NIR-responsive Collagen-based Sponge Coated with Polydimethylsiloxane/Candle Soot for Oil-Water Separation
2022-07-21MinZhangChenchenGuoYuWangYunlongZhangHuiWuLiulianHuangYonghaoNiCuicuiDingLihuiChen
Min Zhang,Chenchen Guo,Yu Wang,Yunlong Zhang,Hui Wu,Liulian Huang,Yonghao Ni,2,Cuicui Ding,Lihui Chen
1.College of Material Engineering, Fujian Agriculture and Forestry University, Fuzhou, Fujian Province,350002,China
2.Department of Chemical Engineering and Limerick Pulp & Paper Centre, University of New Brunswick,Fredericton,E3B 5A3,Canada
3.College of Ecological Environment and Urban Construction, Fujian University of Technology,Fuzhou,Fujian Province,350108,China
Abstract: To fabricate an oil-water separation material that is rich in source,eco-friendly, and responsive, in this study, we successfully developed a collagen-based sponge for application to oil-water separation based on a green and facile strategy.In this design,widely-available collagen(COL)was used as the substrate: it was immersed in polydimethylsiloxane (PDMS)suspension with candle soot (CS) nanoparticles, followed by hot curing.The resultant sponge (CS/PDMS-COL) possessed good hydrophobicity with a water contact angle of 148.3° under a low PDMS concentration of 2%.The results from field emission scanning electron microscope, Fourier transform infrared spectrometer, X-ray photoelectron spectrometer, and X-ray diffractometry demonstrated the successful coating of CS and PDMS on the surface of COL substrate.The CS/PDMS-COL can adsorb eight oils,with the adsorption capacity for trichloromethane reaching 95 g/g.With benzene as the target adsorbent, the separation efficiency was maintained at no less than 95%even after recycling 20 times.CS/PDMS-COL was also used to separate oil-in-water emulsion.Moreover,the sponge killed bacteria effectively due to its excellent near-infrared photothermal responsiveness.This study provides new insight into the preparation of facile oil-water separation materials based on naturally occurring biomaterials effortlessly.
Keywords: collagen sponge; oil-water separation; candle soot; photothermal effect; antibacterial property
1 lntroduction
Oil leakage and the discharge of oily sewage cause serious damage to the environment.Traditionally,adsorption materials such as wool fabric[1], zeolite[2],and activated carbon (AC)[3]are used to manage the resulting oil-water mixtures.However, there is need to find solution to the disadvantages, including weak adsorption capacity and poor recoverability.Recently,a series of adsorption materials with special wettability has been developed,with improved adsorption capacity and selective adsorption performance for oil or organic solvents[4-6].Nevertheless, fabricating a facile oil-water separation material that is extensive in availability,ecofriendly, and intelligently responsive to external stimuli in a facile way,is still challenging.
Collagen (COL) is the most abundant structural protein widely distributed in tissues such as the tendon,skin, intestine, cornea, and blood vessels of some mammals and marine organisms[7].Due to its excellent characteristics, including processability, biocompatibility,and degradability, collagen has great application potential in the leather industry, tissue engineering, pharmaceuticals, foods, and cosmetics[8-9].Specifically, native collagen can be molded into different forms, including fibers, membranes, sheets, gels, tubes, pellets, powders,nanoparticles, and sponges[8,10-11].Of these, the collagen sponge, which can be easily harvested from the freezedrying process for collagen solution, provides a crucial network for bulk storage of oil.Meanwhile, the abundant active groups (such as amino, carboxyl, and hydroxyl groups) on the collagen molecular chains lay a structural foundation for recombining collagen with other components to achieve low wettability and versatility.Based on the advantages mentioned above,the collagen sponge can be regarded as a promising candidate for oil-water separation.
Organosilicon compounds are commonly used in the fabrication of hydrophobic materials.An example is polydimethylsiloxane (PDMS), a non-toxic silicone polymer with low surface energy.Due to its good dispersibility in solvents and the facilein-situcuring ability, PDMS has gained wide popularity in the hydrophobic modification of oil-water separation materials through a simple dip-coating method.For example, Zhai et al[12]prepared a PDMS@SiO2@tungsten disulfide (WS2) sponge with a water contact angle of 158.8° by an immersion coating method, in which PDMS coating was used to fix mixed WS2particles and hydrophobic SiO2nanoparticles onto the sponge.Shi et al[13]combined titanium dioxide (TiO2)particles with AC to form AC-TiO2powder.Afterward,PDMS was grafted onto hydrophilic AC-TiO2powder to prepare superhydrophobic coatings under UV light.This material shows good oil-water separation and antifouling ability.Li et al[14]developed a high-performance separation membrane by embedding UiO-66 as sizesieving sites within the supramolecular fiber structure of collagen fiber membrane (CFM), followed by coating with PDMS.
Candle soot (CS), a low-cost particle material, is produced by incomplete combustion in the middle area of the flame[15-16].Compared with other inorganic particles,such as graphene and carbon nanotubes, CS has the advantages of convenient preparation and scalable production[17].Because CS is composed of carbon black,it has good inherent lipophilicity.In addition, CS can accumulate on the surface to form a coral-like rough structure, leading to the hydrophobic effect without modifying low surface energy substances.Over the past decade, CS has received rapidly increasing attention in fabricating various hydrophobic interfaces[18-21].However,the poor stability of CS coating makes it prone to destruction[22]; therefore, a reinforcement strategy is essential to overcome this defect.
In this study, PDMS and CS were mixed to make a precursor suspension in the first step, after which the collagen sponge was immersed in the mixture.Following curing under heating, the sponge was covered in silane containing CS nanoparticles to prepare a hydrophobic collagen-based material.In this design, the coral-like CS particles were firmly fastened to the cured PDMS to form a rough structure with low surface energy.The resultant modified sponge has a high absorption capacity for a wide variety of oils fromoil-water mixtures.Moreover, the inclusion of CS endowed the modified collagen sponge with an excellent photothermal effect.This study gives new insight into the preparation of intelligent collagenbased sponges for oil-water separation through a facile strategy.
2 Experimental
2.1 Materials
COL was extracted and refined from bovine split according to a previously described method[23]; CS was collected from candle flame with a glass slide.PDMS(Sylgard 184) was purchased from Dow Corning Corporation; the candle was purchased from a local market;benzene (AR) was produced by Vokai Biological Co.,Ltd.(Shanghai, China);n-hexane, dichloromethane(AR) were produced by Xilong Science Co., Ltd.(Shantou, China); Sudan Red III was purchased from Aladdin Co., Ltd.(Shanghai, China); methyl orange and methyl blue were provided by Innochem Co., Ltd.(Beijing,China);Escherichia coli(E.coli,ATCC 8739)andStaphylococcus aureus(S.aureus, ATCC 29213)were provided by Luwei Technology Co., Ltd.(Shanghai, China).The water used in all the experiments was deionized.
2.2 Preparation of CS/PDMS-COL sponge
A volume of 2 mL of the COL solution (10 mg/mL with 0.05 mol/L acetic acid as solvent) was added to a 10-mL glass bottle, sonicated in an ice bath until the bubbles were eliminated completely, and freeze-dried for 24 h to obtain the porous COL sponge.PDMS and its curing agent(10∶1,V/V)were diluted withn-hexane to achieve a series of concentrations (0.1%, 0.3%, 0.5%,1%, and 2%,V/V).CS was weighed (0.04, 0.06, 0.08,0.10, and 0.12 g) and added to 10 mL of PDMS solution,followed by ultrasonic treatment in an ice bath for 2 h to make it evenly dispersed in PDMS solution.PDMS (2%) was added dropwise into the COL sponge until it is saturated, and then it was cured in an oven at 50℃ for 6 h.The cured PDMS-COL sponge was immersed in the CS-PDMS mixed solution for 10 s, and again,it was cured in an oven at 50℃ for 6 h to obtain a CS/PDMS modified collagen(CS/PDMS-COL)sponge.
2.3 Structural characterization of CS/PDMS-COL sponge
The chemical structure of the sample was determined using the Fourier transform infrared spectrometer (FTIR, Nicolet IS10; Thermo Fisher Scientific, USA) with a range of 400-4000 cm-1.The elemental composition on the surface of the sample was examined using X-ray photoelectron spectrometer (XPS, ESCALAB250XI;Thermo Fisher Scientific, USA).The crystal structure was analyzed using an X-ray diffractometry (XRD,Xpert3; CEM, USA) with Al-Kαas the X-ray source.The morphology was observed with a field emission scanning electron microscope (FE-SEM, Navo FEI Nano SEM 230; FEI, USA), and the element distribution on the surface was measured with the equipped X-ray energy dispersive spectrometer (EDS,Elect Super;EDAX,USA).
2.4 Hydrophobicity and dynamic adhesion performance of CS/PDMS-COL sponge
A series of liquids was dropped onto the surface of the CS/PDMS-COL sponge to observe the state of the water droplets.The contact angles from 3 different areas for each sample were measured with a contact angle meter (DSA 30; Kruss, Germany).The dynamic adhesion of CS/PDMS-COL sponge to water was also examined.
2.5 Oil-Water separation performance of CS/PDMSCOL sponge
2.5.1 Static selective oil-water separation
Light oil (n-hexane) and heavy oil (dichloromethane)were selected to assess the static oil absorption capacity of CS/PDMS-COL sponge.The two oils were dyed separately with Sudan Red III for observation purposes.After adding the oil phase to water for 30 s, a piece of CS/PDMS-COL sponge was clamped with a tweezer to adsorb the oil in the water.
2.5.2 Dynamic continuous oil-water separation
The water andn-hexane dyed with methyl blue and Sudan Red III, respectively, were poured into beaker Awith a volume ratio of 1∶1.The CS/PDMS-COL sponge was trimmed into a thin slice and placed onto the mouth of an empty beaker B.The oil-water mixture in beaker A was poured slowly into beaker B to examine the oil-water separation capacity.
In another experiment,the water was dyed with methyl orange.The oil-water separation suction filter device was set up and equipped with a piece of CS/PDMS-COL sponge.Dichloromethane (colorless) and water (yellow)with a volume ratio of 1∶1 were injected into the device in order.A vacuum tube connected to the suction filter port was jointed with a small vacuum pump that provides the external driving force.After the suction filtration was completed, the oil and water phases were collected separately.The separation efficiency (η) was obtained using the following formula[24]:

wherembis the mass of oil before oil-water separation,andmais the mass of oil after oil-water separation.
2.6 Static adsorption of CS/PDMS-COL sponge
2.6.1 Determination of oil absorption capacity
The initial weight (W0) of the CS/PDMS-COL sponge was weighed, and then it was completely immersed in different oil phases.When the adsorption equilibrium was attained, the CS/PDMS-COL sponge was weighed again (W1).The adsorption capacity (Q) is calculated based on the following formula[25]:

The absorption capacity for each type of oil was measured three times in parallel, and the average value was calculated.
2.6.2 Reused performance of CS/PDMS-COL sponge
The reused performance of CS/PDMS-COL sponge was tested with benzene as the oil phase.After the completed adsorption, the oil phase was removed by mechanical extrusion, and the CS/PDMS-COL sponge was carefully washed three times with ethanol and then dried in an oven at 60℃ for reuse.The adsorptiondesorption was repeated 20 times.The water contact angle and oil absorption capacity were recorded after each cycle.
2.7 Emulsion separation performance of CS/PDMSCOL sponge
According to a previous work[26], toluene and water were mixed at 1∶99 (V/V), adding 0.1 mg/mL sodium dodecyl sulfate (SDS) as the emulsifier.After stirring vigorously for 2 h (2000 r/min), the mixture was ultrasonicated in an ice bath for 2 h(300 W)to obtain a stable oil-in-water emulsion.A piece of CS/PDMS-COL sponge was clamped with a tweezer and moved continuously in the emulsion to absorb the oil.
2.8 Near-infrared light (NIR)-responsive antibacterial performance of CS/PDMS-COL sponge
E.coliandS.aureuswere used to examine the NIRresponsive antibacterial ability of the CS/PDMS-COL sponge.First,1 mL of bacterial solution(1×107CFU/mL)was dispersed in a sterilized agar medium.Afterward,CS/PDMS-COL sponge was placed in 1 mL of bacterial suspension and exposed to laser radiation (808 nm,0.7 mW/min) for 2 min.The temperature changes of the samples were recorded, and the NIR camera was used to collect images.The bacterial suspension was spread on an agar plate (Petri dish) and incubated at 37℃ for 24 h.The colony-forming units on the agar plate were counted,and the inhibition rate was calculated based on the following equation:
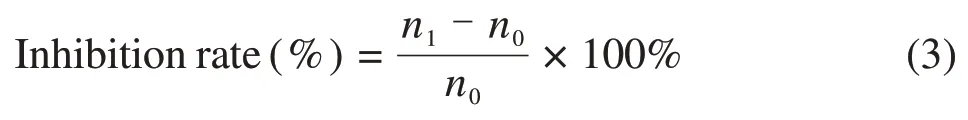
wheren0represents the number of colonies in the blank, andn1represents the number of colonies in the CS/PDMS-COL sponge.
3 Results and discussion
3.1 Structural characterization of CS/PDMS-COL sponge
The mechanism responsible for the superhydrophobicity of the prepared surfaces is illustrated in Fig.1(a).Firstly,the porous COL sponge absorbed the uncured CS/PDMS mixture through a straight dipping process.After that,under the influence of heating(60℃),the PDMS mixture cures rapidly while the CS nanoparticles get embedded into the cured surface.The CS nanoparticles thatpartially stuck out of the surface combined with the inherent low surface energy of the PDMS and provided an excellent hydrophobicity property to the surface of CS/PDMS-COL.

Fig.1 Schematic of co-deposition of CS and PDMS onto the COL sponge (a); FT-IR spectra (b), XPS spectra (c), and XRD patterns(d)of the samples
Fig.1(b) shows the FT-IR spectra of COL, CS, CS/PDMS, and CS/PDMS-COL sponge.In COL, the peaks at 1652,1546,and 1238 cm-1are the characteristic peaks of native COL indicative of amide I, II, and III bands,respectively[27].The strong and broadband in CS at approximately 3430 cm-1is caused by the stretching vibration of the —OH group.The peak at 1622 cm-1indicates the existence of —OH bending vibration and skeletal vibration[28].The band at 2918 cm-1is attributable to the—CH asymmetric stretching vibration of—CH2,and the band at 2848 cm-1is attributed to the symmetric and asymmetric vibrations of the —CH3group[29-30].Similarly, the peak at 1385 cm-1reflects the contribution of C—H stretching vibration, which corresponds to the —CH2functional group[31-32].The peaks at 884, 833, and 752 cm-1are related to the substituted aromatic C—H groups, which can enhance hydrophobicity[31].In the spectrum of CS/PDMS, there are Si—C vibration peaks at 1261 and 802 cm-1and Si—O—Si vibration peaks at 1024 and 1293 cm-1[33],indicating the combination of CS and PDMS.CS/PDMS-COL sponge has the characteristic peaks from both COL and CS/PDMS, indicating that the CS/PDMS mixture was successfully coated on the COL substrate.
XPS was used to analyze the surface status of the CS/PDMS-COL sponge (Fig.1(c)).The COL contained the elements C,N,and O[34],while the peaks of C,O,and Si were observed in the spectrum of PDMS[35].The peaks of CS appear at 531 and 283 eV, indicative of the O and C elements[36].For CS/PDMS-COL sponge, the peaks of C 1s,O 1s,Si 2s,and Si 2p were also observed,implyingthat CS/PDMS was successfully composited onto the COL sponge surface.
Fig.1(d) shows the XRD patterns of COL, CS, and CS/PDMS-COL sponge.The unmodified COL has no sharp peak due to its amorphous structure.The peaks of CS were found at 2θ=24.3°and 42.5°,corresponding to the hexagonal graphite lattice[37-38].The intense, broad peak at 2θ=24.3° is also a characteristic of the amorphous state of the material[39-40].Compared with the pristine COL and CS, CS/PDMS-COL sponge showed the diffract peaks comprised of both of the two components, which further demonstrated that CS was successfully compounded with the COL substrate.
3.2 Microstructure of CS/PDMS-COL sponge
The morphology of CS/PDMS-COL sponge was characterized by FE-SEM (Fig.2(a)).The porosity and average pore size of CS/PDMS-COL sponge were 67.4% and 52.7 µm (CS dosage was 0.08 g and PDMS concentration was 1%), respectively, as determined using the Image J software.These results show that CS/PDMS-COL sponge is highly porous and thus can provide sufficient storage space for the oil phase.The inner surface of the hole was rough,which was due to the coating of irregular CS nanoparticles.Upon increasing the magnification, it was observed that the CS nanoparticles were not naked but covered with a thin PDMS layer, which confirmed the co-deposition of PDMS and CS.Meanwhile, CS was steady due to the fixed action of the cured PDMS coating.From the EDS images of CS/PDMS-COL sponge (Fig.2(b)), it can be observed that C, O, and Si elements were uniformly distributed on the skeleton structure, further testifying to the successful combination of the materials.
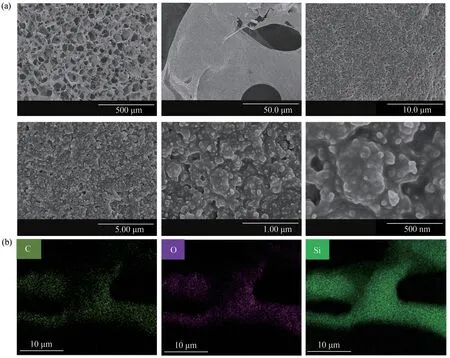
Fig.2 FE-SEM(a)and EDS(b)images of CS/PDMS-COL sponge
3.3 Influence of CS and PDMS on the hydrophobicity of CS/PDMS-COL sponge
As the two most important factors in the preparation,the influences of CS dosage and PDMS concentration on the hydrophobicity of CS/PDMS-COL sponge were studied.With the increase in CS dosage from 0.04 g to 0.08 g (in 10 mL PDMS), the water contact angle of CS/PDMS-COL sponge increased rapidly (Fig.3(a)).The increased hydrophobicity is related to the successful construction of rough surfaces with low surface energy.In addition, as shown in Fig.3(b), when a low PDMS concentration (0.1%) was used, a high value(145.6°) of water contact angle for the CS/PDMS-COL sponge could be obtained.As PDMS concentration increased to 2.0%, the water contact angle increased slightly to 148.3°.
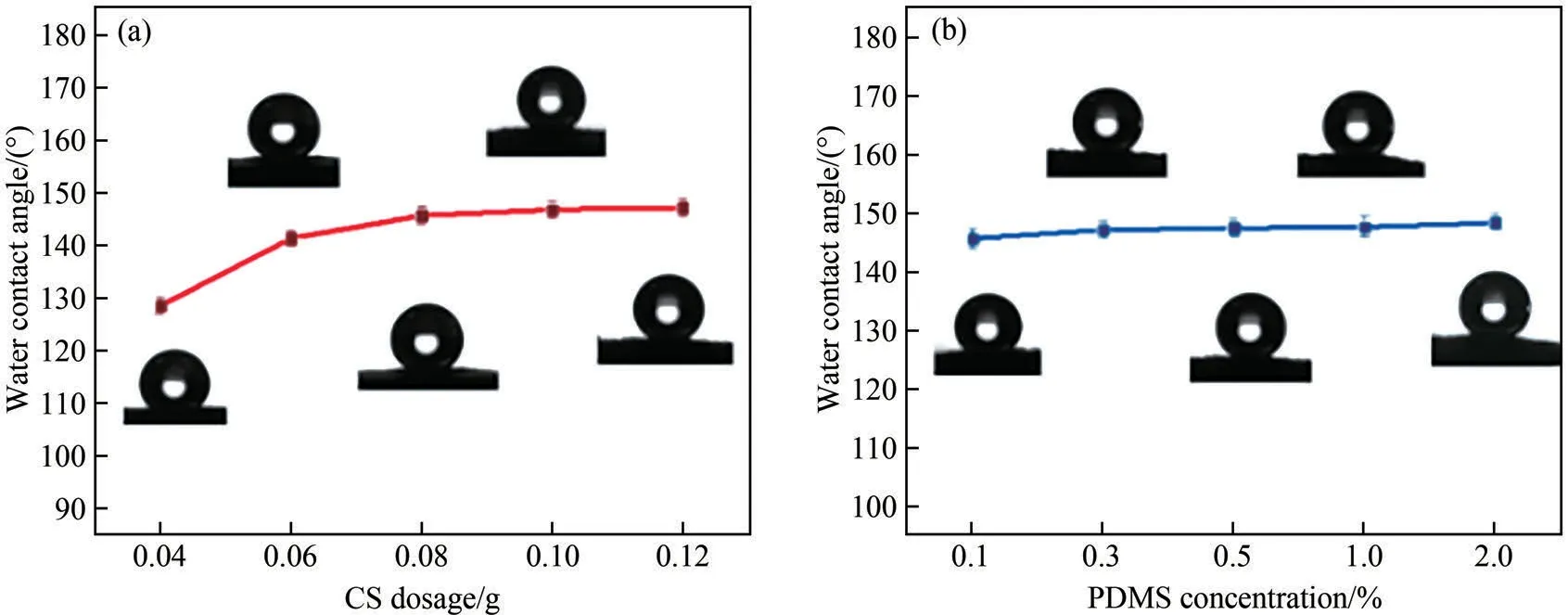
Fig.3 Influences of CS dosage(a)and PDMS concentration(b)on the water contact angle of CS/PDMS-COL sponge
3.4 Hydrophobicity and dynamic adhesion of CS/PDMS-COL sponge
A series of droplets, including aqueous acid (1 mol/L HCl), syrup, green tea, milk tea, milk, brine, coffee,and aqueous alkali (1 mol/L NaOH), were added to the surface of CS/PDMS-COL sponge to examine its hydrophobicity.As shown in Fig.4(a), all the droplets were near-spherical on the surface of CS/PDMS-COLsponge, suggesting that the sponge possessed an excellent tolerance to different chemical environments of solutions.
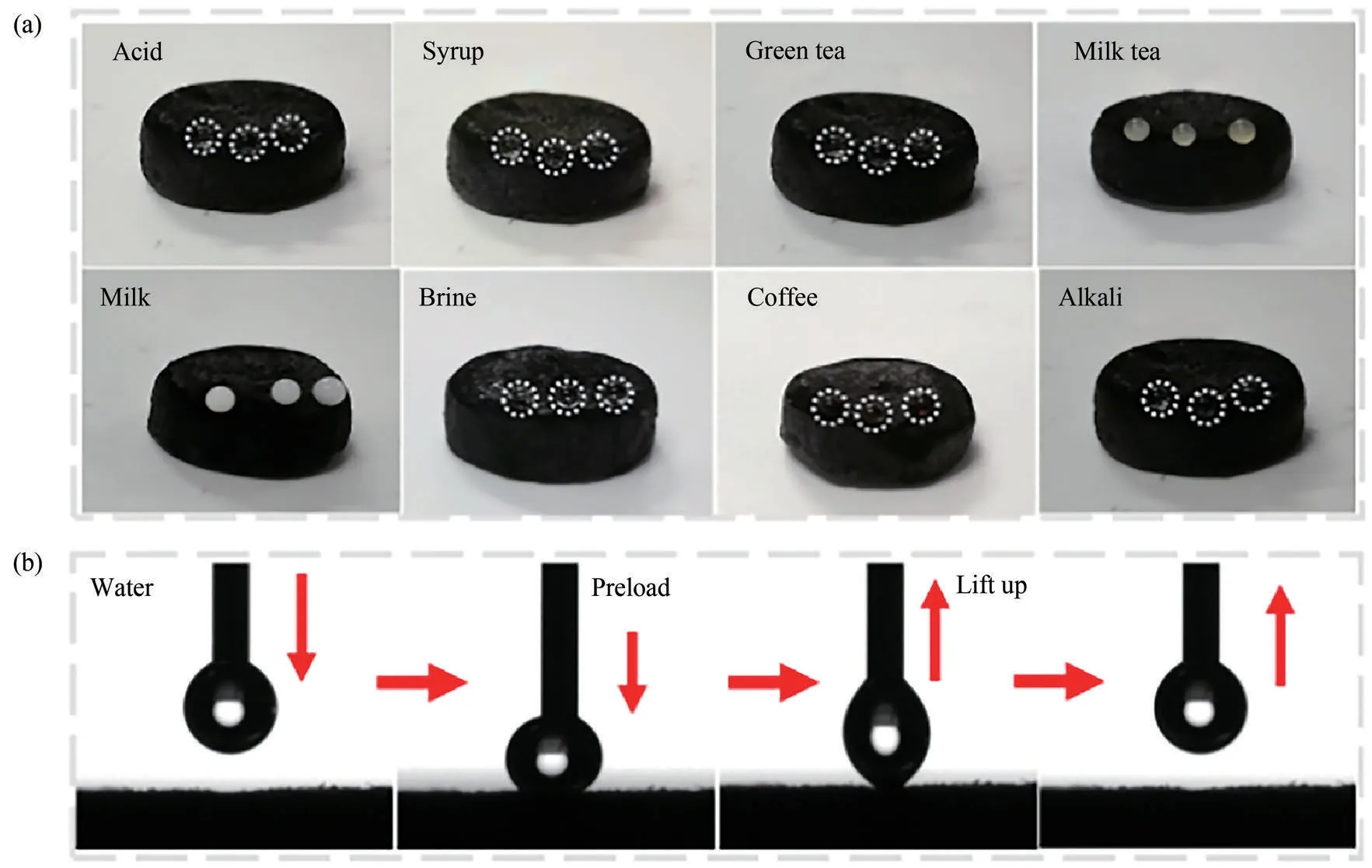
Fig.4 (a)Hydrophobic performance of CS/PDMS-COL sponge with different kinds of droplets;(b)dynamic adhesion performance of CS/PDMS-COL sponge in the air
As shown in Fig.4(b), the dynamic adhesion of water droplets in the air was tested.During the process of preloading and lifting up, no residual water could be observed on the surface of the CS/PDMS-COL sponge.The low adhesion property of the coating in the air is attributed to the low surface energy of PDMS and the rough CS micro-nano structures on the wall of the COL sponge substrate.
3.5 Oil-Water separation performance of CS/PDMSCOL sponge
As shown in Fig.5(a), CS/PDMS-COL sponge easily absorbed the low-density light oil phase (n-hexane)floating on the water.With regards to high-density oil(dichloromethane) (Fig.5(b)), the CS/PDMS-COL sponge could also completely remove the oil phase.The oil phases captured in the sponges were separated conveniently through manual squeezing.Thus, upon applying the CS/PDMS-COL sponge, it not only achieved rapid oil-water separation but also recovered the separating materials conveniently.
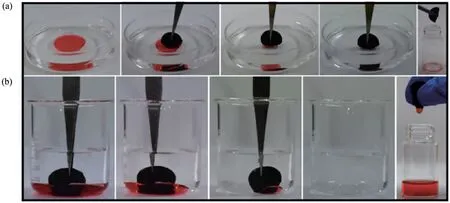
Fig.5 Separation of light oil-water mixture(a)and heavy oil-water mixture(b)by CS/PDMS-COL sponge
As shown in Fig.6(a), a piece of CS/PDMS-COL sponge as an oil-water separation medium was placed above an empty beaker.When pouring the oil-water mixture into the sponge sheet through a glass rod, the redn-hexane passed through the sponge sheet quickly and reached the bottom of the beaker.Meanwhile, the blue water still stayed above the CS/PDMS-COL sponge during the pouring process without filtering out together with the oil phase.As shown in Fig.6(b), when the vacuum pump is started, the colorless dichloromethane chloride is filtered out while the yellow water is kept in the pipe above the device.As the separation of oil and water was completed, the vacuum pump was turned off.No dripping was observed within 30 s after this operation.
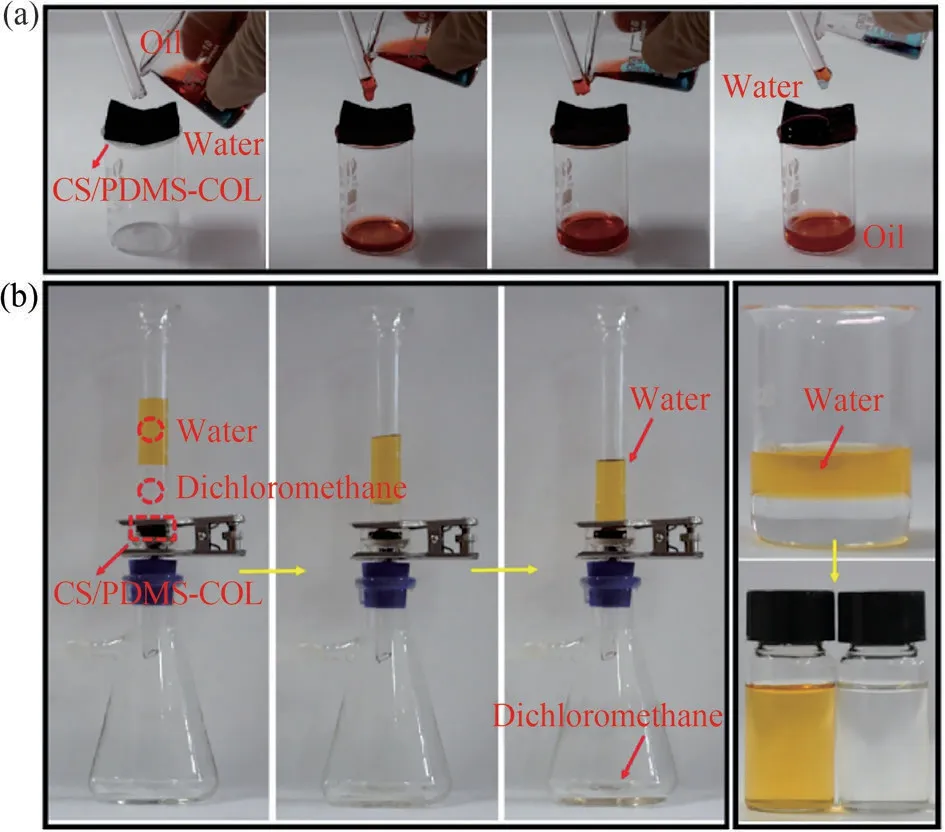
Fig.6 (a) Continuous oil-water separation performance in sponge sheet; (b) continuous oil-water separation performance under external drive
3.6 Qualitative analysis of oil-water separation
As reflected in Fig.7(a), CS/PDMS-COL sponge can effectively adsorb various organic solvents, and the adsorption capacity ranges from 57 (kerosene) to 95(trichloromethane) times its initial weight.Among these oils, the adsorption capacity towards benzene reached 78 g/g, which decreased slowly after 20 cycles of adsorption-desorption(Fig.7(b)).Fig.7(c)and Fig.7(d) show that the separation efficiency and water contact angle of CS/PDMS-COL sponge were slightly reduced after 20 cycles of reuse.These results indicate that CS/PDMS-COL sponge possessed favorable recovery ability and mechanical durability and thus could maintain good hydrophobicity after recycling.
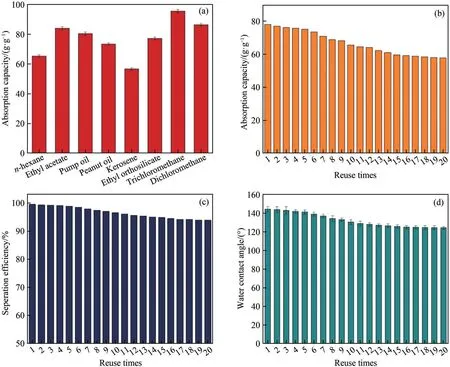
Fig.7 (a) Absorption capacity of CS/PDMS-COL sponge for different solvents; (b) adsorption capacity of CS/PDMS-COL sponge towards benzene for 20 cycles of reuse; (c) separation efficiency after each recycling of CS/PDMS-COL sponge towards benzene;(d)water contact angle after each recycling of CS/PDMS-COL sponge towards benzene
3.7 Emulsion separation performance of CS/PDMSCOL sponge
CS/PDMS-COL sponge was applied to separate the oil-in-water emulsion.As shown in Fig.8(a), through the continuous compression-adsorption process, themilky solution became transparent gradually, indicating that CS/PDMS-COL sponge removed the emulsified oil droplets effectively.When the emulsion was absorbed into the inner space of the hydrophilic CS/PDMS-COL sponge, demulsification occurred, leading to the coalescence of the oil droplets in the CS/PDMSCOL sponge and phase separation.Therefore, the resulting water phase was repelled by the hydrophobic effect of the CS/PDMS-COL sponge, while the oil phase was retained by the CS/PDMS-COL sponge as a result of its high porosity and underwater lipophobicity.Under the optical microscope, no emulsion droplet was observed from the emulsion treated by CS/PDMS-COL sponge(Fig.8(b)).

Fig.8 (a) Photos depicting the oil-in-water emulsion separation process; (b) optical microscope images from emulsion before and after treatment by CS/PDMS-COL sponge
3.8 Photothermal antibacterial property of CS/PDMS-COL sponge
The photothermal effect of CS/PDMS-COL sponge under NIR radiation could be further used in inhibiting the growth of bacteria and even causingthe death of bacteria.Fig.9(a) shows the temperature change of CS/PDMS-COL sponge immersed in the bacterial solution within 2 min.From the specific heating curves (Fig.9(b)), CS/PDMS-COL sponge could be heated up from 22.5℃ to 66.4℃ under NIR radiation; meanwhile, the temperature of other samples did not change significantly after irradiation.It can be observed that CS/PDMS-COL sponge irradiated by near-infrared has an obvious antibacterial effect onE.coli(Fig.9(d)) andS.aureus(Fig.9(e)), while the other control samples showed bacterial colonies similar to that of the blank(Fig.9(c)), which shows that the antibacterial activity was induced by the NIR photothermal effect of CS.As determined, the antibacterial rate of CS/PDMSCOL sponge against bothE.coli(Fig.9(d)) andS.aureus(Fig.9(e)) was greater than 95%, suggesting an excellent antibacterial property of CS/PDMSCOL sponge.
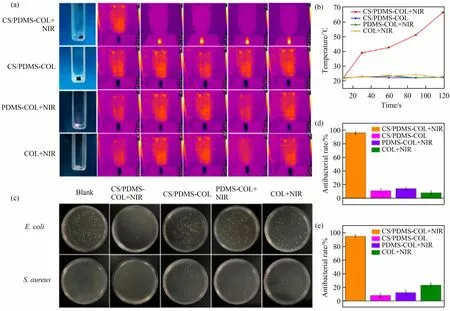
Fig.9 Temperature change and antibacterial property of sponges under NIR irradiation.Four groups were set:CS/PDMS-COL+NIR,CS/PDMS-COL(without NIR), PDMS-COL+NIR, and COL+NIR.(a) Photos and temperature images under NIR irradiation; (b) curves of the temperature of the bacterial liquid treated with different conditions as a function of time;(c)antibacterial effects(antibacterial rate against E.coli(d)and S.aureus(e)(n=3))of blank,CS/PDMS-COL+NIR,CS/PDMS-COL sponge,PDMS-COL+NIR,and COL+NIR
4 Conclusions
In this study, a collagen (COL)-based sponge for application to oil-water separation based on a green and facile strategy was successfully developed.In this design,widely-available COL was used as the substrate: it was immersed in polydimethylsiloxane (PDMS) suspension with candle soot (CS) nanoparticles, followed by hot curing.The resultant sponge (CS/PDMS-COL) can adsorb a variety of oil phases,and its separation efficiency reached 99.0%.There is no obvious change in the water contact angle after 20 cycles of recycling (towards benzene),suggesting good reusability.It can separate oilwater mixture under static and dynamic adsorption and separate oil-in-water emulsions effectively.Furthermore,the composite sponge is responsive to near-infrared light, and it can kill bacteria due to the photothermal responsiveness.This study provides new insight into the preparation of facile oil-water separation materials based on naturally occurring biomaterials effortlessly.
Acknowledgments
We are grateful for the financial support provided by the National Natural Science Foundation of China(Grant Nos.22178056 & 22078060), the Natural Science Foundation of Fujian Province (Grant Nos.2020J01555 & 2020J01881), the Opening Project of Guangxi Key Laboratory of Clean Pulp & Papermaking and Pollution Control (2019KF09), and Special Fund for Science and Technology Innovation of Fujian Agriculture and Forestry University (Grant Nos.CXZX2019108S&CXZX2019116G).
杂志排行
Paper and Biomaterials的其它文章
- Nanocomposite Hydrogel Materials for Defective Cartilage Repair and Its Mechanical Tribological Behavior—A Review
- Review of Carbon Dots from Lignin:Preparing,Tuning,and Applying
- Octadecylamine Graft-modified Cellulose Nanofiber and Its Reinforcement to Poly(butylene adipate-co-terephthalate)Composites
- Formation and Collapse of Cellulose Nanocrystals and Hydrophobic Association-induced Dual Cross-linked Nanocomposite Hydrogels:A Rheological Study
- Stabilizing Pickering Emulsions Using Octenyl Succinic Anhydride Modified with Cellulose Nanofibrils
- Technical Evaluation of Hybrid Clones of Corymbia spp.to Produce Market Pulp
