The mechanism study of low-pressure air plasma cleaning on large-aperture optical surface unraveled by experiment and reactive molecular dynamics simulation
2022-07-13YuhaiLI李玉海QingshunBAI白清顺YuhengGUAN关宇珩HaoLIU刘昊PengZHANG张鹏BuerlikeBATELIBIEKE巴特勒别克RongqiSHEN沈荣琦LihuaLU卢礼华XiaodongYUAN袁晓东XinxiangMIAO苗心向WeiHAN韩伟andCaizhenYAO姚彩珍
Yuhai LI (李玉海),Qingshun BAI (白清顺),Yuheng GUAN (关宇珩),Hao LIU (刘昊),Peng ZHANG (张鹏),3,∗,Buerlike BATELIBIEKE (巴特勒·别克),Rongqi SHEN (沈荣琦),Lihua LU (卢礼华),Xiaodong YUAN (袁晓东),∗,Xinxiang MIAO (苗心向),Wei HAN (韩伟) and Caizhen YAO (姚彩珍)
1 School of Mechatronics Engineering,Harbin Institute of Technology,Harbin 150000,People’s Republic of China
2 Research Center of Laser Fusion,China Academy of Engineering Physics,Mianyang 621900,People’s Republic of China
3 Chongqing Research Institute,Harbin Institute of Technology,Chongqing 401135,People’s Republic of China
Abstract Low-pressure air plasma cleaning is an effective method for removing organic contaminants on large-aperture optical components in situ in the inertial confinement fusion facility.Chemical reactions play a significant role in plasma cleaning,which is a complex process involving abundant bond cleavage and species generation.In this work,experiments and reactive molecular dynamics simulations were carried out to unravel the reaction mechanism between the benchmark organic contaminants of dibutyl phthalate and air plasma.The optical emission spectroscopy was used to study the overall evolution behaviors of excited molecular species and radical signals from air plasma as a reference to simulations.Detailed reaction pathways were revealed and characterized,and specific intermediate radicals and products were analyzed during experiments and simulation.The reactive species in the air plasma,such as O,HO2 and O3 radicals,played a crucial role in cleaving organic molecular structures.Together,our findings provide an atomic-level understanding of complex reaction processes of low-pressure air plasma cleaning mechanisms and are essential for its application in industrial plasma cleaning.
Keywords: organic contaminants,large-aperture optical components,low-pressure air plasma,plasma cleaning,reactive species,reactive molecular dynamics
1.Introduction
Over the past few decades,inertial confinement fusion (ICF)as a controlled fusion is expected to bring possible solutions to the energy shortages and environmental pollution on the Earth [1].Clean and sustainable energy can be obtained by driving the fusion through multiple large-aperture optical components (>400 mm) in ICF facilities,which transfer and focus high-energy laser beams [2].At present,the contamination damage of large-aperture optical components is the bottleneck limiting the output energy improvement of the ICF facility [3].The surface quality and optical properties of the contaminated optical components are seriously degraded[4].Organic contaminants are an essential component of surface contaminants of optical components,mainly from vacuum pump oil and rubber components.According to the detection of contaminants in the ICF facility [3],organic contaminants were adsorbed on the surface of large-aperture optical components after serving in a vacuum environment for a long time.
Currently,ex situ wet cleaning (deionized water,acid treatments,ultrasonic,megabound cleaning,etc) of organic contaminants is generally used for large-aperture optical components after manufacturing and coating [3].Due to the limitation of the disassembly and installation of the largeaperture optical components in the ICF facility,it is challenging to remove organic contaminants in situ using existing wetting cleaning methods.At the same time,in the long-time vacuum operation environment of the ICF facility,the surface of optical components will be repeatedly contaminated.Thus,it is urgent to explore new technology to clean large-aperture optical components in situ in the ICF facility.Low-pressure air plasma cleaning (LPAPC) is a promising alternative to removing organic contaminants.Traditional dry cleaning technology (such as laser [5],UV/O3[6],CO2/SNOW [7],etc) is not considered for in situ cleaning of optical components due to low cleaning efficiency and uncontrolled cleaning process.With the development of plasma technology,low-pressure plasma can realize large-area uniform discharge,high-efficiency cleaning,no damage and secondary contamination to the optical surface in the vacuum environment[8].Low-pressure plasma is considered an ideal in situ cleaning technology for large-aperture optical components,which has been initially applied in ITER[9,10],synchrotron beamline optics [11],extreme ultraviolet (EUV) [12],microelectronics[13]facilities.Contaminants such as carbon,Sn and Si produced a reduction reaction with hydrogen plasma to generate CHx,SnH4and SiH in synchrotron beamline optics,EUV facilities.Inert gases (such as helium,argon,and neon) plasmas removed beryllium and tungsten particles from the surface of optical components in ITER by physical bombardment [14].
Although plasma cleaning is a mature commercial technology,few studies elucidate the interaction between plasma and organic contaminants.To understand the plasma cleaning mechanism,including reaction frequency factor,activation energy,cleaning rate,the dissociation evolution characteristics of organic contaminants were studied using reaction kinetics [15] and finite element analysis [16].Although Arrhenius’s theorem in reaction kinetics described the coupling of the activation reaction and energy of various reactive species in the plasma quantitatively,it cannot provide pathway on cleavage and forming chemical bonds in the detailed reaction process at an extremely short period [17].It is still challenging to fully understand the fundamental chemical reactions between the plasma and organic contaminants and how reactive species in air plasma affect cleavage behaviors by experimental detections.Recently,reactive molecular dynamics (RMD) based on a reactive force field (ReaxFF)simulation have been performed to clarify the mechanisms of interactions between plasma-generated species and various biomolecules[18,19].It could provide insights into the entire interwoven reaction pathway of the plasma cleaning at the atomic level compared with the traditional theoretical calculation that researches a single reaction [20].
This study conducted an in situ low-pressure air plasma cleaning experiment on the large-aperture optical components to remove the organic contaminant.Reactive species and excitation signals in low-pressure air plasma were studied using optical emission spectroscopy (OES).Then,the chemical composition and components content of the surface organic contaminants were also analyzed by attenuated total reflection flourier transformed infrared spectroscopy (FTIR)and X-ray photoelectron spectroscopy (XPS) to provide a reference to simulation results.We aim to understand the plasma cleaning mechanism by RMD simulation,which provides a possible reaction pathway for the dissociation of organic contaminants by reactive species in the plasma.
2.Experimental methods and simulation details
2.1.Experimental methods
SiO2sol-gel antireflective (AR) film is widely coated on the surface of large-aperture optical components to improve the transmittance and laser-induced damage threshold(LIDT)[2].In this experiment,the fused quartz samples (50×50×5 mm3)were prepared with the same surface quality as the largeaperture optical components in the ICF facility.According to previous research [21],the sol-gel AR film was deposited on the fused quartz substrates using the dip-coating method at 355 nm.The fused quartz was immersed in the sol-gel liquid and then pulled at a constant speed(~80 mm min-1).After the post-treatment of hexamethyldisilazane and ammonia,the dense film was obtained,consistent with the actual coating method of large-aperture optical components [22].
The samples were placed in a vacuum chamber to contaminate for 5 h with a high vacuum(10-5Pa)to simulate the organic contamination environment in the ICF facility.Dibutyl phthalate(DBP)has the benzene ring,ester group and carbon chain,representing the chemical properties of most organic contaminants (alkanes,aromatic hydrocarbons,esters),which are found in the high energy facilities [23].Therefore,DBP was chosen as a typical contaminant in our research.
Figure 1 outlines the experimental equipment designed to verify in situ plasma cleaning of large-aperture optical components,which consisted of a power system,laser path,vacuum system and four electrodes(made of copper plates of 20×5×430 mm3).A large area of uniform plasma was generated by discharging between electrodes and the cavity wall in the vacuum chamber.The air was excited by an audio frequency power supply and the plasma was dispersed on the optical surface (430×430 mm2).The cleaning parameters are listed in table 1.The discharges use frequencies usually in the radio frequency band,such as 13.56 MHz.The plasma sheath voltage can reach hundreds or even thousands of volts in a capacitively coupled discharge plasma reactor.When the ions pass through the sheath,they will be accelerated by the sheath voltage to bombard the surface of the optical component with high energy,which is easy to cause surface damage.According to previous research on grating cleaning[24],oxygen plasma is overaggressive and challenging to control accurately.Thus,we choose more mild air as the plasma gas source to avoid surface damage to large-aperture optical components.At the same time,the audio-frequency(20 kHz) discharge structure is straightforward and suitable for mounting on the cavity wall of the laser path with limited space.
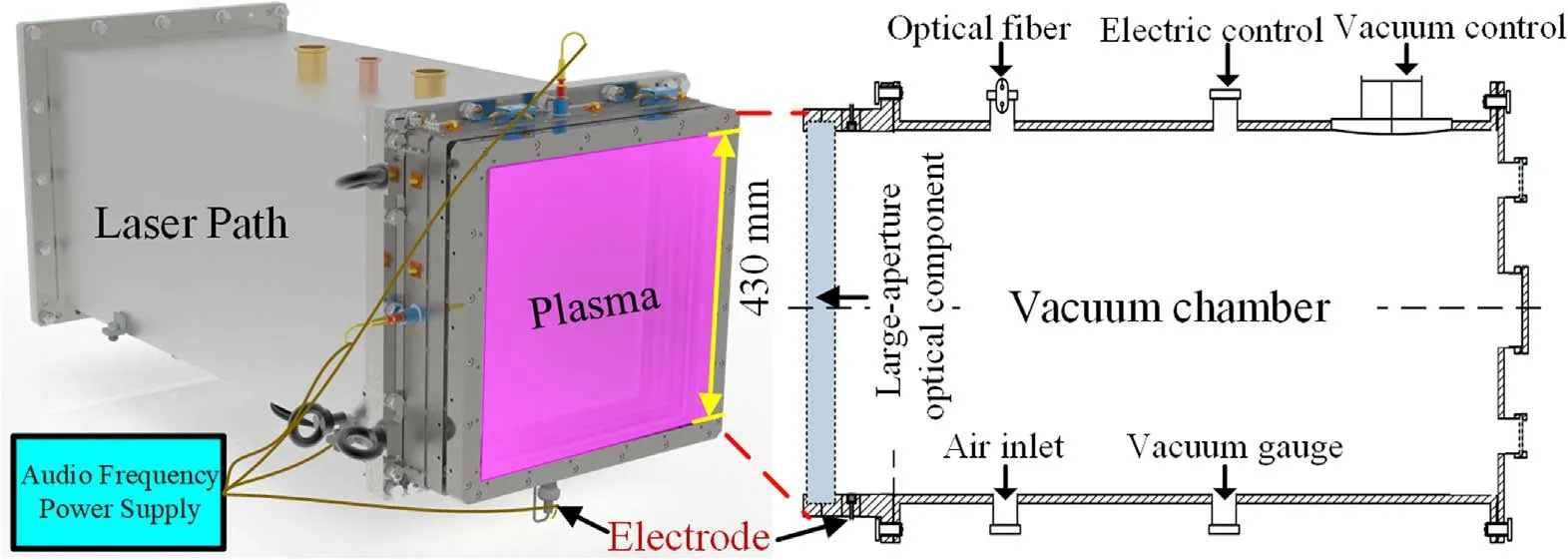
Figure 1.Experiment setup for low-pressure air plasma cleaning.

Figure 2.Molecular model of a chemical reaction between plasma and organic contaminants.
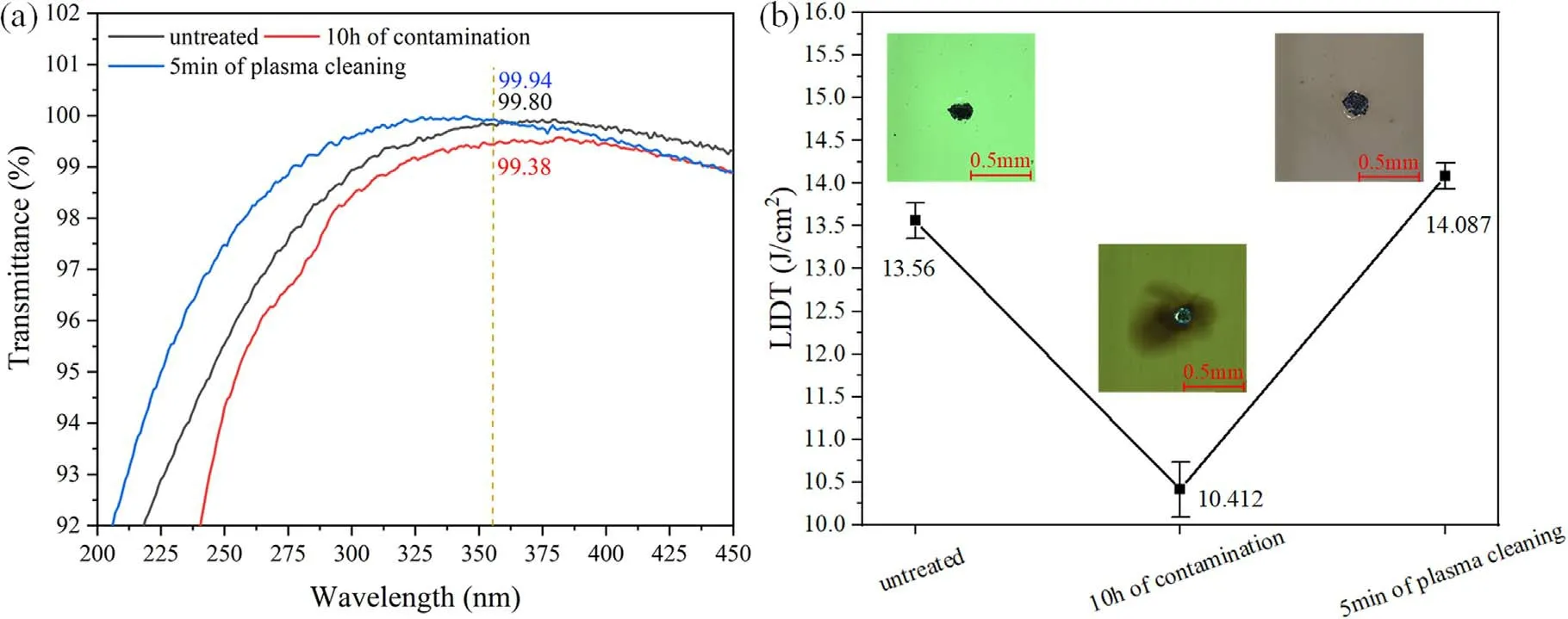
Figure 3.(a) Transmittance and (b) LIDT of optical components.
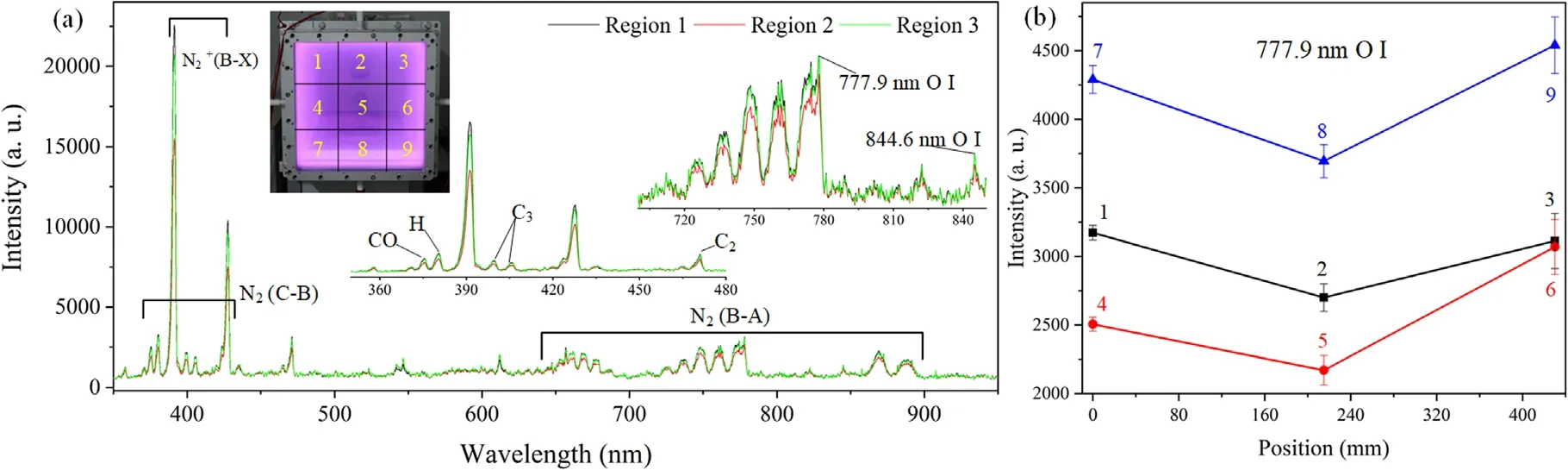
Figure 4.The excited species in air plasma.(a) The full optical emission spectra of air plasma,(b) an emission line of O I.
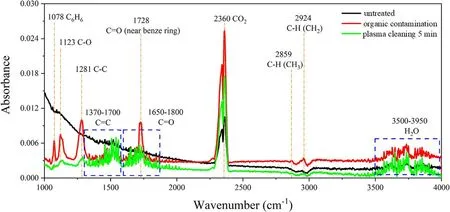
Figure 5.FTIR spectra of sol-gel AR film with different treatments.
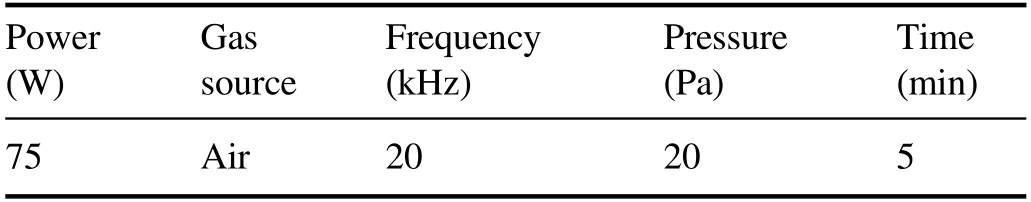
Table 1.Low-pressure plasma cleaning parameters.
The transmittance of optical components coated with the sol-gel AR film is measured by the spectrophotometer(Perkin Eimer Lambda 950).Single-pulse Nd:YAG laser(wavelength 355 nm) measures the LIDT of optical components by the 1-on-1 method.Optical emission spectra (Maya 2000 pro)were used to measure reactive species and their concentration during air plasma cleaning.The measurement range of spectrum signal was from 165 to 1100 nm and the optical resolution can reach approximately 0.035 nm.The atomic force microscope (PSIA XE-100) was used to measure the surface morphology and roughness of optical components.A total reflection accessory of FTIR(Thermo Nicolet 5700)was conducted to examine the formation and cleavage bonding information after plasma cleaning.It is challenging to detect infrared absorption spectra of tiny organic contaminants on the surface of SiO2sol-gel AR film.Thus,the sample surface was coated with a thin layer of DBP.An XPS(Thermo Fisher ESCALAB 250Xi)was conducted to analyze surface element content and functional groups quantitatively.XPS spectra were obtained using an Al Kα (1486.7 eV) X-ray source at 300 W.High-resolution spectra (0.1 eV) were calibrated by C(1s) signal at 285.0 eV [25].
2.2.Simulation details
Plasma cleaning simulation is based on RMD,a bond orderbased potential proposed by van Duin [26].ReaxFF uses a general relationship between the bond distance and bond order,bond energy,leading to proper dissociation of bonds to separated atoms.Although RMD does not explicitly simulate electrochemical reactions involving electrons (e-) transfer,it is beneficial to construct the entire interwoven chemical reactions in the plasma cleaning and the mass transfer in the femtosecond time range [20].In RMD simulations,the general system energy is divided into various partial energy contributions.Each expression of the bond order function is demonstrated in equation (1).
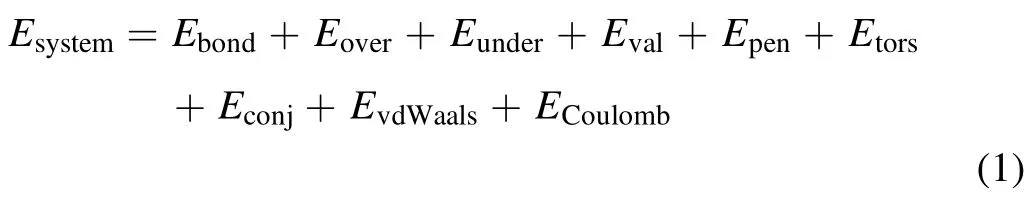
Here,Esystemrepresents the system energy.The Ebond,Eover,Eunder,Eval,Epen,Etors,Econj,EvdWaals,ECoulombdenote the bond energy,over-/under-coordination energy,valence angle energy,penalty energy,torsion energy,conjugation energy,non-bonded van der Waals interactions and Coulomb interactions,respectively.A ReaxFF potential comprising C/H/O parameters is employed to accurately describe the plasma cleaning model.
In this study,plasma concentration refers to the concentration of reactive species involved in chemical reactions.Plasma temperature refers to the gas temperature,which is mainly determined by the average kinetic energy of the ions during elastic collisions.According to previous experimental results,plasma composition is mainly considered reactive oxygen species (e.g.,O,OH,O3,H2O2,and HO2) compared to reactive nitrogen species [27].O2is regarded as the main component of the gas source for comparing destruction capability with other exciting species.Therefore,five DBP molecules and two hundred reactive species were randomly placed in a sealed box,as shown in figure 2.The plasma concentration was much higher than the actual plasma concentration,but this setting was widely accepted to explain the chemical reaction mechanism between plasma and organic contaminants from a microscopic perspective [18].Periodic boundary conditions were applied to simulate an infinite system.Thus,energy minimization was performed by a smart minimizer to make the model more reasonable.After that,the model was simulated in the canonical ensemble (NVT) at room temperature for 1 ns and the time step was set at 0.25 fs.
3.Results analysis
3.1.Optical properties of sol-gel AR films
The ultimate goal of low-pressure air plasma in situ cleaning is to restore the optical properties of large-aperture optical components.The transmittance of the sol-gel AR film decreased rapidly from about 99.80% to 99.38% after contamination for 10 h,as shown in figure 3(a),which was unacceptable for high-energy laser transmission.It was noted that the transmittance of the optical component recovered after 5 min of plasma cleaning,which indicated that organic contaminants were removed by the plasma from the surface.The LIDT of optical components in the high-energy laser facility is an essential factor to limit the improvement of the load capacity ability of the facility [2].The LIDT of the optical component decreased by nearly one-third(from ~13.6 to ~10.4 J cm-2)after 10 h of contamination,with significant ablations in the damaged area,as shown in figure 3(b).This is because organic contaminants are adsorbed in the pores of sol-gel AR films and carbonized under high-energy laser irradiation.After 5 min of plasma cleaning,the LIDT was recovered and ablation regions caused by organic contaminants were eliminated.Although the optical properties of the components were degraded by organic contamination in the high vacuum environment,the optical properties of the components recovered completely after plasma cleaning.
3.2.Optical emission spectra of air plasma
OES analysis of air plasma is conducted in situ in different regions within the window to obtain reactive species information,as shown in figure 4.OES is an effective method to analyze excited species,vibrational temperature,electron density and temperature during plasma excitation,which has no disturbance to the plasma compared to the Langmuir probe technique in the in situ diagnosis[28,29].It can be found that there are mainly sharp emission peaks (like H,O,N2,CO,N2+,C2,C3) and wide emission bands (like N2,) by measuring the full spectrum of low-pressure air plasma,which is in good accordance with previous results [30-32].Among them,the signal of O I was found in spectra,which was the main reactive species driving chemical reaction and induced the production of other species (e.g.,OH,O3,H2O2,and HO2).
In addition,we found that air plasma concentration distribution in the in situ plasma cleaning was different in the large-aperture window according to purple intensity in figure 4(a).So the window was divided into nine regions to measure the emission spectra of O I.The plasma concentration was positively related to the spectral intensity,according to equation (2) [32].

where nAis the concentration of reactive species A,Iλis the light emission intensity integrated against line width,and αλAis the coefficient related to electron velocity and emission wavelength.
Among them,the most typical emission line of O I was 777.9 nm (3p5P→3s5S),which was a direct excitation from the ground state[33].The line 844.6 nm(3p3P→3s3S)of O I was not used to evaluate plasma excitation due to a weak signal.The plasma concentration distribution showed a high concentration of O radical around the window and a low concentration in the middle,consistent with the diffusion law of charged particles in plasma [32].The highest O I radical spectral intensity in region 9 was ~4540.5,twice that in region 5,representing the higher excitation degree of air in region 9.The plasma species spread toward the middle region of low density through the capacitive coupling charge from the four sides of the window.It is necessary to study the influence of the contributions of air plasma concentration on the plasma cleaning effect in future research.
3.3.Chemical composition evolutions on optics surfaces
To understand the composition of chemical elements on the surfaces of sol-gel AR film,the surface chemical bond information and element contents were detected by FTIR and XPS,as shown in figures 5 and 6.Figure 5 shows FTIR spectra of cleavage and formation bond information before and after plasma cleaning.It can be noted that abundant C-H bonds were found on the surface of sol-gel AR film after organic contamination.In DBP molecular structure,1078 cm-1and 1123 cm-1correspond to the in-plane swing deformation vibration of the benzene ring and the stretching vibration of the C-O single bond near the C=O double bond.1281 cm-1refers to the in-plane swing vibration of the benzene ring and the stretching vibration of the C-C bond between the C atom in the benzene ring and C=O.2859 cm-1and 2924 cm-1represent the stretching vibrations of the C-H bond on CH3and CH2,dropping below the untreated level after LPAPC.The results showed that plasma could remove most contamination from the surface in situ.Besides,2360 cm-1and 3500-3950 cm-1correspond to CO2and H2O(gaseous) in the background environment,which increased significantly relative to the untreated surface.The plasma is widely used to clean surfaces containing trace amounts of organic contaminants,and it is still difficult to completely remove contaminants coated with a layer of DBP.
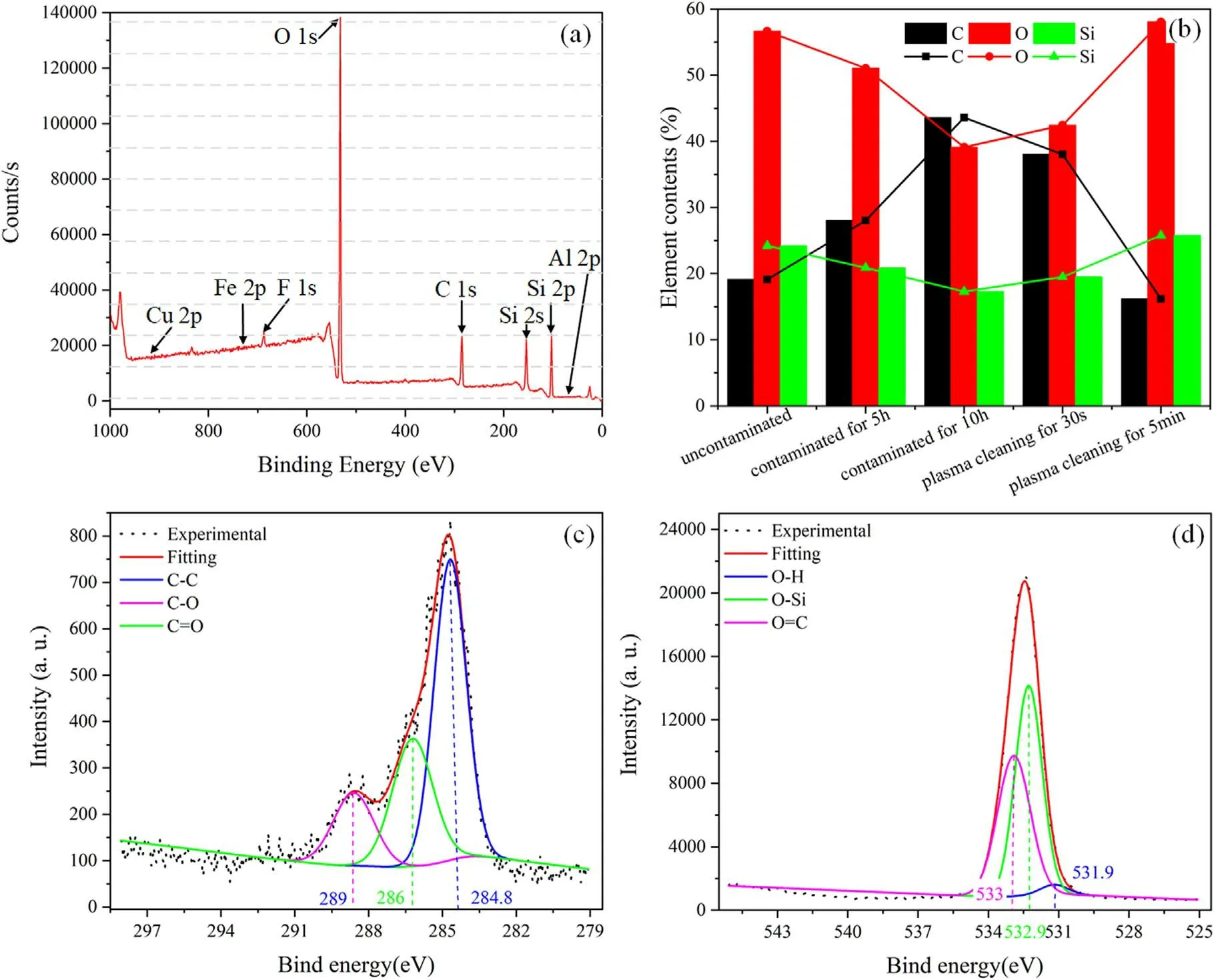
Figure 6.High-resolution XPS spectra of the film before and after low-pressure air plasma cleaning.(a) The full spectrum of XPS after plasma cleaning for 5 min,(b)element contents,(c)fitting of the C(1s)signal after plasma cleaning for the 30 s,(d)fitting of the O(1s)signal after plasma cleaning for 5 min.
Furthermore,both C-C (1281 cm-1) and C-O(1123 cm-1) bonds were easily oxidized to C=O and C=C double bonds after plasma cleaning[34],indicating that DBP molecular structure was destroyed.A wide range of C=C was stretching vibration frequency at 1370-1700 cm-1,mainly the π bond in the benzene ring and olefin,with a vibration frequency of 1370-1610 cm-1and 1620-1700 cm-1,respectively.These results indicated that unsaturated bonds were produced during the cleaning process.Different types of C=O bonds (such as lipid group,aldehyde group) were generated in the peak positions of 1650-1800 cm-1,which was due to the intermediate oxidation products of the carbon chain,which had been proved by Bonova et al [35].Therefore,plasma removed organic contaminants by destroying CH and C-C bonds and oxidizing to generate small molecular groups such as C-O,C=O,C=C,O-H,CO2and H2O.
A high-resolution XPS analysis of samples was performed to understand the changes in element contents and physicochemical properties of sol-gel AR film before and after plasma cleaning.Figure 6(a) shows the XPS signals C(1s),O(1s),Si(2 s),Si(2p),Al(2p),Fe(2p) and Cu(2p),which contain the components contents and binding energy.Since the electrode(copper plate),the cavity wall(aluminum alloy)and the screw(stainless steel) are made of metal.The elements of Al(2p),Fe(2p),and Cu(2p) were not found in all XPS signals after plasma cleaning for 5 min in figure 6(a),which indicated that the surface of sol-gel AR film was not contaminated by the metal during plasma cleaning.However,it is inevitable that the electrode sputter metal particles after multiple cleaning cycles [9].Since the discharge in this device is between the electrode and the cavity wall,the electrode sputtering can be blocked by coating the electrode surface or adding borosilicate glass.In this work,it is proposed for the first time to use audiofrequency capacitive coupled air plasma to remove organic contaminants on the surface of large-aperture optical components in situ.Future research aims to reduce and avoid metal contamination and damage on the surface of optical components after multiple cycles of cleaning.

Figure 7.Schematic diagram of low-pressure air plasma cleaning mechanism.
The main elements of the sol-gel AR film surface contaminated by DBP were C(1s)and O(1s).Among them,C(1s)content increased rapidly with the increase of contamination time because the main component of DBP was C(1s),as shown in figure 6(b).As the surface of sol-gel AR films consisted of O(1s) and Si,the content was reduced after contamination because of forming a layer of oil film on the surface.The organic contaminants were not completely removed due to a slight C(1s)decrease after plasma cleaning for the 30 s.After cleaning for 5 min,C(1s)content was lower than that of the uncontaminated surface and the content of O(1s)and Si reached the uncontaminated level.Furthermore,the C(1s)and O(1s) element signals were analyzed to quantitatively evaluate the chemical composition changes of the products during the plasma cleaning.Figure 6(c)shows that the C(1s)represents the middle product during the chemical reaction after plasma cleaning for 30 s,characterized by having three well-defined components,284.8 eV,286 eV,and 289 eV,respectively [36].These signals indicated that large amounts of C-O,C=O bonds were produced during the plasma cleaning due to plasma oxidation,consistent with the FTIR results.Similarly,it was possible to assign three components to the O(1s) signal placed at 531.9 eV,532.9 eV and 533 eV,corresponding to oxygen atoms in different chemical states after plasma cleaning for 5 min O(1s)had a characteristic signal of 531.9 eV,assigned to oxygen atoms bonded with hydrogen forming hydroxide groups(O-H).At the same time,there were still more C=O bonds left after plasma cleaning,which indicated that C=O was not easy to decompose and formed the final residuals.
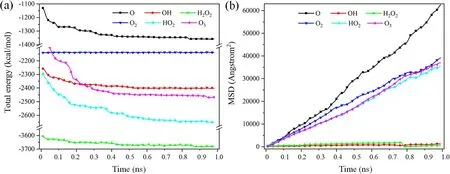
Figure 8.The interaction characteristics between reactive species and DBP in the reaction process.(a) Total energy evolution,(b) mean square displacement of reactive species.
4.Discussion
4.1.Analysis of low-pressure air plasma cleaning mechanism
When an audio-frequency alternating current electric field was applied to the discharge electrodes,free electrons were accelerated in the electric field and gained kinetic energy.Electrons with certain energy collided with atoms and molecules in the gas in the process of movement.In inelastic collisions,electrons transferred energy to atoms and molecules to ionize them and produce abundant reactive species,as shown in figure 7.When these particles accelerated through the sheath to bombard the solid surface,they transferred energy to organic contaminants and substrates,facilitating the desorption of organic contaminants on the surface.Among them,secondary electron emission has a significant influence on the generation and concentration of reactive species,but it has no influence on the dissociation mechanism of organic contaminants by reactive species.Previous studies revealed that oxidation and ultraviolet reaction,ion and electrons collision,magnetic fields attraction mainly occurred during plasma treatment,chemical reaction played a crucial role in plasma cleaning[27].In plasma cleaning,ions energy is usually reduced to avoid surface damage,so the contribution of physical bombardment to plasma cleaning can be ignored.
A layer of oil film was formed on the sol-gel AR film after contamination,as the AFM detection shows in figure 7.Reactive species in low-pressure air plasma combined with organic contaminants to form new unstable radicals.The FTIR and XPS test results proved that the organic contaminants on the surface were removed with significant destruction of C-H and C-C bonds after plasma cleaning.Further,those chemical bonds were oxidized to C=C,C=O and O-H bonds during plasma cleaning.New free radicals were generated on the surface,along with the formation of small molecules.These chemical reactions continued until the hydrocarbons were decomposed entirely into small molecules such as H2O and CO2,which have been examined in previous research [37].Then,these volatile small molecules were extracted easily from the surface by a vacuum pump due to weak van der Waals and Coulomb forces between small molecules and the surface.After plasma cleaning,the surface morphology and roughness of sol-gel AR film were restored and showed the loose porous structure without damage,as shown in figure 7.Finally,organic contaminants on the surface of large-aperture optical components were removed by in situ plasma cleaning.
Although the reactive species and the intermediate products of plasma cleaning can be found through various experimental detection methods,it is still challenging to obtain the evolution behaviors and formation mechanisms of chemical bonds.Especially,some functional groups,such as C=O(carboxylic acid,ester,aldehyde,ketone,in 1650-1800 cm-1) and C=C (olefins,aromatic hydrocarbons,in 1370-1700 cm-1),have overlapping wavelengths making it impossible to distinguish the product species.Thus,RMD was conducted to study the evolution of cleavage and the formation of chemical bonds during plasma cleaning at the atomic level.
4.2.Reaction data analysis
The reaction statistics between air plasma and organic contaminants were analyzed further to elucidate the chemical reaction characteristic at the atomic level.Figure 8(a)shows the total energy change process of a chemical reaction within 1 ns during RMD simulation.Although the total energy of the system had not reached equilibrium,the primary chemical reaction was complete in plasma cleaning,including richer reaction information.When reactive species were involved in plasma cleaning,a cascade of bond cleavage and formation events within approximately 0.5 ns reached the reaction scope.This phenomenon was that the plasma concentration in the system decreased and the reaction gradually stopped as the plasma reacted with organic contaminants.O2molecules did not destroy the DBP structure because they exhibited weak,non-bond interactions during plasma cleaning.Notably,O,O3,and H2O radicals reacted with the DBP like H2O2and OH radicals,with the only difference being that O,O3,and H2O radicals reacted violently with DBP in 0.2 ns,resulting in a significantly decreased total energy.
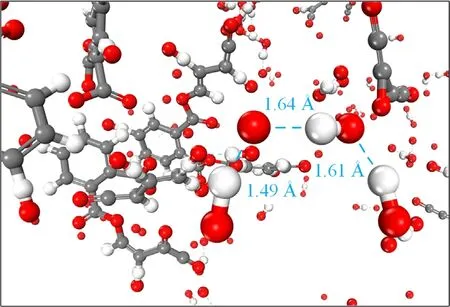
Figure 9.Temporal snapshots of hydrogen bond formation between atoms.The dashed blue lines are hydrogen bonds.
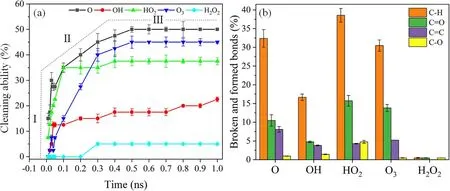
Figure 10.The cleaning capability of reactive species was evaluated.(a)The cleaning capability of different reactive species in air plasma is considered within 1 ns,which defines the cleavage of C-C bonds compared to total C-C bonds,(b)statistics of C-H bond dissociation and C=O,C=C,and C-O bond formation upon interaction with reactive species and organic contaminants.The percentages are calculated by breaking and forming bonds divided by the total bonds present in the model.
The translational mobility of reactive species during plasma cleaning was examined by the mean squared displacement (MSD).This work calculated the MSD of reactive species based on each species’ center of mass,as shown in figure 8(b).The slope of the MSD curve is related to the mobility of reactive species,which means greater mobility of reactive species with a higher slope [21].The dynamic properties of these reactive species were related to the chemical characteristics and density of reactive species.Oxygen atoms abstracted hydrogen atoms from contaminants to form OH radicals,which tended to form long chains due to the formation of hydrogen bonds,as shown in figure 9.The concentration of OH generated in the oxygen radicals reaction model was less than that of other models,which indicated that oxygen radicals had the most significant mobility characteristics.O2,O3,and HO2had similar mobility characteristics due to similar molecular densities after dissociation.Many hydrogen bonds were formed between reaction products of H2O2,which resulted in the strong interaction between molecules.Therefore,O radical had better mobility characteristics during chemical reactions and reacted more sufficiently with organic contaminants during long-time plasma cleaning.
Furthermore,statistics regarding hydrogen abstraction reactions and carbon chains oxidation were conducted after the chemical reaction.Figure 10 shows that the total number of broken and formed bonds is calculated based on a simulation time of 1 ns.Figure 10(a)evaluates the evolution of the cleaning capability of reactive species in the air plasma along with reaction time,which can be divided into three stages.The reaction between the reaction species and the organic contaminants was the most intense in stage I(within 0.05 ns).The reactive species had a similar strong cleaning capability except for the H2O2radicals.With the consumption of reactive species in the system,the reaction rate gradually decreased from 0.05 ns to 0.35 ns in stage II.Eventually,the chemical reaction basically stopped at stage III after the plasma concentration dropped to a certain level.The reaction between OH and DBP continued throughout the whole reaction process,while the cleaning capability of OH was slightly weaker than those of O,O3,and HO2.O radicals reacted most strongly with organic contaminants and had the most vital cleaning capability,followed by O3and HO2.High plasma concentration was adopted in the simulation to shorten the reaction time,which resulted in the inconsistency between the reaction time in the experimental time and the simulation,as shown in figures 6(b) and 10(a).
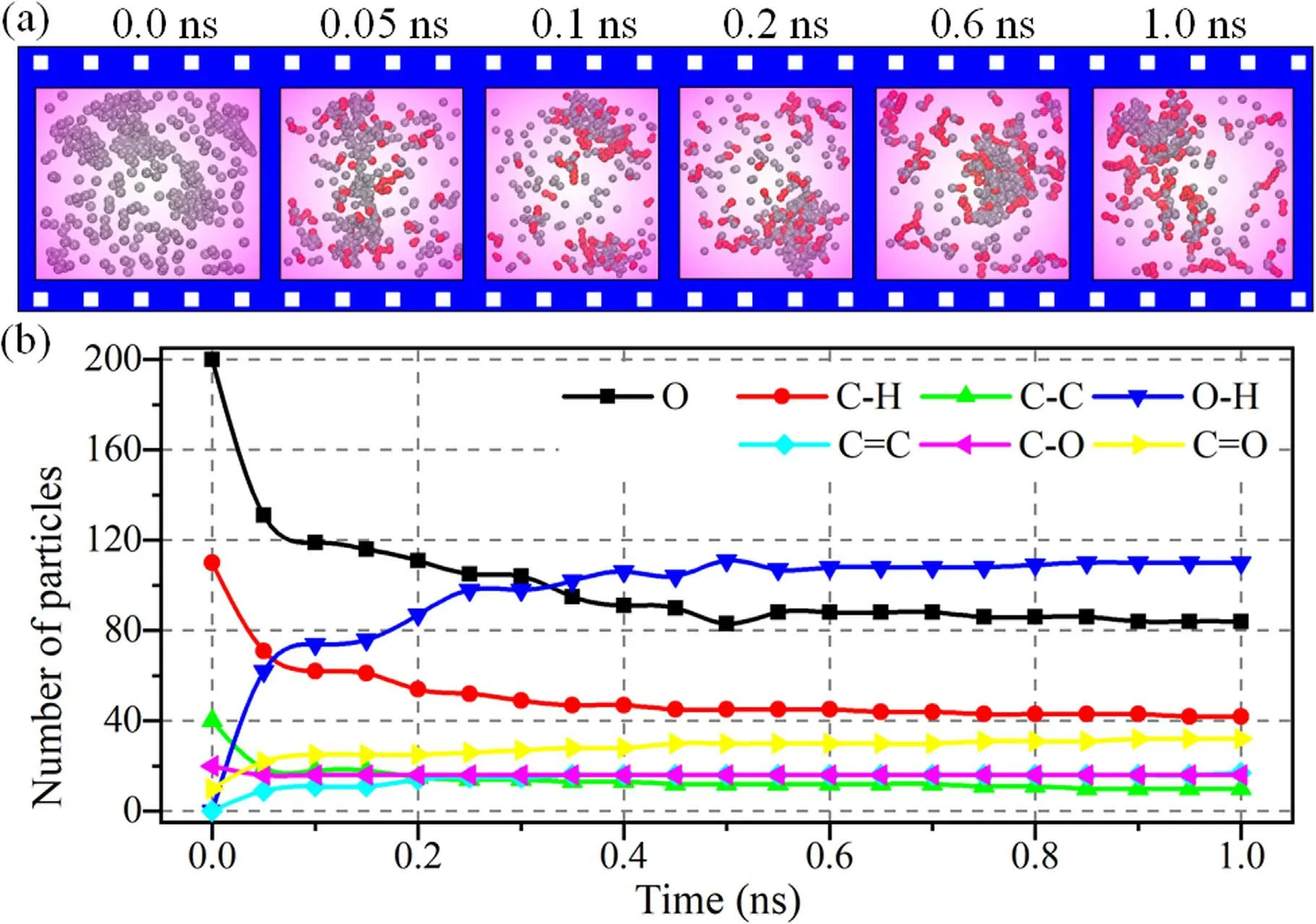
Figure 11.Real-time dynamics of air plasma cleaning.(a)Snapshots of the partial cleaning system extracted from the RMD simulation of air plasma cleaning.The red color represents new generated molecules and bonds.The gray color represents the molecules that were not involved in the reaction.(b) Time dependences of the numbers of oxygen and main molecular species in real-time RMD simulation.
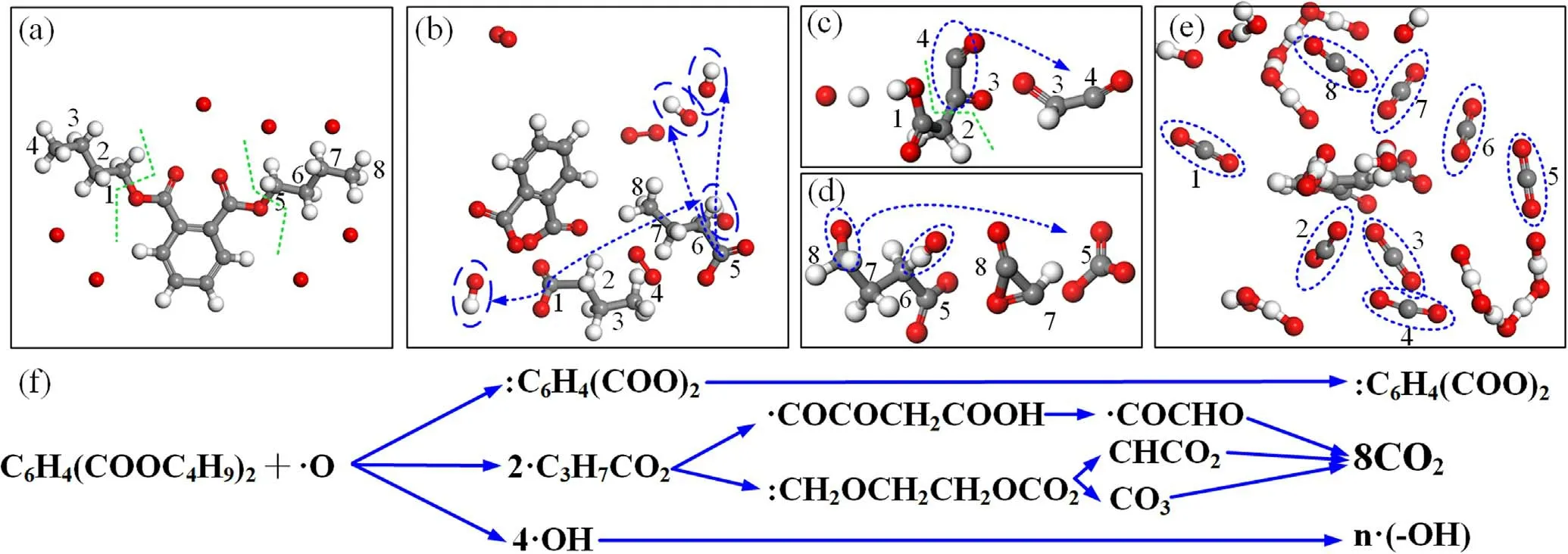
Figure 12.Temporal snapshots DBP dissociation mechanism induced by O radicals.(a)Dissociation of DBP,(b)O-H bonds formation,(c)and (d) C-C bonds cleavage,(e) DBP dissociated products,(f) main reaction pathways in the plasma cleaning.
In addition,the effects of reactive species on chemical bond breaking and formation after plasma cleaning were evaluated.HO2radicals triggered more hydrogen abstraction reactions (~39%) than other reactive species,indicating that the HO2radicals played a more crucial role in the bond cleavage of C-H,followed by O and O3radicals.Meanwhile,this characteristic was also observed in the capability of HO2atoms to form C=O bonds (~16%).The number of C-O bonds was little after plasma cleaning because C-O bonds were unstable and easily oxidized to C=O because of high plasma concentration.However,more C=C bonds were found in the FTIR and XPS results due to low plasma concentrations in the actual reaction process.Therefore,abundant C-O,C=C,and C=O bonds were generated in air plasma cleaning,in which O,O3,and HO2played a leading role compared with OH and H2O2radicals.
4.3.Possible reaction pathway
The plasma cleaning reaction has a similar pathway through breaking the molecular structure into small groups between various reactive species.As the representation of chemical reactions,figure 11 shows the time-dependent progression of O radicals and the main molecular species during the RMD simulation.After 1 ns,about 120 O were consumed,and about 45 C-H and 25 C-C were destroyed,which indicated that the DBP molecule structure had already been destructed except benzene ring.At the same time,about 110 O-H,20 C=C,and 30 C=O were generated after the chemical reaction.DBP molecules were destructed gradually slightly with the O consumed quickly,especially the chemical reaction almost stopped after 0.4 ns.Because oxygen concentration progressively reduced along with chemical reaction,the carbon chain had been oxidated into C=C and C=O.It was challenging to have further oxidation of benzene rings.Therefore,the final products of plasma cleaning were benzene ring,C=C,and C=O,consistent with the XPS results.
The primary reaction was hydrogen abstraction from the carbon chain,which triggered the hemolytic cleavage of important bonds (i.e.,C-C and C-H bonds) and formed double bonds(i.e.,C-O,C=O,and C=C bonds).Therefore,cleavage and formation of mechanisms of DBP are discussed with statistics of O radicals.Plasma cleaning is a complex chemical reaction that involves abundant reaction pathways and products during the process.An O radicals-based DBP dissociation main pathway is illustrated in figure 12.Most of the reaction species in each figure were removed to analyze the products more clearly during the chemical reaction.Due to the high concentration of O atoms and the intense oxidation,the C-O bond on carbon 1 and carbon 5 was broken firstly,which indicated that C-O-C in the ester structure of the DBP molecule was the most easily destroyed.At the same time,two hydrogen atoms on carbon were taken away by O to form O-H bonds,which further abstracted hydrogen from the carbon chain to become an H2O molecular (see green lines,blue dash circles in figures 12(a)and(b)).Due to the high concentration of oxygen radicals,OH radicals were further oxidized into groups such as HO2and H2O2.Subsequently,carbon 1 and carbon 5 connected two oxygen atoms,one of which formed a C=O double bond.The hydrogen atoms were abstracted away from the carbon chain,resulting in C≡O and C=O bonds forming.Further,the carbon chain length was broken down (green line,blue dashed circle in figures 12(c) and (d)).Therefore,these changes led to the complete oxidation and destruction of four CO2molecules of a carbon chain (blue dashed circle in figure 12(e)).Finally,the two DBP branched chains were oxidized into CO2and OH radicals except for the benzene ring,which completely dissociated organic contaminants by plasma.The main reaction pathways of plasma cleaning are provided in figure 12(f).In the actual reaction process,there is a large amount of intermediate residue on the surface of the reaction due to the limitation of plasma concentration and cleaning time.The benzene ring is difficult to destroy in the plasma cleaning process,but the removal efficiency can be improved with increasing temperature and cleaning time [38,39].
5.Conclusion
Experiments demonstrated that the organic contaminants on the surface of large-aperture components (430×430 mm2)were removed by low-pressure air plasma in situ without secondary contamination.Abundant reactive species were detected in optical emission spectra during plasma cleaning.Unsaturated bonds,such as C-O,C=C,C=O,and O-H bonds,were generated during the cleaning process and C=O was the final production.Besides,the cleaning mechanism of the organic contaminants with various reactive species in air plasma was illustrated by experiments and the reactive molecular dynamics simulation.In the process of plasma cleaning,O,HO2,and O3radicals had a more robust cleaning capacity in dissociating organic contaminants than H2O2and OH.O radicals had the most cleaning ability due to significant mobility and reacted more sufficiently with organic contaminants during the reaction process.The rate of chemical reaction slowed down gradually with the decrease of the plasma concentration.Reactive molecular dynamics simulations revealed the main pathway that the DBP molecular structure was oxidized into small groups by oxygen radicals and elucidated the dissociation and formation mechanism of the chemical bonds.The primary reaction pathway was hydrogen abstraction from the carbon chain,which triggered the hemolytic cleavage of important bonds (i.e.,C-C and C-H bonds) and formed unsaturated groups (i.e.,C-O,C=C and C=O),consistent with the experimental results.Aromatic hydrocarbons are more difficult to dissociate than alkanes and remain on the surface after plasma cleaning.
Acknowledgments
This work was supported by the Joint Funds of National Natural Science Foundation of China and China Academy of Engineering Physics (NSAF) (No.U2030109) and National Natural Science Foundation of China (No.52075129).
猜你喜欢
杂志排行
Plasma Science and Technology的其它文章
- Investigation on the multi-hole directional coupler for power measurement of the J-TEXT ECRH system
- Neutronic analysis of Indian helium-cooled solid breeder tritium breeding module for testing in ITER
- Investigation on the electrode surface roughness effects on a repetitive selfbreakdown gas switch
- Roll-to-roll fabrication of large-scale polyorgansiloxane thin film with high flexibility and ultra-efficient atomic oxygen resistance
- Effect of shock wave formation on propellant ignition in capillary discharge
- The application of a helicon plasma source in reactive sputter deposition of tungsten nitride thin films
