Review of Carbon Dots from Lignin:Preparing,Tuning,and Applying
2022-07-21TaoZhangHaimingLiJingpengZhouXingWangLingpingXiaoFengshanZhangYanzhuGuo
Tao Zhang,Haiming Li,Jingpeng Zhou,Xing Wang,Lingping Xiao,Fengshan Zhang,Yanzhu Guo,,3,*
1.Liaoning Key Lab of Lignocellulose Chemistry and BioMaterials, Liaoning Collaborative Innovation Center for Lignocellulosic Biorefinery, College of Light Industry and Chemical Engineering,Dalian Polytechnic University,Dalian,Liaoning Province,116034,China
2.Huatai Group Corp.Ltd.,Dongying,Shandong Province,257335,China
3.Key Laboratory of Pulp and Paper Science & Technology of Ministry of Education, Qilu University of Technology (Shandong Academy of Sciences), Ji'nan, Shandong Province, 250353,China
Abstract: Carbon dots (CDs), emerging carbon materials with unique physical and chemical properties, have drawn extensive attention from researchers.In recent years, many carbon sources have been used as precursors for preparing CDs.In contrast to other types of precursors, lignin,as a renewable and available source of natural aromatic biopolymers, is believed to be a low-cost precursor for the large-scale preparation of CDs.However,the preparation of CDs with excellent optical properties from lignin has some drawbacks because of the complex structure of lignin.Hence, the methods for preparing the CDs from lignin are summarized in this paper,and the mechanism and physical and chemical properties of lignin-based CDs are discussed.Moreover, some approaches to tuning the optical properties of lignin-based CDs have been proposed.Additionally, the use of lignin-based CDs in the fields of sensing, supercapacitor, bioimaging, anti-counterfeiting,and information encryption is reviewed.
Keywords: lignin; carbon dots; fluorescence tuning; sensing; supercapacitor;bioimaging
1 lntroduction
As a spherical zero-dimensional carbon material with nanoscale size and abundant functional surface groups,carbon dots (CDs) have great potential in various fields, such as sensing probe[1], cell imaging[2], vivo imaging[3], drug delivery[4], and photocatalysis[5],which are alternatives to traditional materials[5-8].Since the discovery of CDs by Xu et al[9]in 2004, extensive methods for preparing the CDs have been reported in the literature.In recent decades, various carbon sources,including engineered carbonaceous nanomaterials (e.g.,carbon nanotubes[10]and graphene[11]), molecular precursors (e.g., citric acid[12], phenylenediamine[13],and phthalic acid[14]), and biomass (e.g., cellulose[15],chitosan[16],leaves[17],milk[18],and lignin[19])have been used as raw materials to prepare CDs.In contrast to other carbon sources, biomass is considered a potential carbon source to promote the large-scale production of CDs owing to its eco-friendly, abundant, and inexpensive properties[20].
Lignin, an abundant natural polymer with a unique aromatic structure[21], has drawn considerable attention as a carbon source for preparing CDs.Chen et al[22]first prepared fluorescent CDs with an emission wavelength of 430 nm by the simple and rapid hydrothermal treatment of lignin.Ding et al[23]achieved the gram-scale preparation of CDs from lignin via a combination of oxidized cleavage and hydrothermal treatment.Nair et al[24]prepared blueemissive CDs with a quantum yield(QY)of up to 31%by incorporating Mn, N, and S into the CDs.Although various promising methods for preparing CDs from lignin have emerged, there are still several challenges in the preparation of CDs with excellent optical properties.For instance, lignin-based CDs typically exhibit a wide particle size distribution owing to the complexity of the lignin structure, which is not beneficial to the formation of CDs with stable optical properties[25].Additionally, some methods for tuning the optical properties of traditional CDs are ineffective for lignin-based CDs because of the stable chemical properties of lignin[26].Further, differences in lignin sources and production methods and the lack of a summary on improving the optical properties of ligninbased CDs limit the development of which with excellent optical properties.
2 Methods of preparing CDs from lignin
Generally, the methods for preparing CDs are usually divided into two types,including"bottom-up"and"topdown" methods[27].Lignin, as a biomass macromolecule with a complex chemical structure, is not suitable to be applied in the "top-down" method because of its severe reaction processes and conditions.Therefore,the "bottom-up" approach is typically applied to prepare CDs from lignin owing to its unique advantages, e.g., simplicity, universality, controllability, and cost-effectiveness.
2.1 Hydrothermal treatment
Currently, hydrothermal treatment is the most popular method for preparing CDs from lignin, and it exhibits amounts of favorable advantages, such as low cost,simplicity, controllability, and environmental friendliness.In a typical hydrothermal treatment process,lignin is initially suspended or dissolved in water or organic solvents, which are typically mixed with passivants to tune the fluorescent properties of the CDs.Afterward, the mixtures are transferred into Teflon containers wrapped with stainless steel and heated at a predetermined temperature for a specific time[28].Alkali lignin,a by-product of the alkali pulping process, is widely applied as a carbon source becauseof its excellent solubility in water, which prevents the use of organic solvents or sodium hydroxide to facilitate the dissolution of lignin during the hydrothermal process.Gao et al[29]prepared CDs for monitoring Fe3+in water via the hydrothermal treatment of alkali lignin.The direct preparation of CDs from black liquor, a by-product of the pulping process, is an efficient, energy-saving, and environmentally friendly method.Zhao et al[30]prepared CDs by the hydrothermal treatment of black liquor obtained from poplar sulfate pulping process.Further, several auxiliary approaches have been developed to improve the efficiency of the hydrothermal treatment of lignin.Microwaves were employed in the hydrothermal treatment process because of their excellent heating efficiency, which reduces the reaction time required for hydrothermal treatment.Rai et al[31]prepared ligninbased CDs from lignosulfonate by microwave treatment at 600 W for 10 min.However, microwaveassisted hydrothermal treatment is restricted by the demanding equipment.
The progress of the formation of lignin-based CDs by the hydrothermal treatment is complex.To date,there have been few analyses on the formation mechanism of lignin-based CDs.Therefore, based on previous studies[32],the formation mechanism of ligninbased CDs was summarized herein.During the hydrothermal or microwave-assisted hydrothermal treatment, intermolecular collisions, dehydration reactions, and the breakage of ether bonds occurred in the lignin precursor under high pressure-temperature conditions, which generated crosslinking polymers, as illustrated in Fig.1.As the reaction temperature and reaction time gradually increased, the polymer chains of lignin became entangled with each other.During the hydrothermal treatment, the structure of the ligninbased CDs became more stable owing to the carbonization of lignin[33].The lignin-based CDs in this state possess abundant functional groups, luminescent sites, and a stable nanosized carbon core, thereby possessing excellent optical properties.Nevertheless,with further increases in the reaction temperature and time, the number of functional groups and luminescent sites on the CDs gradually decreased.The formed lignin-based CDs were approximate with states of a"bare core", which displayed unique electrochemical performance because of the existence of abundant microcrystalline regions and lattices in the carbon core.However, the optical properties of the lignin-based CDs in the bare core state were poor; hence, the optimization of reaction conditions (e.g., reaction time and temperature) was critically required to generate the CDs with excellent optical properties.Zhang et al[28]prepared green emission carbon quantum dots (CQDs)with a QY of more than 16% from alkali lignin at 180℃ for 12 h and observed that an inappropriate reaction time or temperature would afford reduced QY values of CQDs.

Fig.1 Illustration of hydrothermal cross-linking polymerization
2.2 Other methods
In addition to hydrothermal pretreatment, other methods have been reported for generating CDs from lignin.Myint et al[34]first prepared carbon nanoparticles from kraft lignin using liquid CO2, and the carbon nanoparticles were further treated by acid oxidation and carbonization to obtain CDs.Shi et al[35]prepared CDs by the direct carbonization of lignin at 300℃ for 2 h under a nitrogen atmosphere without a solvent.Xu et al[36]treated agricultural straw using an ionic liquid to produce CDs, fermentable sugars, and cellulose enzymatic lignin simultaneously.Niu et al[37]obtained CDs from corn lignin through self-assembly at room temperature, which was green, environmentally friendly, rapid,and simple.
3 Approaches to tuning the optical properties of lignin-based CDs
Hydrothermal treatment is the main method for generating CDs from lignin, and the prepared CDs possesssimilar chemical and physical properties compared to traditional CDs, e.g., stable nano size, unique optical properties,abundant functional groups,and low toxicity[38].To date, the fluorescence of CDs from lignin has been mainly based on blue and green lights.The challenge of tuning the fluorescence of lignin-based CDs to long-wavelength light exists owing to the complex molecular structure and stable linkage of lignin.Therefore,researchers have focused on developing approaches to tune the optical properties of ligninbased CDs.
3.1 Doping heteroatoms
Various heteroatoms, e.g., nitrogen[39], sulfur[40], phosphorus[41],and fluorine[42],have been successfully introduced into CDs, which would generate new surface states on the CDs, facilitate a high yield of radiative recombination, and eventually result in increased QY values.
Nitrogen, which has an atomic radius similar to that of carbon, is easily doped into the carbon skeleton,which endows CDs with excellent QY values, wide spectra, and red-shifted emission wavelengths[43].Hence, nitrogen doping is the most important and popular method for tuning the optical properties of CDs.Table 1 shows the QY values and emission wavelengths (Em) of lignin-based CDs with different heteroatoms.It can be concluded that various passivants, such as ammonia and ethylenediamine,have been employed in the hydrothermal process of lignin to dope nitrogen into CDs.The QY value of NCDs obtained from ammonia passivants was 8.2%,which is four times that of the QY value before doping(2.5%)[44].Zhang et al[28]adopted ethylenediamine as a dopant to prepare lignin-based CDs with a QY value of 17.6% and anEmof 525 nm, which is a significant improvement over other lignin-based CDs.The rearrangement of electron holes and radiation theory is mainly used to explain the fluorescence mechanism of heteroatom-doped CDs.The formed electron-donating groups (—NH2) on the surface of the CDs can enhance the conjugation degree of CDs and increase the electron transition from the ground state to the excited state[45].In addition to changing the surface state, the introduction of nitrogen affected the carbon core of the CDs.Pyrrole N and pyridine N are considerably distributed at the edge and center of the carbon core,and graphite N is regularly doped in the skeleton of the carbon core.The introduction of graphite N decreases the energy gap of the CDs, which leads to a redshift in the emission wavelength[39].
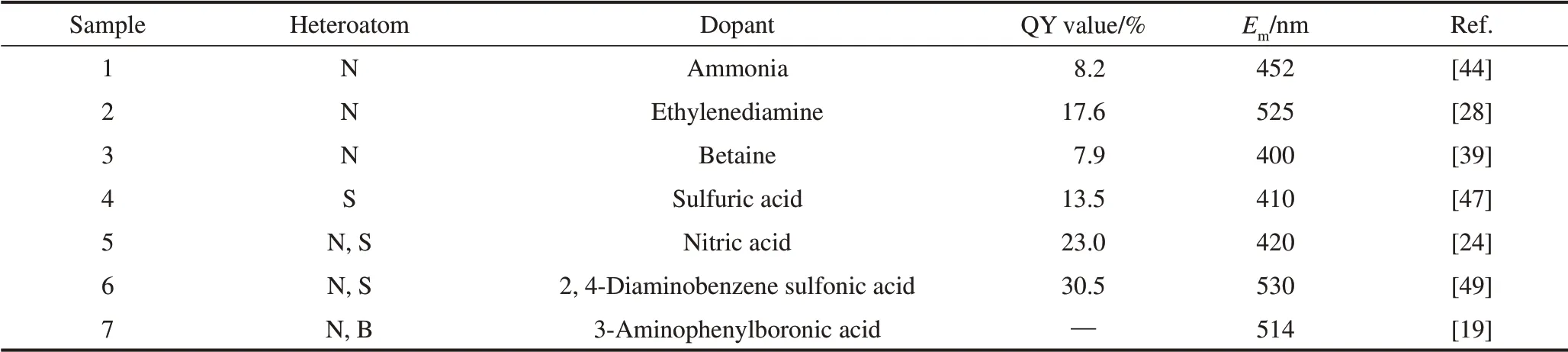
Table 1 Properties of heteroatoms doped lignin-based CDs
In addition to nitrogen, other types of non-metallic elements,such as sulfur and boron,have been doped into lignin-based CDs.Further, sulfur can be used as an electron donor; however, it is difficult to introduce sulfur into the carbon framework, which causes its weak performance in the enhancement of optical properties[46].Yang et al[47]developed a rapid approach to dope sulfur onto lignin-based CDs using sulfuric acid as a passivant.The introduction of sulfur enabled the CDs to become stable in an acidic environment.Furthermore, Dong et al[48]reported a synergistic effect between nitrogen and sulfur in CDs.The introduction of sulfur can enhance the doped nitrogen surface state,change the energy gap, and significantly increase the QY value of the CDs.Zhu et al[49]enhanced the QY value of lignin-based CDs to 30.5% andEmto 530 nm by adjusting the addition of different passivantscontaining nitrogen or sulfur.The simultaneous introduction of boron and nitrogen has been reported to increase the number of emission sites of CDs.Zhu et al[19]prepared triple-emission lignin-based CDs by introducing boron and nitrogen on the surface of CDs.Additionally, fluorine, the most electronegative element, is an effective doping element for tuning the optical properties of CDs[50].The primary function of fluorine doping is to form hydrogen bonds in CDs.Fluorine-doped lignin-based CDs have not yet been reported.However, CDs from other carbon sources doped with fluorine have been successfully prepared.Zuo et al[51]successfully red-shifted theEmof CDs from 500 nm to 550 nm via fluorine doping.Therefore,fluorine doping is a potential method for tuning the optical properties of lignin-based CDs.
Though he knew it would take some hours to get through the forest, he was so anxious to be at his journey s end that he resolved to go on; but night overtook him, and the deep snow and bitter frost made it impossible for his horse to carry him any further
3.2 Pretreatment of lignin
In contrast to other precursors, lignin has structural advantages and disadvantages in the preparation of CDs.The stable linkage between the units in lignin and its heterogeneous molecular weight affords CDs with nonuniform particle sizes, which further affects their optical properties.Contrarily, the aromatic ring in lignin endows lignin-based CDs with a relatively large sp2carbon domain, which enhances the optical properties of CDs[52].To avoid defects in the chemical structure of lignin,some pretreatments have already been performed on lignin to make it an excellent carbon source.Wang et al[38,53]successfully obtained lignin-based nanoparticles from lignin depolymerized using o-aminobenzenesulfonic acid, which was employed as a carbon source to prepare CDs.Furthermore, the generated CDs had smaller particle sizes and more narrow distributions than the other CDs.Zhu et al[54]depolymerized lignosulfonate with nitric acid.CDs prepared using depolymerized lignin as a carbon source had an increased QY value and uniform particle size.Xu et al[55]treated lignosulfonate with nitric acid and ultrasonicated it for 12 h to generate lignin-based CDs with QY value of up to 21%.Notably, the CDs generated using this approach had higher QY value than those reported in other studies.
Meanwhile, introducing other functional groups to lignin before hydrothermal treatment is considered an effective method to tune the optical properties of CDs.Shi et al[35]introduced amine groups into lignin structures by Mannich and Michael's addition reactions.Subsequently, nitrogen-doped CDs with excellent fluorescence stability over a wide range of pH values were prepared using modified lignin as the carbon source.Wang et al[56]introduced nitrogen and sulfur to alkali lignin by co-heating with o-aminobenzenesulfonic acid.The CDs prepared from the modified lignin exhibited excitation-dependent luminescence over a wide range of 377-576 nm.
4 Properties of lignin-based CDs
4.1 Structural properties
The nanoscale morphology of lignin-based CDs is typically measured by transmission electron microscopy(TEM)and is an important physical feature that enables the application of lignin-based CDs in various fields.Fig.2 shows the typical TEM images and size distribution histograms of the lignin-based CDs[37,57].Meanwhile, the lattice structure of lignin-based CDs was measured by high-resolution TEM(HRTEM),which revealed the graphite core structure of the lignin-based CDs.The preparation of samples is critical for obtaining accurate and suitable TEM images.A typical process involves dropping the CD solutions at concentrations ranging from 1 mg/mL to 6 mg/mL on the ultrathin carbon film and distributing it evenly by airdrying.According to previous studies[58], the diameter of lignin-based CDs is in the range of 2-10 nm, which exhibits excellent dispersing ability and a quasi-spherical shape.

Fig.2 TEM image (a) of lignin-based CDs[37] (Copyright 2017 American Chemical Society.); TEM (b), HRTEM (c), and size distribution histogram(d)of magnesium,nitrogen-doped CQDs[57]
Additionally, the chemical structures of lignin-based CDs have been widely characterized using Fouriertransform infrared (FT-IR), X-ray photoelectron spectroscopy (XPS), and Raman spectroscopy.Fig.3 shows typical FT-IR, XPS, and Raman spectra of nitrogen-doped CQDs[28].FT-IR and XPS were used to confirm the chemical structure of the lignin-based CDs.Lignin-based CDs generally contain —OH, C—H,C=O, and C=C groups in lignin and —NH2, C—N,C—S, and —SO2groups, which are typically formed during the passivation process.FT-IR analysis is conventionally used to compare the functional groups in lignin-based CDs and lignin.XPS analysis is widely used for the qualitative determination of functional groups on the surface of lignin-based CDs.Raman spectroscopy is commonly employed to determine the vibrational properties of carbon materials.The Raman spectrum of lignin-based CDs typically shows two typical bands at approximately 1380 and 1600 cm-1,which are associated with the D band related to the sp3defects of carbon and the G band related to the in-plane vibration of sp2carbon, respectively.Meanwhile, the intensity ratio of the D and G bands (ID/IG) was calculated to confirm the graphitization degree of the CDs.
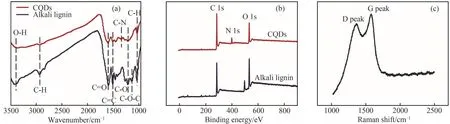
Fig.3 FT-IR(a),XPS(b),and Raman spectra(c)of CQDs[28](Copyright 2019 John Weily and Sons Inc.)
4.2 Optical properties
Ultraviolet-visible (UV-vis) spectroscopy is typically used to characterize the energy level transitions ofchromophores in lignin-based CDs, and the main functional groups that affect the optical properties of the lignin-based CDs can be revealed.For instance, a typical UV-vis absorption spectrum of a sulfur-doped CDs (SCQDs) prepared from lignin is shown in Fig.4(a).The UV-vis absorption spectrum of ligninbased CDs typically has an obvious and strong peak located between 230 and 270 nm, which can be ascribed to the π-π* transition of C=C or C=N bonds[23].The absorption peak between 270 and 360 nm corresponds to the n-π* transition of the C=O bonds.Moreover, the absorption peaks in the region of 360-600 nm are generally related to the transition of the functional ligands on the surface of the lignin-based CDs[54].
Lignin-based CDs have unique fluorescent properties,which make them promising materials for applications in various fields.In most cases,the emission wavelength of lignin-based CDs is longer than the corresponding absorption wavelength, indicating that the emission energy is lower than the absorption energy[18].At present,the optimal emission wavelengths of the prepared ligninbased CDs are mainly located in the blue and green regions, and the optimal excitation wavelengths are mostly at approximately 365 nm.The lignin-based CDs obtained using different approaches typically exhibit similar excitation-emission wavelengths but different fluorescence properties, such as excitation-dependent behaviors.As shown in the photoluminescence (PL)emission spectra of the CDs prepared from prehydrolyzed lignin(Fig.4(b)),the maximum PL emission peak of the CDs is centered at 405 nm under a 320 nm excitation wavelength[47].Meanwhile, the emission wavelength was slowly red-shifted, accompanied by a decrease in the fluorescence intensity as the excitation wavelength increased from 280 nm to 460 nm.This excitation-dependent behavior can be explained by the differences in the emissive trap sites on each CDs[59-60].Yellow CQDs (Y-CQDs)[49]with invariable emission peak positions show almost excitation-independent behavior, indicating that they possess relatively few surface-state defects.

Fig.4 UV-vis absorption (a) and PL emission spectra (b) of CDs[47]; UV-vis absorption and PL emission spectra (c) of Y-CQDs[49](Copyright 2021 American Chemical Society.)
5 Applications
Owing to their excellent properties such as stable nanoscale size, unique fluorescent properties, low cytotoxicity, and outstanding biocompatibility, lignin-based CDs have been widely applied in various fields, as illustrated in Fig.5.
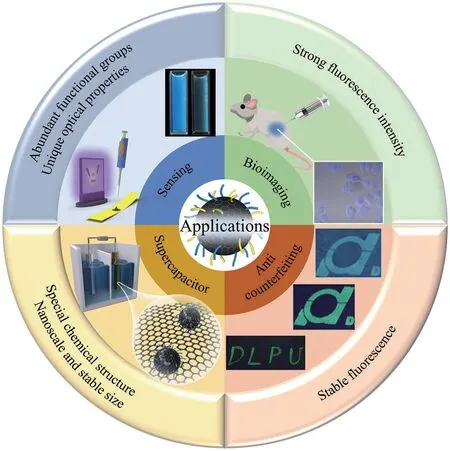
Fig.5 Schematic diagram of the application of lignin-based CDs
5.1 Sensing
Lignin-based CDs exhibit excellent optical properties with negligible changes in intensity, even after longterm storage.This extraordinary and long-term stability of optical properties makes CDs promising candidates for application in the fields of detectors or sensors for pH values, metal ions, and gases[37].Photoinduced electron transfer, resonance energy transfer,photoinduced charge transfer, and the inner filter effect are the main mechanisms that illustrate the sensing ability of CDs[61].Lignin-based CDs have abundant functional groups, such as hydroxyl, carboxyl, and amino groups, which can be effectively coupled withtarget ions, leading to the fluorescence quenching of lignin-based CDs.Yang et al[47]prepared lignin-based CDs (SCQDs) to detect Sudan I (Fig.6(a) and Fig.6(b)).The lowest limit of detection (LOD) was calculated to be 0.12µmol/L.Moreover,the introduction of lignin-based CDs into other functional materials can impart other functions into lignin-based CDs while maintaining their optical properties.Wang et al[62]fabricated a composite film from nitrogen-doped CQDs and poly(vinyl alcohol) to detect formaldehyde (FA) in water and air through color change.The LOD for the FA solution was estimated to be 4.64 mmol/L.The fluorescence of the CQD film changed significantly after exposure to gaseous FA.As shown in Fig.6(c),Yang et al[57]used magnesium and nitrogen-doped CQDs as sensors to detect the pH values of solutions,which exhibited different emission wavelengths at different pH values.

Fig.6 PL emission spectra at various Sudan I concentrations(a);linear relationship between I0/I(I0 and I denote the PL intensities of SCQDs solution in the absence and presence of Sudan I,respectively)and Sudan I concentration(C)[47](b);fluorescence of magnesium,nitrogen-doped CQDs at different pH values(c)[57]
5.2 Supercapacitor
Owing to their excellent electron transfer/reservoir behavior, nano-scale size, uniform dispersion, and edge quantum properties, lignin-based CDs have been developed to improve the electrochemical performance of supercapacitors[63].Furthermore, the introduction of heteroatoms, such as nitrogen or oxygen can promote redox reactions with the electrolyte and increase the additional pseudocapacitance contribution.Cui et al[64]dispersed lignin-based CDs into graphene sheets via p-p stacking and electrostatic interactions to prepare asupercapacitor with a relatively high specific capacitance.Ding et al[65]constructed a heterojunction of graphene quantum dots (GQDs) and graphene from lignin to boost the high specific capacitance and ultrafast charging speed because the addition of GQDs afforded highly dispersed graphene sheets, allowing for increased electron storage capacity and efficient diffusion.
5.3 Bioimaging
Owing to the better biocompatibility and lower toxicity than other CDs derived from small organic molecules,lignin-based CDs have great potential for applications in the field of bioimaging.Furthermore, their stable nanoscale size, high water solubility, and unique optical properties facilitate the use of lignin-based CDs in the field of bioimaging.Wang et al[53]used the ligninbased CDs as bioimaging agents in cells, as ligninbased CDs can emit bright light under excitation.
5.4 Anti-counterfeiting and information encryption
According to previous studies, lignin-based CDs exhibit outstanding stability under complex conditions and strikingly excellent solubility in different solvents (e.g.,water, ethanol, and dimethylsulfoxide (DMSO)),making them suitable candidates for fluorescent inks.After the ink is written onto the substrate (e.g., certain flexible films, paper, textiles, and clothes), the ligninbased CDs are homogeneously dispersed on the surface of the substrate and retain their excellent fluorescence properties[66].Wang et al[53]wrote "50" on currency using lignin-based CDs solution.The "50" is invisible under daylight but illuminated by UV light.Luminescence printing is typically used in combination with the lignin-based CD fluorescent ink because of its convenience, facile design, simplicity, and easy handling.Zhu et al[49]printed Y-CQD inks prepared from alkali lignin on paper.The pattern printed on the paper exhibited green fluorescence under the excitation of UV light; however, the pattern was invisible under daylight.Owing to their outstanding durability, ecofriendliness, and reliability, lignin-based CDs exhibit great potential for anti-counterfeiting and information encryption.
6 Summary and outlook
In this review, the preparation methods, formation mechanism, physical and chemical properties, and approaches to tuning the optical properties of ligninbased carbon dots (CDs) are summarized.The ligninbased CDs are typically prepared by the "bottom-up"method, which has the advantages of simplicity,versatility, controllability, and low cost.The crosslinking polymerization and dehydration is the main reaction throughout the "bottom-up" method and promotes the formation of CDs with nanoscale morphology.Hydrothermal treatment is widely used to prepare lignin-based CDs.Meanwhile,the passivation of heteroatoms and the pretreatment of lignin to improve the optical properties of lignin-based CDs have been introduced.Moreover, the applications of lignin-based CDs in sensing, supercapacitors, bioimaging, anticounterfeiting, and information encryption are summarized.In conclusion, this paper highlights the value-added utilization of lignin and provides new ideas for developing alternatives to semiconductor QDs.
Author contributions
The manuscript was written through contributions from Yanzhu Guo and Tao Zhang, with input from all authors.Data were collected by Tao Zhang, Haiming Li,and Xing Wang.The manuscript has been proofread by Yanzhu Guo, Jingpeng Zhou, Lingping Xiao, and Fengshan Zhang.All the authors approved the final version of the manuscript.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation (22078036), China Postdoctoral Science Foundation (2021M691106), Shandong Postdoctoral Innovation Project (202102050), the Foundation(KF202022)of Key Laboratory of Pulp and Paper Science & Technology of Ministry of Education of China,and Liaoning BaiQianWan Talents Program.
杂志排行
Paper and Biomaterials的其它文章
- Nanocomposite Hydrogel Materials for Defective Cartilage Repair and Its Mechanical Tribological Behavior—A Review
- Octadecylamine Graft-modified Cellulose Nanofiber and Its Reinforcement to Poly(butylene adipate-co-terephthalate)Composites
- NIR-responsive Collagen-based Sponge Coated with Polydimethylsiloxane/Candle Soot for Oil-Water Separation
- Formation and Collapse of Cellulose Nanocrystals and Hydrophobic Association-induced Dual Cross-linked Nanocomposite Hydrogels:A Rheological Study
- Stabilizing Pickering Emulsions Using Octenyl Succinic Anhydride Modified with Cellulose Nanofibrils
- Technical Evaluation of Hybrid Clones of Corymbia spp.to Produce Market Pulp
