抑制MCL-1可逆转PD-L1敲除引起的结直肠癌细胞对司美替尼化疗敏感性下降*
2022-07-06孙磊洪楚原尹雪霞郭雄波张实钟北平王国强
孙磊, 洪楚原, 尹雪霞, 郭雄波, 张实, 钟北平, 王国强
抑制MCL-1可逆转敲除引起的结直肠癌细胞对司美替尼化疗敏感性下降*
孙磊, 洪楚原, 尹雪霞, 郭雄波, 张实, 钟北平, 王国强△
(广州医科大学附属第二医院胃肠外科,广东 广州 510260)
明确程序性死亡配体1programmed death ligand-1,-敲除是否可引起结直肠癌(colorectal cancer, CRC)细胞对司美替尼化疗敏感性下降,并探寻逆转-敲除引起的司美替尼化疗敏感性下降的方法。人结肠癌RKO细胞及敲除-的RKO细胞接受司美替尼处理后,Western blot检测两组细胞中活化的胱天蛋白酶3(cleaved caspase-3, C-Casp-3)蛋白水平的差异,流式细胞术及细胞集落形成实验分别检测两组细胞的凋亡率及细胞存活率。沉默髓细胞白血病基因1(myeloid cell leukemia-1,-)或AZD5991联合司美替尼处理后,Western blot检测RKO及敲除-的RKO细胞中C-Casp-3蛋白水平,流式细胞术及细胞集落形成实验分别检测细胞的凋亡率及细胞存活率。司美替尼联合AZD5991处理小鼠结肠腺癌MC38细胞及沉默-的MC38细胞,Western blot检测细胞中C-Casp-3蛋白水平,流式细胞术及细胞集落形成实验分别检测细胞的凋亡率及细胞存活率。Western blot结果发现,司美替尼可显著降低磷酸化细胞外信号调节激酶(phosphorylated extracellular signal-regulated kinase, p-ERK)蛋白水平,敲除-可显著降低细胞中司美替尼诱导产生的C-Casp-3,而-shRNA或联合使用AZD5991可显著增加细胞中司美替尼诱导产生的C-Casp-3。流式细胞术结果发现,敲除-显著降低了司美替尼诱导产生的细胞凋亡,而-shRNA或联合使用AZD5991均可显著增加司美替尼诱导产生的细胞凋亡。细胞集落形成结果发现,敲除-显著提高了低浓度司美替尼条件下细胞的存活率,而-shRNA或联合使用AZD5991均可显著降低细胞的存活率。更重要的是,-shRNA或联合使用AZD5991均有效逆转了-敲除引起的C-Casp-3、细胞凋亡及细胞存活率的改变。敲除-可引起CRC细胞对司美替尼化疗敏感性下降,沉默-基因或联合使用AZD5991可逆转-敲除引起的CRC细胞对司美替尼化疗敏感性下降。
髓细胞白血病基因1;程序性死亡配体1;司美替尼;结直肠癌;化疗敏感性
在我国,结直肠癌(colorectal cancer, CRC)的发病率在所有恶性肿瘤中排第三位,且发病率逐年升高。靶向治疗是CRC综合治疗的重要组成部分,然而药物耐药严重影响了患者的治疗效果,成为影响患者预后的重要因素之一。因此,明确CRC化疗耐药的相关机制并探寻相应的治疗靶点,这对改善CRC患者的预后具有重要的研究意义。
肿瘤细胞表面的程序性死亡配体1(programmed death ligand-1, PD-L1;又称为CD274或者B7H1)是重要的免疫检查蛋白,可通过与T细胞表面受体程序性死亡蛋白1(programmed death-1, PD-1)特异性结合引起免疫逃逸,而阻断PD-L1/PD-1之间的相互作用可有效抑制多个肿瘤的发展。目前针对PD-L1/PD-1的免疫治疗在多个肿瘤中显示出巨大的临床价值,其中肿瘤组织PD-L1蛋白表达阳性被认为是可采取抗PD-L1/PD-1治疗的重要标记物[1]。然而,在CRC中仅有约10%的患者肿瘤组织PD-L1蛋白表达阳性,绝大部分患者难以从抗PD-L1/PD-1免疫治疗中获益[2-3]。PD-L1除了发挥其免疫检查点的功能外,其自身可通过调节相关信号通路在肿瘤发生发展中扮演重要角色。研究发现,PD-L1在多个肿瘤细胞中发挥抗凋亡作用并参与肿瘤的化疗耐药[4]。在卵巢癌中,PD-L1可通过抑制自噬及激活mTOR信号通路抑制卵巢癌细胞的增殖,并在免疫正常的小鼠中发现沉默-可显著抑制肿瘤的生长[5]。在黑色素瘤中,PD-L1通过mTOR信号通路促进肿瘤细胞的增殖[6]。在乳腺癌中,PD-L1可促进细胞增殖并通过JAK2/STAT3信号通路参与肿瘤的化疗耐药[7];在胃癌中,沉默-可影响肿瘤细胞的增殖及迁移[8]。以上研究结果表明PD-L1除了发挥其免疫功能外,在肿瘤的增殖、凋亡及化疗耐药中均可发挥重要的作用。因此,明确PD-L1在CRC中的非免疫功能有着重要的研究价值和临床意义。本研究拟探讨在CRC细胞中敲除-对司美替尼化疗敏感性的影响,并探寻逆转-敲除引起的司美替尼化疗敏感性下降的治疗方法。
材料和方法
1 细胞、主要试剂和仪器
人结肠癌RKO细胞、小鼠结肠腺癌MC38细胞及敲除-的RKO和MC38细胞株均由美国梅奥诊所秦波教授赠送。
慢病毒感染相关试剂购自Sigma;所有抗体均购自Cell Signaling Technology;Annexin V和PI购自Becton Dickinson;Gimasa试剂购自Sigma;细胞培养箱购自Thermo;流式细胞仪购自Bio-Techne。
2 方法
2.1慢病毒感染细胞培养于6 cm培养皿中,当细胞达到80% 融合时更换含有6 mg/L polybrene的1 mL新鲜培养基,并加入2 mL含髓细胞白血病基因1(myeloid cell leukemia-1,-) shRNA质粒的慢病毒悬液,培养箱孵育12 h后,更换含嘌呤霉素(1∶5 000)的新鲜培养基进行筛选,待细胞数量足够时采用Western blot测定感染效率。
2.2Western blot收集培养皿中的所有细胞至15 mL离心管,1 500 r/min离心5 min,弃去上清后冷的PBS洗涤细胞,加入SDS裂解液裂解细胞并100 ℃煮沸20 min。样本上样到SDS-PAGE凝胶孔中,100 V电泳1 h,蛋白转至PVDF膜后用5%牛奶室温封闭30 min。PBST洗膜3次后加入Ⅰ抗4 ℃孵育过夜,PBST洗膜3次后加入Ⅱ抗孵育1 h,PBST洗膜3次后加显影剂显影曝光并扫描拍照。
2.3流式细胞术每个样本设置3个重复孔,药物处理48 h后,收集培养皿中所有细胞至15 mL离心管,1 500 r/min离心5 min,弃去上清,冷的PBS洗细胞2次,100 μL 1× Binding Buffer重悬细胞,每管分别加入Annexin V和PI染色,以上操作全部在冰上完成。室温静置30 min后上流式细胞仪测定结果。
2.4细胞集落形成实验细胞融合度约为80%时收集细胞至15 mL离心管,用0.25%胰蛋白酶消化后吹打成单个细胞,1 500 r/min离心5 min,PBS洗涤后无血清培养基重悬细胞,细胞计数后使用不同药物浓度的无血清培养基将细胞密度稀释至5×105/L,2 mL细胞悬液加入6孔板中,每个浓度设3个重复孔。14 d后,弃培养基,PBS洗涤细胞后加入甲醛固定细胞5 min,吸去甲醛加入Gimsa染常温染色30 min,自来水缓流洗去染色液,孔板干燥后计数细胞集落的数量。
3 统计学处理
采用SPSS 16.0软件行统计分析。所有数值均采用均数±标准差(mean±SD)表示。组间均数比较采用独立样本检验。以<0.05为差异有统计学意义。
结果
1 敲除PD-L1降低CRC细胞对司美替尼的化疗敏感性
RKO细胞及敲除-的RKO细胞经过司美替尼的处理后,Western blot检测相关蛋白的表达,结果发现司美替尼可显著降低p-ERK蛋白水平,表明司美替尼可有效阻断MEK/ERK信号通路,并且司美替尼可显著抑制PD-L1蛋白的表达,这与之前的研究结果一致[9];重要的是,敲除-可显著降低细胞中C-Casp-3蛋白水平(图1A)。流式细胞术结果发现,敲除-可显著降低司美替尼引起的细胞凋亡(图1B)。细胞集落形成实验证实,在低浓度的司美替尼培养基中,敲除-可显著提高RKO细胞的存活率(图1C)。以上结果表明,敲除-可降低CRC细胞对司美替尼的化疗敏感性。
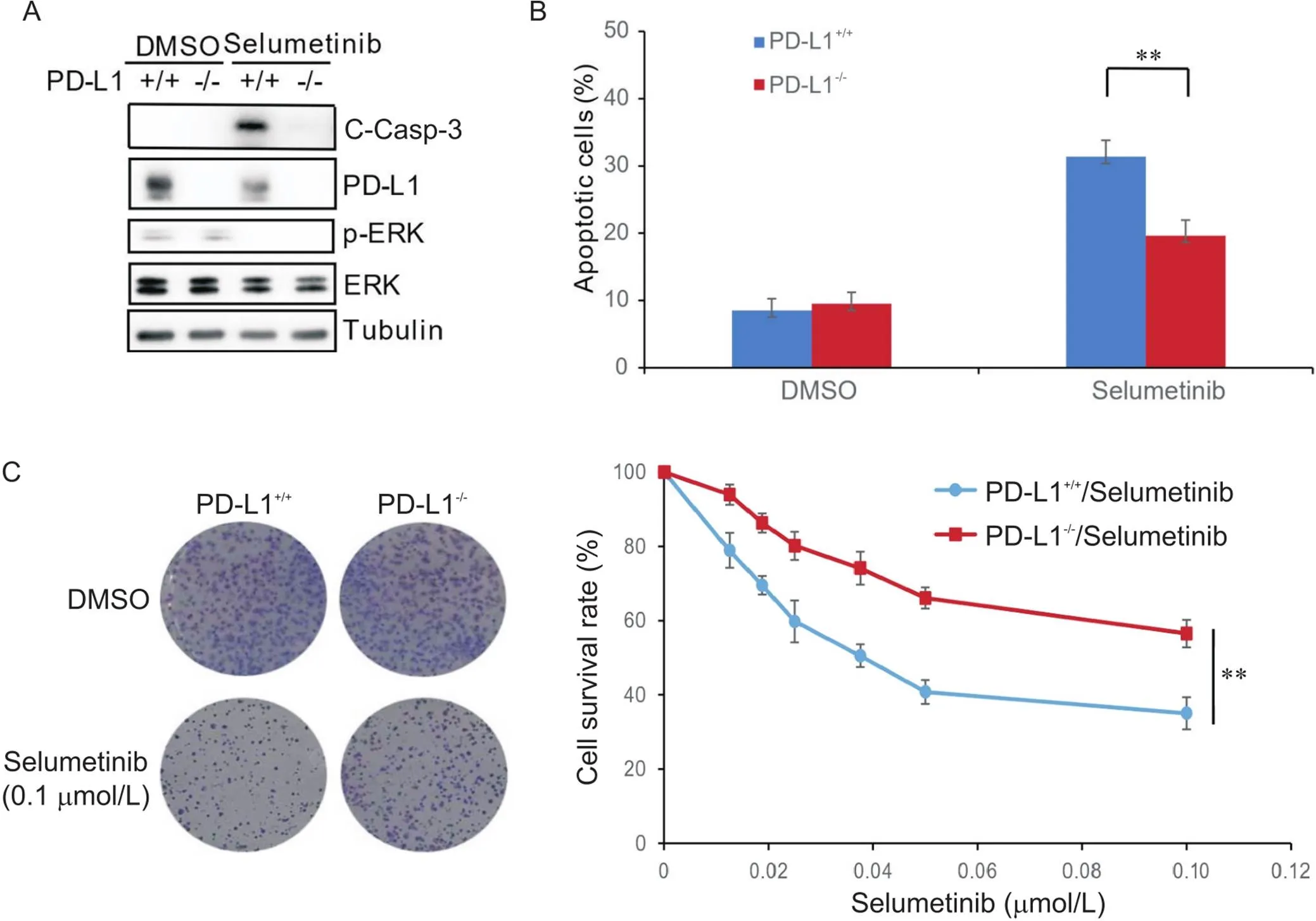
Figure 1. Knockout of PD-L1 decreased chemosensitivity of human colon carcinoma RKO cells to selumetinib. A: Western blot results showed that selumetinib significantly decreased p-ERK protein level, and knockout of PD-L1 significantly decreased selumetinib-induced cleaved caspase-3 (C-Casp-3) protein level; B: flow cytometric results showed that knockout of PD-L1 significantly increased the apoptotic rate after selumetinib treatment; C: colony formation assay results showed that knockout of PD-L1 significantly increased the cell survival rate after selumetinib treatment. Mean±SD. n=3. **P<0.01.
2 沉默MCL-1可逆转PD-L1敲除引起的CRC细胞对司美替尼化疗敏感性下降
MCL-1可通过结合Bim蛋白抑制线粒体途径的细胞凋亡,而司美替尼可通过激活caspase通路诱导细胞凋亡。因此,我们推测沉默-可能会逆转敲除-引起的CRC细胞对司美替尼的耐药性。首先,在RKO及敲除-的RKO细胞中慢病毒感染沉默-,然后加入司美替尼处理细胞48 h。Western blot结果发现,沉默-可显著升高两组细胞中C-Casp-3蛋白水平,且两组细胞之间C-Casp-3蛋白水平未见显著差异(图2A)。流式细胞术结果发现,沉默-可显著增加司美替尼引起的细胞凋亡,并逆转了-敲除引起的两组细胞凋亡的差异(图2B)。
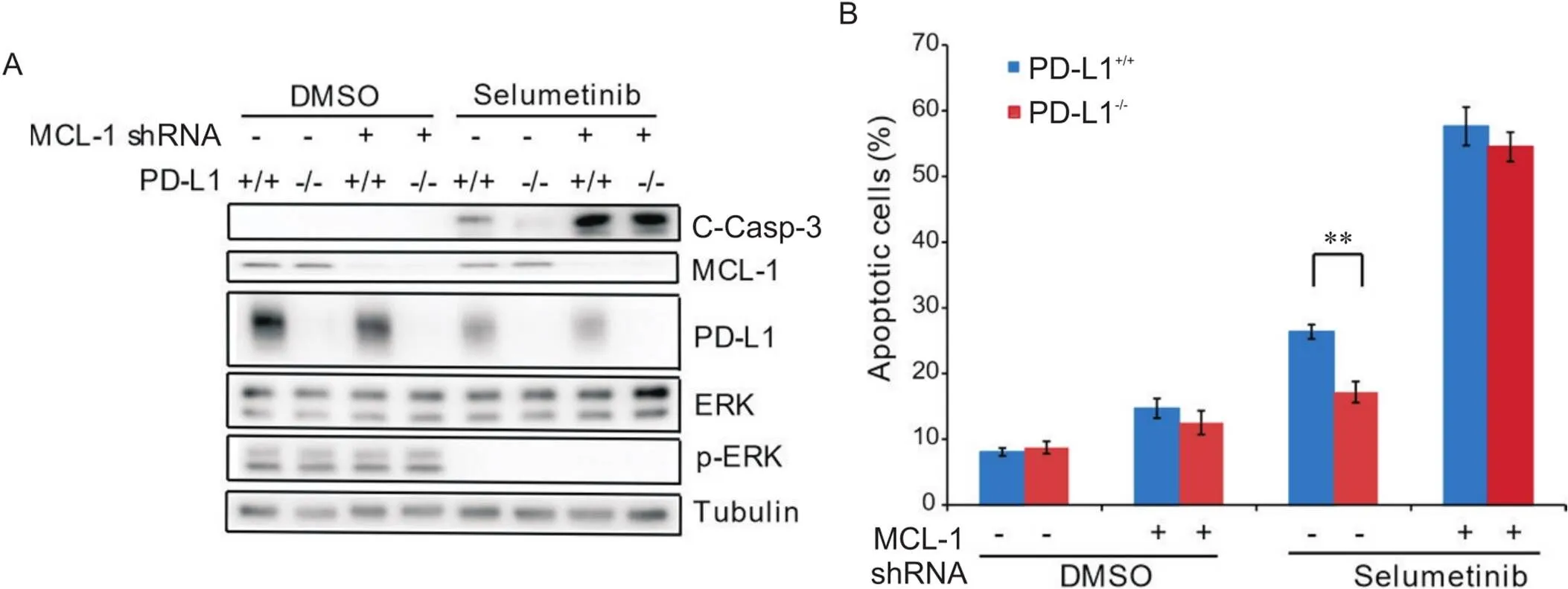
Figure 2. Knockdown of MCL-1 reversed PD-L1 knockout-decreased chemosensitivity of human colon carcinoma RKO cells to selumetinib. A: Western blot results showed that knockdown of MCL-1 significantly increased selumetinib-induced C-Casp-3 protein level in both RKO and PD-L1 knockout RKO cells, and no significant difference was shown in the two cell lines; B: flow cytometric results showed that knockdown of MCL-1 significantly increased the apoptosis of both RKO and PD-L1 knockout RKO cells induced by selumetinib, and no significant difference was shown in the two cell lines. n=3. **P<0.01.
3 联合使用MCL-1抑制剂AZD5991可逆转PD-L1敲除引起的司美替尼化疗敏感性下降
AZD5991是MCL-1的特异性抑制剂,我们联合使用AZD5991和司美替尼共处理RKO细胞48 h。Western blot结果发现,联合使用AZD5991可显著升高两组细胞中C-Casp-3蛋白水平,两组细胞之间C-Casp-3蛋白水平未见显著差异(图3A)。流式细胞术结果表明,联合使用AZD5991可显著增加司美替尼引起的细胞凋亡,并逆转了-敲除引起的细胞凋亡的差异(图3B)。细胞集落形成实验结果发现,联合使用AZD5991可显著降低低司美替尼浓度下的细胞存活率,并逆转了-敲除引起的细胞存活率的差异(图3C)。
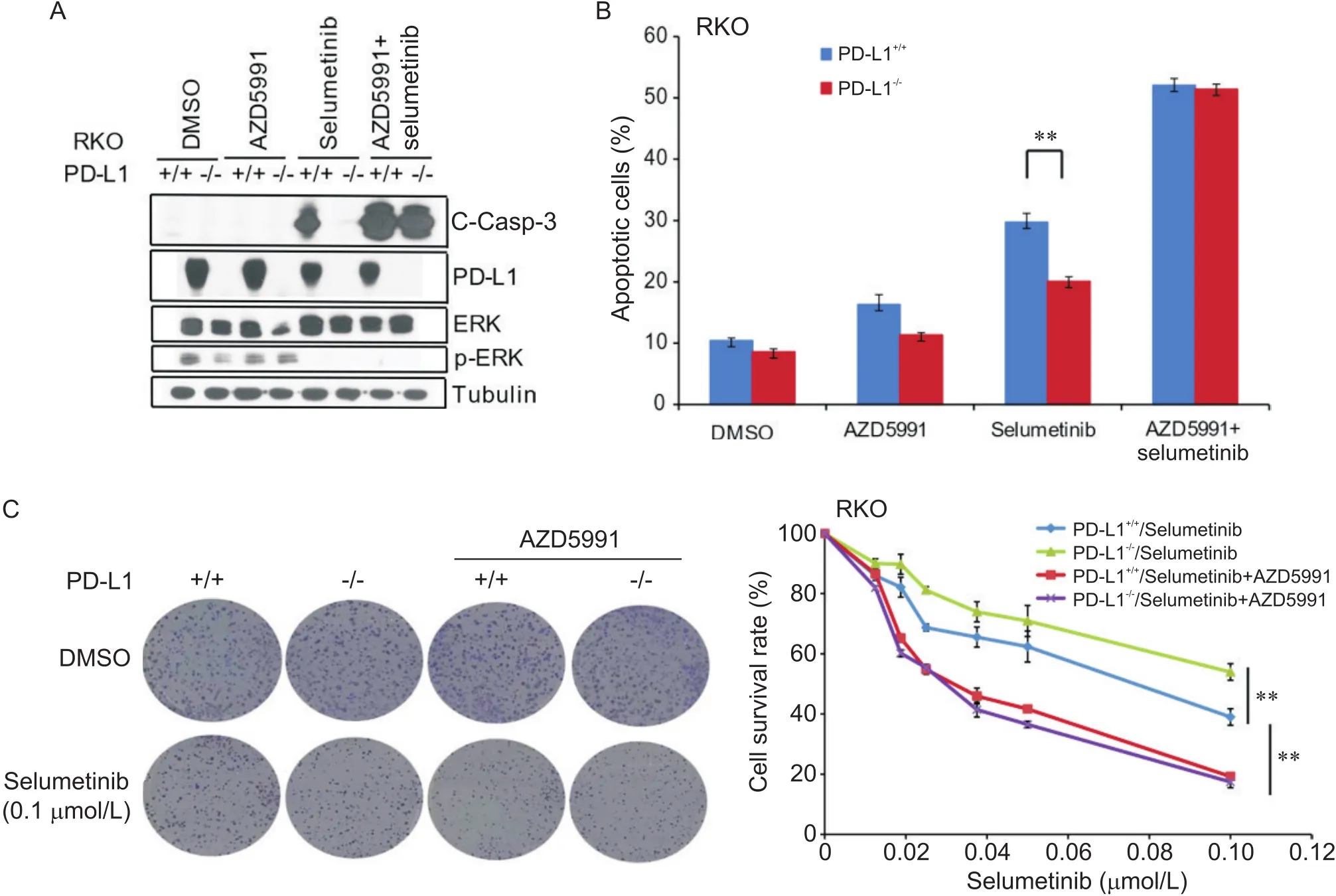
Figure 3. Combination treatment with AZD5991 reversed PD-L1 knockout-decreased chemosensitivity of human colon carcinoma RKO cells to selumetinib. A: Western blot results showed that combination treatment with AZD5991 significantly increased selumetinib-induced cleaved caspase-3 (C-Casp-3) protein level in both RKO and PD-L1 knockout RKO cells, and no significant difference was shown in the two cell lines; B: flow cytometric results showed that combination treatment with AZD5991 significantly increased selumetinib-induced apoptosis of both RKO and PD-L1 knockout RKO cells, and no significant difference was shown in the two cell lines; C: colony formation assay results showed that combination treatment with AZD5991 significantly decreased the survival rate of both RKO and PD-L1 knockout RKO cells, and no significant difference was shown in the two cell lines. Mean±SD. n=3. **P<0.01.
为进一步证实以上研究结果,我们在小鼠结肠腺癌MC38细胞中重复上述实验,结果发现,沉默-可显著降低细胞中C-Casp-3蛋白水平及司美替尼引起的细胞凋亡,增加了低司美替尼浓度下的细胞存活率;联合使用AZD5991可逆转-敲除引起的C-Casp-3、细胞凋亡及细胞存活率的差异(图4)。
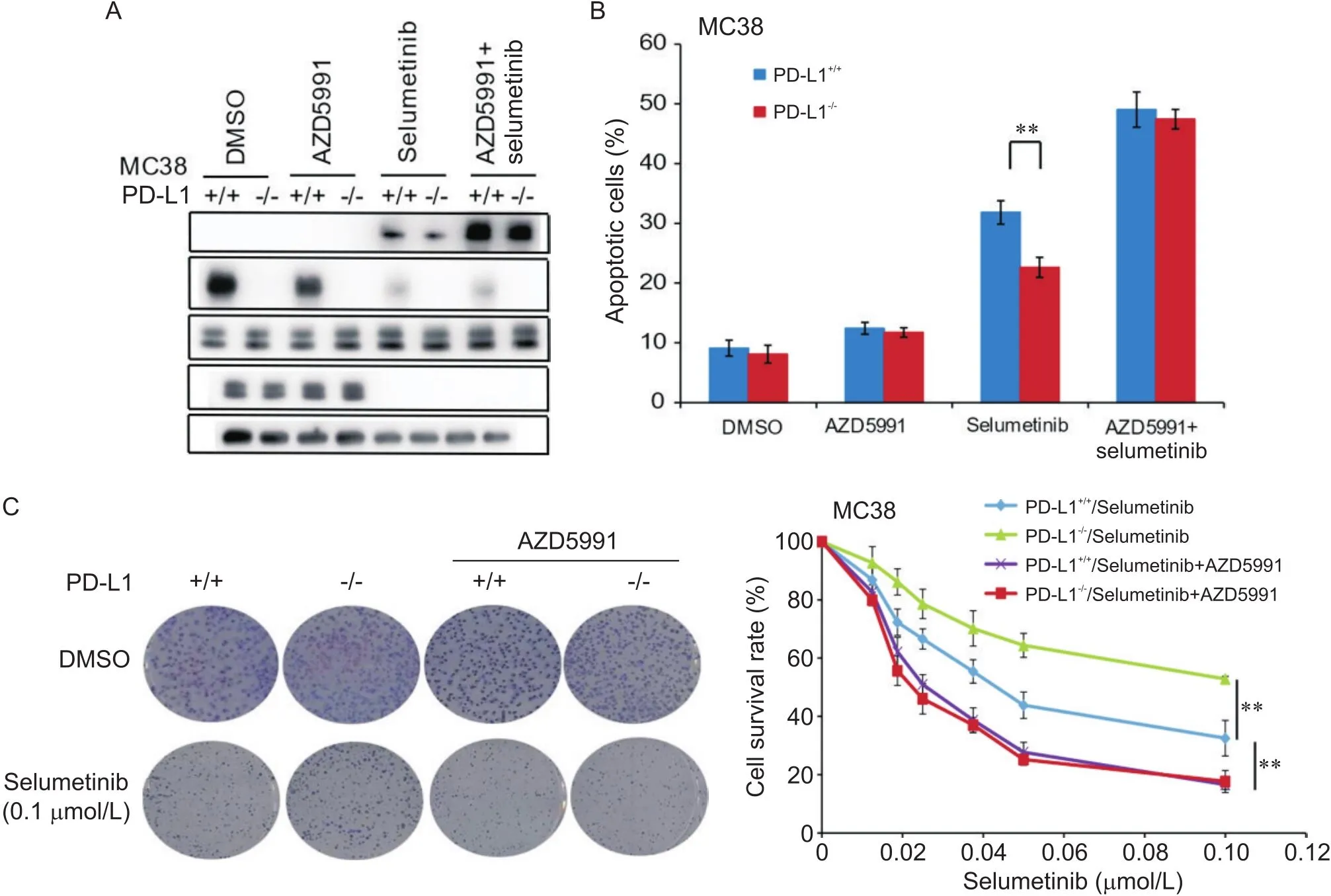
Figure 4. Combination treatment with AZD5991 reversed PD-L1 knockout-decreased chemosensitivity of mouse colon adenocarcinoma MC38 cells to selumetinib. A: Western blot results showed that combination treatment with AZD5991 significantly increased selumetinib-induced cleaved caspase-3 (C-Casp-3) protein level in both MC38 and PD-L1 knockout MC38 cells, and no significant difference was shown in the two cell lines; B: flow cytometric results showed that combination treatment with AZD5991 significantly increased selumetinib-induced apoptosis of both MC38 and PD-L1 knockout MC38 cells, and no significant difference was shown in the two cell lines; C: colony formation assay results showed that combination treatment with AZD5991 significantly decreased the survival rate of both MC38 and PD-L1 knockout MC38 cells, and no significant difference was shown in the two cell lines. Mean±SD. n=3. **P<0.01.
以上实验结果表明,联合AZD5991可显著提高两组细胞对司美替尼的化疗敏感性,并逆转了-敲除引起的细胞对司美替尼化疗敏感性下降。
讨论
PD-L1是重要的免疫检查点,并且抗PD-L1/PD-1治疗在部分肿瘤患者中取得令人瞩目的疗效,因此,PD-L1与PD-1结合后诱导的免疫逃逸机制成为目前的研究热点。然而,除了免疫检查点的功能外,PD-L1自身在肿瘤的发展中同样发挥着重要的作用。PD-L1在肿瘤细胞或者免疫细胞中的表达水平被认为是抗PD-L1/PD-1治疗有效的预测因子[10],然而,在CRC组织中,PD-L1表达的阳性率较低[2-3],并且部分表达阳性患者对抗PD-L1/PD-1治疗无效。因此,明确PD-L1自身在肿瘤发展中的机制同样有着重要的作用。在胰腺癌小鼠模型中发现,抑制PD-L1可通过抑制PI3K/Akt/mTOR信号通路抑制肿瘤的生长及转移[11];在人头颈部鳞癌及肺腺癌组织中发现,PD-L1可促进上皮间质变,细胞中E-cadherin表达下降,而vimentin表达增加[12-13]。此外,PD-L1在肿瘤化疗耐药中同样发挥重要的作用,在卵巢癌细胞中,沉默-通过抑制P-gp及细胞周期蛋白D1进而增加细胞对顺铂的化疗敏感性[14],在头颈部鳞癌中,沉默-可显著增加顺铂耐药细胞的化疗敏感性[15]。目前,PD-L1在CRC化疗耐药中的作用及机制的研究较少。司美替尼是MEK1/2信号通路特异性抑制剂,目前已批准应用于多个肿瘤的治疗,其作用机制主要是通过抑制ERK1/2的磷酸化阻断下游通路,并通过激活caspase通路诱导细胞凋亡。本研究首次发现,沉默-可引起CRC细胞对司美替尼化疗耐药,进一步证实了PD-L1除了发挥免疫检查点的功能外,还可通过其自身的表达水平影响CRC的化疗敏感性。
MCL-1是Bcl-2家族中重要的抗凋亡蛋白,主要通过与相关蛋白相互作用参与细胞凋亡的调节。经典的线粒体途径凋亡中,肿瘤细胞在外部刺激下可通过多个信号通路激活Bim蛋白,活化的Bim蛋白从微管蛋白复合体上释放,移位于线粒体膜,活化BAK/BAX促凋亡因子,使得寡聚化的BAX/BAK插入线粒体外膜,导致其通透性改变并释放细胞色素C,细胞色素C与凋亡蛋白酶活化因子1形成凋亡小体,凋亡小体与凋亡蛋白caspase作用并进一步激活caspases级联反应,最终导致细胞凋亡[16]。正常情况下,肿瘤细胞内的抗凋亡蛋白MCL-1通过与Bim蛋白结合抑制肿瘤细胞的凋亡,但在某些药物的作用下,MCL-1与Bim蛋白的结合力下降并促进Bim的释放,进而激活线粒体途径凋亡信号通路促进细胞凋亡[17],研究证实,MCL-1与多个肿瘤的化疗耐药相关。在乳腺癌中,MCL-1的过表达与化疗耐药明显相关[18]。在胰腺癌中,MCL-1与细胞对吉西他滨化疗耐药相关,沉默-可显著增加细胞对吉西他滨的化疗敏感性[19]。在卵巢癌中,MCL-1与细胞对卡铂化疗耐药明显相关[20]。因此,MCL-1在肿瘤的化疗耐药中发挥重要作用。本研究首次发现,无论PD-L1的表达水平如何,沉默-或联合使用AZD5991均可显著增加CRC细胞对司美替尼的化疗敏感性,并可逆转-敲除引起的对司美替尼化疗敏感性下降,这对于PD-L1表达阴性的CRC患者提高对司美替尼化疗敏感性有着重要的临床指导价值。
综上所述,我们研究发现沉默-可引起CRC细胞对司美替尼化疗敏感性下降,沉默-或者联合使用AZD5991均可逆转-敲除引起的CRC细胞对司美替尼化疗敏感性下降。PD-L1/MCL-1影响CRC细胞司美替尼化疗敏感性的分子机制尚待进一步明确。目前,MEK抑制剂已应用于V600E突变型CRC的三线姑息治疗,鉴于CRC组织中PD-L1的低表达率,MEK抑制剂联合MCL-1抑制剂有可能成为部分CRC患者新的治疗策略。
[1] Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy[J]. Nat Rev Cancer, 2019, 19(3):133-150.
[2] Inaguma S, Lasota J, Wang Z, et al. Clinicopathologic profile, immunophenotype, and genotype of CD274 (PD-L1)-positive colorectal carcinomas[J]. Mod Pathol, 2017, 30(2):278-285.
[3] Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, et al. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes[J]. Mod Pathol, 2016, 29(9):1104-1112.
[4] Azuma T, Yao S, Zhu G, et al. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells[J]. Blood, 2008, 111(7):3635-3643.
[5] Clark CA, Gupta HB, Sareddy G, et al. Tumor-intrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma[J]. Cancer Res, 2016, 76(23):6964-6974.
[6] Kleffel S, Posch C, Barthel SR, et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth[J]. Cell, 2015, 162(6):1242-1256.
[7] Liu H, Tekle C, Chen YW, et al. B7-H3 silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3 phosphorylation[J]. Mol Cancer Ther, 2011,10(6):960-971.
[8] Li J, Chen L, Xiong Y, et al. Knockdown of PD-L1 in human gastric cancer cells inhibits tumor progression and improves the cytotoxic sensitivity to CIK therapy[J]. Cell Physiol Biochem, 2017, 41(3):907-920.
[9] Feng D, Qin B, Pal K, et al. BRAF(V600E)-induced, tumor intrinsic PD-L1 can regulate chemotherapy-induced apoptosis in human colon cancer cells and in tumor xenografts[J]. Oncogene, 2019, 38(41):6752-6766.
[10] 陈淑芬,侯婧瑛,吴淑云,等. IFN-γ上调人胃癌细胞株PD-L1表达[J]. 中国病理生理杂志, 2013, 29(11):1952-1956.
Chen SF, Hou JY, Wu SY,et al. Interferon-γ up-regulates programmed death ligand1 in human gastric cancer cells[J]. Chin J Pathophysiol, 2013, 29(11):1952-1956.
[11] Zhao L, Li C, Liu F, et al. A blockade of PD-L1 produced antitumor and antimetastatic effects in an orthotopic mouse pancreatic cancer model via the PI3K/Akt/mTOR signaling pathway[J]. Onco Targets Ther, 2017, 10:2115-2126.
[12] Ock CY, Kim S, Keam B, et al. PD-L1 expression is associated with epithelial-mesenchymal transition in head and neck squamous cell carcinoma[J]. Oncotarget, 2016, 7(13):15901-15914.
[13] Kim S, Koh J, Kim MY, et al. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung[J]. Hum Pathol, 2016, 58:7-14.
[14] Zuo Y, Zheng W, Liu J, et al. MiR-34a-5p/PD-L1 axis regulates cisplatin chemoresistance of ovarian cancer cells[J]. Neoplasma, 2020, 67(1):93-101.
[15] Shen B, Huang D, Ramsey AJ, et al. PD-L1 and MRN synergy in platinum-based chemoresistance of head and neck squamous cell carcinoma[J]. Br J Cancer, 2020, 122(5):640-647.
[16] 黄晔,廖阳,沈杨炳,等. 槲皮素通过抑制c-Jun的表达水平增强5-氟尿嘧啶对胃癌细胞凋亡的诱导活性[J]. 中国病理生理杂志, 2018, 34(2):6.
Huang Y, Liao Y, Shen YB, et al. Quercetin enhances 5-fluorouracil-induced apoptosis in gastric cancer by down-regulating c-Jun expression[J]. Chin J Pathophysiol, 2018, 34(2):6.
[17] Fletcher JI, Huang DC. Controlling the cell death mediators Bax and Bak: puzzles and conundrums[J]. Cell Cycle, 2008, 7(1):39-44.
[18] Balko JM, Giltnane JM, Wang K, et al. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets[J]. Cancer Discov, 2014, 4(2):232-245.
[19] Wei SH, Dong K, Lin F, et al. Inducing apoptosis and enhancing chemosensitivity to gemcitabine via RNA interference targeting Mcl-1 gene in pancreatic carcinoma cell[J]. Cancer Chemother Pharmacol, 2008, 62(6):1055-1064.
[20] Wu X, Luo Q, Zhao P, et al. MGMT-activated DUB3 stabilizes MCL1 and drives chemoresistance in ovarian cancer[J]. Proc Natl Acad Sci U S A, 2019, 116(8):2961-2966.
Inhibition of MCL-1 reverses poor chemosensitivity of colorectal cancer cells to selumetinib induced by-knockout
SUN Lei, HONG Chu-yuan, YIN Xue-xia, GUO Xiong-bo, ZHANG Shi, ZHONG Bei-ping, WANG Guo-qiang△
(,510260,)
To determine the effects and mechanism of programmed death ligand-1-on chemosensitivity to selumetinib in colorectal cancer (CRC) cells.After human colon carcinoma RKO cells and-knockout RKO cells were treated with selumetinib, cleaved caspase-3 (C-Casp-3) protein level was detected by Western blot, and the apoptosis rate and cell survival rate were tested by flow cytometry (FCM) and colony formation assay, respectively. The RKO and-knockout RKO cells were treated with selumetinib after myeloid cell leukemia-1(-) knockdown, or co-treated with selumetinib and AZD5991, C-Casp-3 protein level was detected by Western blot, and the apoptosis rate and cell survival rate were tested by FCM and colony formation assay, respectively. After mouse colon adenocarcinoma MC38 cells and-knockout MC38 cells were co-treated with selumetinib and AZD5991, C-Casp-3 protein level was detected by Western blot, and the apoptosis rate and cell survival rate were tested by FCM and colony formation assay, respectively.Western blot showed that selumetinib significantly decreased phosphorylated extracellular signal-regulated kinase (p-ERK) protein level. Knockout of-significantly reduced selumetinib-induced C-Casp-3 protein level.-shRNA or co-treatment with AZD5991 significantly increased selumetinib-induced C-Casp-3 protein level. The FCM results showed that knockout of-significantly reduced the apoptosis induced by selumetinib, and-shRNA or co-treatment with AZD5991 significantly increased apoptosis induced by selumetinib. Colony formation assay showed that knockout of-significantly increased cell survival rate after treatment with low concentration of selumetinib, and-shRNA or co-treatment with AZD5991 significantly reduced cell survival rate. More importantly,-shRNA or co-treatment with AZD5991 eliminated the differences in C-Casp-3, apoptosis and cell survival rate caused by-knockout.Knockout of-decreases the chemosensitivity of CRC cells to selumetinib. Both genetic and pharmaceutical inhibition of MCL-1 reverses poor chemosensitivity of CRC cells to selumetinib induced by-knockout.
Myeloid cell leukemia-1; Programmed death ligand-1; Selumetinib; Colorectal cancer; Chemosensitivity
R363; R73-36; R735.3+5
A
10.3969/j.issn.1000-4718.2022.06.007
1000-4718(2022)06-1008-07
2021-11-25
2022-01-25
广州市属高校科研项目(No.1201630159);广州市科技计划市校联合项目(No.202102010062);广州医科大学附属第二医院精英人才配套项目(No. 010M07060)
Tel: 020-34153251; E-mail: wgqjyh@163.com
(责任编辑:余小慧,李淑媛)
