′,5′),104.7 (C-10),98.8 (C-6),98.4 (C-1″),94.3 (C-8),71.6 (C-4″),70.3 (C-3″),70.1 (C-2″),69.9 (C-5″),17.9 (C-6″)。以上数据与文献[9]基本一致,故鉴定为山柰酚-7-O-α-L-鼠李糖苷。
化合物3:黄色针晶,ESI-MSm/z:579.2[M+H]+。1H-NMR (400 MHz,DMSO-d6)δ:12.61 (1H,s,5-OH),10.27 (1H,s,4′-OH),7.79 (2H,d,J=8.8 Hz,H-2′,6′),6.92 (2H,d,J=8.8 Hz,H-3′,5′),6.79 (1H,d,J=2.0 Hz,H-8),6.46 (1H,d,J=2.0 Hz,H-6),5.55 (1H,d,J=1.6 Hz,H-1″),5.30 (1H,d,J=1.1 Hz,H-1‴),1.13 (3H,d,J=6.1 Hz,7-rha,H-6″),0.80 (3H,d,J=5.5 Hz,3-rha,H-6‴);13C-NMR (100 MHz,DMSO-d6)δ:178.0 (C-4),161.7 (C-7),161.0 (C-5),160.2 (C-4′),157.8 (C-2),156.1 (C-9),134.5 (C-3),130.7 (C-2′),130.7 (C-6′),120.3 (C-1′),115.5 (C-3′),115.5 (C-5′),105.8 (C-10),101.9 (C-1″),99.5 (C-1‴),98.4 (C-6),94.6 (C-8),71.6 (C-4″),71.1 (C-4‴),70.7 (C-3″),70.3 (C-2‴),70.2 (C-2″),70.1 (C-5″),70.1 (C-3‴),69.8 (C-5‴),17.9 (C-6″),17.5 (C-6‴)。以上数据与文献[10]基本一致,故鉴定为山柰酚-3,7-O-α-L-二鼠李糖苷。
化合物4:黄色针晶,ESI-MSm/z:565.2[M+H]+。1H-NMR (400 MHz,DMSO-d6)δ:12.61 (1H,s,5-OH),10.26 (1H,s,7-OH),8.13 (2H,d,J=8.8 Hz,H-2′,6′),6.89 (2H,d,J=8.8 Hz,H-3′,5′),6.83 (1H,d,J=2.0 Hz,H-8),6.45 (1H,d,J=2.0 Hz,H-6),5.56 (1H,brs,H-1‴),5.36 (1H,d,J=5.1 Hz,H-1″),3.84 (1H,brs,H-2‴),3.75 (1H,dd,J=11.7,5.0 Hz,H-2″),3.66 (1H,m,H-4″),3.63 (1H,m,H-3‴),3.58 (1H,m,H-5″),3.52 (1H,m,H-3″),3.41 (1H,dt,J=12.3,6.1 Hz,H-5‴),3.30 (1H,m,H-4‴),3.21 (1H,d,J=9.3 Hz,H-5″),1.12 (3H,d,J=6.1 Hz,H-6‴);13C-NMR (100 MHz,DMSO-d6)δ:177.7 (C-4),161.6 (C-7),160.9 (C-5),160.2 (C-2),156.8 (C-4′),155.9 (C-9),133.8 (C-3),131.1 (C-6′),131.1 (C-2′),120.5 (C-1′),115.3 (C-3′),115.3 (C-5′),105.6 (C-10),101.2 (C-1″),99.4 (C-6),98.3 (C-1‴),94.6 (C-8),71.6 (C-4‴),71.5 (C-3″),70.8 (C-2″),70.3 (C-3‴),70.1 (C-5‴),69.8 (C-2‴),66.0 (C-4″),64.3 (C-5″),17.9 (C-6‴)。以上数据与文献[11]基本一致,故鉴定为山柰酚-3-O-α-L-阿拉伯糖-7-O-α-L-鼠李糖苷。
化合物5:黄色针晶,ESI-MSm/z:595.2[M+H]+。1H-NMR (400 MHz,DMSO-d6)δ:7.34 (1H,d,J=1.3 Hz,H-2′),7.29 (1H,dd,J=8.3,1.3 Hz,H-6′),6.88 (1H,d,J=8.3 Hz,H-5′),6.76 (1H,d,J=1.4 Hz,H-8),6.45 (1H,d,J=1.4 Hz,H-6),5.55 (1H,s,H-1‴),5.26 (1H,s,H-1″),3.98 (1H,brs,H-2‴),3.84 (1H,brs,H-2″),3.64 (1H,d,J=8.8 Hz,H-3‴),3.52 (1H,d,J=7.8 Hz,H-3″),3.45 (1H,m,H-5‴),3.40 (1H,m,H-4‴),3.22 (1H,m,H-5″),3.15 (1H,m,H-4″),1.13 (3H,d,J=6.0 Hz,H-6‴),0.83 (3H,d,J=6.0 Hz,H-6″);13C-NMR (100 MHz,DMSO-d6)δ:178.0 (C-4),161.7 (C-5),160.9 (C-7),157.9 (C-2),156.1 (C-9),148.6 (C-4′),145.3 (C-3′),134.5 (C-3),121.2 (C-1′),120.6 (C-6′),115.7 (C-5′),115.5 (C-2′),105.7 (C-10),101.9 (C-6),99.5 (C-1″),98.4 (C-1‴),94.5 (C-8),71.6 (C-4″),71.2 (C-4‴),70.6 (C-3″),70.4 (C-3‴),70.2 (C-2″),70.1 (C-2‴),70.0 (C-5″),69.8 (C-5‴),17.9 (C-6″),17.5 (C-6‴)。以上数据与文献[12]基本一致,故鉴定为槲皮素-3,7-O-α-L-二鼠李糖苷。
化合物6:淡黄色粉末,ESI-MSm/z:411.2[M+H]+。1H-NMR (400 MHz,DMSO-d6)δ:7.38 (1H,d,J=15.9 Hz,H-7′),7.02 (1H,brs,H-5′),6.96 (1H,br.d,J=8.1 Hz,H-6′),6.76 (1H,d,J=8.1 Hz,H-2′),6.10 (1H,d,J=15.9 Hz,H-8′),5.65 (1H,s,1-OH),5.01 (1H,brs,H-5),3.97 (1H,m,H-3),3.88 (2H,m,H-8),3.57 (1H,brs,H-4),2.11 (2H,m,H-2),1.91 (1H,dd,J=13.2,2.4 Hz,H-6α),1.75 (1H,dd,J=12.0,9.9 Hz,H-6β),1.48 (2H,dq,J=13.6,6.9 Hz,H-9),1.23 (2H,dq,J=14.6,7.2 Hz,H-10),0.79 (3H,t,J=7.4 Hz,H-11);13C-NMR (100 MHz,DMSO-d6)δ:173.2 (C-7),165.4 (C-1′),148.5 (C-7′),145.6 (C-3′),145.1 (C-6′),125.4 (C-4′),121.3 (C-9′),115.8 (C-8′),114.6 (C-2′),113.8 (C-5′),73.1 (C-1),71.1 (C-4),69.4 (C-5),66.9 (C-3),64.1 (C-8),30.0 (C-9),18.5 (C-10),13.5 (C-11)。以上数据与文献[13]基本一致,故鉴定为5-O-咖啡酰基奎宁酸丁酯。
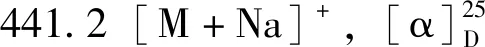
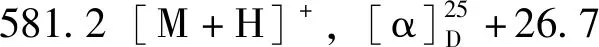
化合物9:白色粉末,ESI-MSm/z:193.1[M-H]-。1H-NMR (400 MHz,DMSO-d6)δ:7.48 (1H,d,J=15.9 Hz,H-7),7.27 (1H,d,J=1.6 Hz,H-2),7.08 (1H,dd,J=8.2,1.6 Hz,H-6),6.79 (1H,d,J=8.2 Hz,H-5),6.36 (1H,d,J=15.9 Hz,H-8),3.81 (3H,s,3-OCH3)。以上数据与文献[16]基本一致,故鉴定为阿魏酸。
化合物10:白色粉末,ESI-MSm/z:163.0[M-H]-。1H-NMR (400 MHz,CD3OD)δ:7.60 (1H,d,J=15.9 Hz,H-7),7.44 (2H,d,J=8.6 Hz,H-2,6),6.80 (2H,d,J=8.6 Hz,H-3,5),6.28 (1H,d,J=15.9 Hz,H-8)。以上数据与文献[17]基本一致,故鉴定为对羟基桂皮酸。
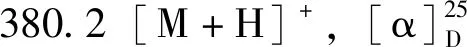
化合物12:白色粉末,ESI-MSm/z:427.4[M+H]+。1H-NMR(400 MHz,CDCl3)δ:5.63 (1H,d,J=6.0 Hz,H-6),3.48 (1H,t,J=5.4 Hz,H-3),1.16 (3H,s,H-23),1.14 (3H,s,H-24),1.09 (3H,s,H-25),1.04 (3H,s,H-26),1.00 (3H,s,H-27),0.99 (3H,s,H-28),0.95 (3H,s,H-29),0.85 (3H,s,H-30);13C-NMR (100 MHz,CDCl3)δ:141.7 (C-5),122.2 (C-6),76.5 (C-3),49.8 (C-10),47.6 (C-8),43.2 (C-18),41.0 (C-4),39.4 (C-22),39.1 (C-13),38.0 (C-14),36.2 (C-16),35.2 (C-19),35.0 (C-9),34.7 (C-21),34.7 (C-29),33.2 (C-11),32.5 (C-30),32.2 (C-15),32.2 (C-28),30.5 (C-12),30.5 (C-17),29.1 (C-24),28.4 (C-20),28.0 (C-2),25.6 (C-23),23.8 (C-7),19.8 (C-26),18.6 (C-27),18.4 (C-1),16.4 (C-25)。以上数据与文献[19]基本一致,故鉴定为粘霉烯醇。
化合物13:白色粉末,ESI-MSm/z:427.4[M+H]+。1H-NMR (400 MHz,C5D5N)δ:5.64 (1H,dd,J=8.2,3.1 Hz,H-15),3.46 (1H,dd,J=10.5,5.7 Hz,H-3),1.27 (3H,s,H-23),1.14 (3H,s,H-24),1.10 (3H,s,H-25),1.03 (3H,s,H-26),1.01 (3H,s,H-27),1.01 (3H,s,H-28),0.98 (3H,s,H-29),0.92 (3H,s,H-30);13C-NMR (100 MHz,C5D5N)δ:158.8 (C-14),117.5 (C-15),78.5 (C-3),56.4 (C-5),49.9 (C-18),49.5 (C-9),42.0 (C-19),39.8 (C-4),39.7 (C-8),38.7 (C-10),38.6 (C-1),38.3 (C-16),38.2 (C-13),37.3 (C-17),36.4 (C-7),35.7 (C-12),34.4 (C-21),33.8 (C-22),33.8 (C-29),30.4 (C-26),30.4 (C-28),29.4 (C-20),29.1 (C-23),28.5 (C-2),26.6 (C-27),21.9 (C-30),19.6 (C-6),18.2 (C-11),16.8 (C-25),16.1 (C-24)。以上数据与文献[20]基本一致,故鉴定为蒲公英赛醇。
化合物14:无色针晶,ESI-MSm/z:427.4[M+H]+。1H-NMR (400 MHz,CDCl3)δ:1.17 (3H,s,H-28),1.04 (3H,s,H-27),1.00 (6H,s,H-26,30),0.95 (3H,s,H-29),0.86 (6H,s,H-23,25),0.72 (3H,s,H-24);13C-NMR (100 MHz,CDCl3)δ:213.4 (C-3),59.6 (C-10),58.4 (C-4),53.2 (C-8),42.9 (C-18),42.3 (C-5),41.7 (C-2),41.4 (C-6),39.8 (C-13),39.4 (C-22),38.4 (C-14),37.6 (C-9),36.1 (C-16),35.8 (C-11),35.5 (C-19),35.2 (C-29),32.9 (C-21),32.6 (C-15),32.2 (C-28),31.9 (C-30),30.6 (C-12),30.1 (C-17),28.3 (C-20),22.4 (C-1),20.4 (C-26),18.8 (C-27),18.4 (C-7),18.1 (C-25),14.8 (C-24),7.0 (C-23)。以上数据与文献[21]基本一致,故鉴定为木栓酮。
4 讨论
竹灵消作为鹅绒藤属植物中的一种,主要化学成分同样也是该属植物的特征性成分——C21甾体类,本研究除了得到了1个C21甾体外,还分离出了13个其他类型的化合物,包括4个黄酮苷类、3个简单苯丙素及其衍生物、2个双四氢呋喃型木脂素、1个生物碱和3个五环三萜。在这些结构类型中,C21甾体、生物碱和三萜已被报道过,而本研究首次从竹灵消中分离得到黄酮苷类、简单苯丙素类和双四氢呋喃型木脂素类化合物,丰富竹灵消中化学成分的结构类型,为竹灵消的进一步开发提供参考。
